Abstract
A relationship between chemical constituents and physicochemical and textural properties of different Korean sweet potato (SP) cultivars were investigated in terms of the hardness of raw and cooked root, alcohol insoluble solid (AIS), starch and amylose content, amylase activities, and pasting properties. Correlation coefficients were analyzed and principal component analysis was performed. The hardness of cooked root was found to correlate with the hardness ratio of cooked/raw SP, AIS content (0.75**), starch content (0.64**), and peak viscosity of SP powder (0.56*). It was discovered that SP cultivars could be classified into mealy (Sincheonmi, Daeyumi, Sinyulmi), intermediate (Dahomi, Sinjami, Geonhwangmi, Yeonjami, Pungwonmi), and waxy (Juhwangmi, Sinhwangmi) types depending on the first principal component (PC1, 68%). Therefore, it was found that the texture types of SP cultivars could be predicted from hardness of cooked root and are in relation to AIS and starch contents, and peak viscosity of raw powder.
Keywords: Sweet potato cultivar, Textural character, Hardness, Correlation coefficient, Principal component analysis
Introduction
Sweet potato (Ipomoea batatas L.), a tuberous root vegetable, is an economically important crop and is preferred as a staple and famine relief food in many tropical and subtropical countries for its high crop yields and wide ecological adaptability; it can survive at a variety of latitudes and temperatures and in different soil types (Han et al., 2013). In addition, sweet potato (SP) is an excellent source of carotene, dietary fiber, and vitamins, as well as the amino acids, lysine and threonine. It is a nutritious and abundant food source for humans and animals as well as a raw material for manufacturing (Lai et al., 2016).
There are numerous SP genotypes with different sensory characteristics such as taste, flesh color, and texture. Distinctive flesh colors (white/cream, deep yellow, orange, and purple) from flesh pigments like anthocyanins, carotenoids, and phenolic compounds which play a role as antioxidants (Teow et al., 2007). Textural characteristics of SP storage roots have two major types, which are mealy (dry, firm) and soggy (moist, waxy) after cooking SP (Kitahara et al., 2017; Sato et al., 2017; Walter et al., 2000). In the current breeding system in Japan, the textural characteristics are divided into five categories: mealy, slightly mealy, intermediate, slightly soggy, and soggy (Kitahara et al., 2017).
Because this sensory evaluation system has some shortcomings, it has become necessary to establish an invariant evaluation system of the texture of the SP by identifying the relationships between the textural characters of the SP and physicochemical properties of its constituents. The textural characteristics of the SP are considered to be affected by the starch concentration, amylolytic enzyme, amylose content, tissues and cells including pectic substances and calcium, and cell wall polysaccharides such as cellulose and hemicellulose (Noda et al., 1994; Nakamura et al., 2015; Takahata et al., 1995; Walter et al., 1975). However, Walter et al. (2000) reported that the textural properties of cooked SPs were not attributable to amylose content, granule size, gelatinization, or pasting properties of starch.
Shin and Ahn (1987) reported that a mealy type SP, Wonki, had higher alcohol insoluble solids, starch, and protopectin contents and amylase activity than a waxy type SP, Chunmi. There are little information about the relationship between chemical constituents of SP and textural properties of cooked SP, and therefore, further investigations are still needed.
In Korea, the SP has been used as a famine relief food, starch source, diet food, health functional food, a base material for starchy noodles (Dangmeon) and a component of distilled liquor (Soju), among others. But most consumers are consuming the SP as raw or cooked SP storage roots themselves. Therefore, textural characters of SP are considered to be most important to breed any new SP cultivars. Various inbred cultivars have been developed (Lee et al., 2010a; 2010b; 2015; 2016) and many studies have investigated the characteristics of SP powders and the physicochemical properties of SP starches (Baek et al., 2014; Garcia and Walter, 1998; Han et al., 2013; Kim et al., 2011; Tian et al., 1991). Nevertheless, the principal components to classify the SP into the textural properties of SP have not yet been identified.
Therefore, the objectives of this study were to investigate amylase activities and starch contents of SP, hardness of raw and cooked SP root, and pasting properties of powders from the ten Korean SP cultivars, to measure the physicochemical and pasting properties of starches and to analyze correlation coefficients and principal components of SP cultivars.
Materials and methods
Materials
Ten different SPes from Korean cultivars that were harvested from an experimental field in 2016 were obtained by Bioenergy Crop Research Institute (Muan, Korea) Korea. The orange-fleshed Sinwhangmi and Juwhangmi cultivars, the purple-fleshed Sinjami and Yeonjami varieties, the white/cream-fleshed Sinyulmi and Sincheonmi, and the newly inbred cultivars Geonwhangmi, Pungwonmi, Dahomi, and Daeyumi were used. The sweet potato (SP) cultivars were harvested between October 12 and 14, 2016 and were stored at room temperature for 5 days. All of these samples were treated for experimental purposes. The texture of SP roots were measured, and the starch was purified from SP on October 20, 2016 and the other samples which were measured enzyme activities and AIS contents, and were made SP powder were stored in an ultralow temperature freezer (− 80 °C, MDF-U52 V, SANYO Electric Co., Ltd., Osaka, Japan).
The α-amylase activity and β-amylase activity assay kits were purchased from Megazyme Ireland Limited (Wicklow, Ireland). Sodium sulfate anhydrous was purchased from Junsei Chemical Co. Ltd. (Saitama, Japan). Purified amylose and amylopectin isolated from potato starch were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Measurement of hardness of sweet potato
The textural properties of raw and cooked SP root flesh were measured with a texture analyzer (TA-XT plus, Stable Micro Systems, Ltd., Surrey, England). Sample was prepared by washing, peeling, and cutting the root flesh (1 × 1 × 1 cm3). The cooked sample was prepared by steaming of cut sample for 10 min. A two-bite compression test with 60% deformation using a cylinder probe (φ 20 mm) was used. The test speed was 1.0 mm/s.
Preparation of sweet potato powder
Raw SP storage roots were washed and peeled manually. Peeled roots were put into water to prevent the browning reaction caused by polyphenol oxidase and diced into an appropriate size for rapid freezing. The pieces of SP roots were freeze-dried at − 80 °C for 5 days using a freeze-drier (Ilshin Biobase Co., Ltd., Dongducheon, Korea). Dried samples were ground and passed through a 100-mesh sieve. The SP powders were stored in a light-protective bag at room temperature until use.
Preparation of sweet potato starch
Starch was isolated from the SP roots using an alkaline steeping method (Kim et al., 2013). The pieces of roots were steeped in a 0.2% NaOH solution (Daejung Chemicals & Metals Co. Ltd., Shiheung, Korea) and pulverized using a food blender (Hanarossak, Daesung Artlon Co., Paju, Korea) for 2 min (× 5 times). The starch slurry was passed through 100 and 270 mesh sieves in sequence. The residue was blended with an alkaline solution and washed to remove protein using 0.2% NaOH until the yellow layer disappeared. The starch slurry was washed with distilled water, neutralized using 1 N HCl, washed again, and centrifuged (VS-21 SMT, Vision Scientific Co., Ltd., Seoul, Korea, 5000×g, 10 min). The precipitated starch was dried at room temperature, ground, and passed through a 100-mesh sieve. The starch was stored in a desiccator until use. The starch content (%) was calculated by percent of starch yield (g) divided by weight of sample SP root (g).
Measurement of properties of sweet potato
Moisture content and color values
The moisture contents of ten different SP roots were measured by AACCI Method 44-15.02 air oven method (2012). The color values of raw SP flesh were measured using a colorimeter (SpectraMagic™ NX, Konica Minolta, Tokyo, Japan). The color values, L (lightness), ± a (redness/greenness), and ± b (yellowness/blueness) values were measured. The instrument was calibrated using a standard white plate (L = 96.82, a = − 0.08, b = − 0.18) and the color difference (ΔE) was calculated using the equation below:
Alcohol insoluble solid content
The diced raw SP root flesh (10 g) was put into a beaker and 99% ethanol (~ 20 mL) was added; then, the ethanol concentration was adjusted to 80% ethanol. The sample was homogenized using a homogenizer (WiseTis HG-15A Homogenizer with Direct Controller, Witeg Labortechnik GmbH, Wertheim, Germany) at 1200 rpm. The supernatant was transferred into another beaker and the residue was washed again with 80% ethanol. The slurry was filtered through a Büchner funnel with Whatman No. 2 filter paper (Whatman Ltd., Maidstone, England). The residue (AIS, alcohol insoluble solid) was dried at room temperature, weighed, and the AIS content (%) was calculated as follows:
Determination of α-, and β-amylase activities
The α-amylase and β-amylase activities of SP root powder were measured using Megazyme kits, Ceralpha method (AACCI, 2012) and Betamyl-3 method (Hagenimana et al., 1992), respectively.
Enzymes were extracted from freeze-dried SP powder (0.5 g) with a solution of 1% sodium chloride, 0.02% calcium chloride, and 0.02% sodium azide, and buffer solution. The enzyme extracts were obtained by stirring for 1 h and filtering through a Whatman GF/B glass fiber filter and a membrane filter. To analyze α-amylase activities, the diluted extract warmed in a 40 °C water bath for 5 min and an amylase HR reagent which contained p-nitrophenyl α-d-maltoheptaoside (blocked) plus a thermostable α-glucosidase (replacing the thermostable α-glucosidase used in the former mixture) solution (0.2 mL) was added to the extract and incubated for 20 min. For β-amylase activities, the extract and 0.2 mL of Betamyl-3® substrate solution were stirred using a vortex mixer and incubated in a 40 °C water bath for 10 min. A stopping reagent (3.0 mL) was added and stirred vigorously. The absorbance of samples was measured at 400 nm using a UV–Vis spectrophotometer (Optizen POP, Mecasys Co., Ltd, Daejeon, Korea) and activities of α-, and β-amylase were calculated by the following equation:
Determination of properties of SP powder and starch
Amylose content and water binding capacity
The apparent amylose content (AAC) of starch was determined using the method of Park et al. (2012), which was modified from that of Williams et al. (1970). A standard curve for amylose content was created using the same method with purified amylose and amylopectin from potato starch (Sigma-Aldrich St. Louis. MO, USA). The water binding capacity (WBC) of SP powder was measured by the method of Medcalf and Gilles (1965).
Rapid Visco-Analyzer
The pasting properties of SP powder and starch were analyzed with a rapid visco analyzer (RVA, TecMaster, Perten Instruments AB, Hagersten, Sweden). The sample (3 g, 14% moisture basis, mb) and distilled water (25 mL) were added into an aluminum canister with stirring. The suspension was held at 50 °C for 1 min, heated to 95 °C at a rate of 12 °C/min, and held at 95 °C for 3 min. It was then cooled down from 95 to 50 °C at a rate of 12 °C/min and held at 50 °C for 2 min. Initial pasting temperature, peak viscosity (P), trough viscosity (T), final viscosity (F), breakdown viscosity (P–T), and setback viscosity (F–T) were measured. All the viscosity parameters were measured twice and the results are presented in centipoise.
Statistical analysis
The data are represented as means and standard deviations of at least triplicate measurements. Statistical analyses were carried out with Student’s t test and Duncan’s multiple range test (p < 0.05). Correlations among the chemical constituents and their properties of SP cultivars were analyzed by Pearson’s correlation coefficients. SPSS 12.0 K (SPSS Inc., Chicago, IL, USA) was used for the analyses.
Principal component analysis (PCA) was performed using the Unscraambler, v.9.1 (Camo, Oslo, Norway) to summarize and visualize differences and similarities among SP from different Korean cultivars according to texture types for various properties of SP.
Results and discussion
Hardness of sweet potato
The hardness values of raw and cooked SP roots are shown in Table 1. The hardness values of raw and cooked SP were significantly different (p < 0.05) with cultivars. From the SP breeders of Bioenergy Crop Research Institute, ten different SP cultivars were divided into these categories, with Sinhwangmi, Juhwangmi, and Yeonjami being waxy, Geonhwangmi and Pungwonmi being intermediate, and Dahomi, Daeyumi, Sinjami, Sinyulmi, and Sincheonmi being mealy (NICS, RDA, 2017). Cooked SP has been arbitrarily classified as moist or yam types, which have a soft, syrupy texture, and dry types, which exhibit a firm, mealy texture (Walter et al., 2000). This property describes the mouthfeel characteristics and is independent of water content (Rao et al., 1974). In the current breeding system in Japan, the textural characteristic of a cultivar or breeding line is sensually determined after cooking by comparison with the textural characteristic of the standard cultivar “Kokei-14”. They are sorted into five categories: mealy, slightly mealy, intermediate (texture of standard cultivar), slightly soggy, and soggy (Kitahara et al., 2017). The chemical constituents, starch, amylolytic enzymes, amylose, pectic substance, cellulose, and hemicellulose have affected by texture of cooked root (Kitahara et al., 2017; Nakamura et al., 2015).
Table 1.
Hardness and hardness ratio of steamed and raw sweet potato cultivars
| Sweet potato cultivars | Hardness (raw) (kg) | Hardness (cooked) (g) | Hardness ratio (%)2) of cooked × 100/raw |
|---|---|---|---|
| Geonhwangmi | 25.44 ± 0.23bc1) | 330.69 ± 7.82f | 1.29 ± 0.04g |
| Dahomi | 24.75 ± 0.25c | 460.24 ± 6.04e | 2.53 ± 0.02e |
| Daeyumi | 18.48 ± 0.44f | 981.01 ± 22.54a | 3.94 ± 0.07b |
| Sinyulmi | 21.75 ± 1.03d | 617.56 ± 15.65d | 2.77 ± 0.16d |
| Sinjami | 26.26 ± 0.14ab | 809.23 ± 10.92b | 3.09 ± 0.04c |
| Sincheonmi | 15.81 ± 0.24g | 751.38 ± 9.22c | 4.71 ± 0.07a |
| Sinhwangmi | 26.25 ± 0.21ab | 233.79 ± 18.28g | 0.89 ± 0.08h |
| Yeonjami | 22.75 ± 0.10d | 255.32 ± 40.67g | 1.12 ± 0.18h |
| Juhwangmi | 20.12 ± 1.33e | 178.03 ± 22.78h | 0.92 ± 0.13h |
| Pungwonmi | 26.85 ± 0.94a | 448.24 ± 19.44e | 1.64 ± 0.10f |
Values are given as mean ± SD
1)a–hValues with different superscripts in the same column are significantly different at p < 0.05 by Duncan’s multiple range test
2)Hardness ratio (%) means hardness of cooked sweet potato flesh divided with hardness of raw sweet potato flesh × 100
In terms of the hardness of raw SP root, the hardness was not related to the texture characteristic of cooked SP. The mealy type Sincheomi cultivar had the lowest hardness value, but the mealy type Sinjami, waxy type Sinhwangmi, and intermediate type Pungwonmi had higher hardness values. Nevertheless, the hardness of cooked SP root showed similar trends of SP breeders’ opinions (NICS, RDA, 2017). Unlike the hardness trends of raw SP roots, the hardness of cooked SP root was lower in the waxy type SP than in the mealy type SP. Daeyumi cultivar had the highest hardness, and Juhwangmi cultivar showed the lowest value among the cultivars. To investigate the relationship between textural characteristics and breeders’ opinion, the hardness of raw and cooked SP and ratio of cooked/raw SP roots were compared. The hardness of cooked root showed similar trends with the hardness ratio of raw and cooked root. Daeyumi, Sinjami, Sincheonmi, and Sinyulmi were higher in hardness of cooked roots and hardness ratio cooked/raw roots than others. Juhwangmi, Sinhwangmi, and Yeonjami had lower values than others. The hardness of Dahomi and Pungwonmi showed similar to the mealy type, but the hardness ratio cooked/raw of Geonhwangmi and Pungwonmi showed similar to the waxy type. From the hardness of cooked root and hardness ratio of cooked/raw root, Korean SP cultivars can be assorted into three categories: mealy, intermediate, and waxy types. However, there are little information about the direct causes affecting textural characters of SP root during cooking.
Alcohol insoluble solid content
Alcohol insoluble solid (AIS) content is influenced by alcohol concentration; however, AIS is composed with macromolecules such as dietary fiber, starch and protein. Kathleen (1970) reported that the correlation coefficient between AIS (80% ethanol) and dry matter (DM) contents was 0.99 to measure maturity of French beans. The AIS of SP root was included in starch, dietary fiber, and protein, because low molecular weight materials, free sugar, amino acid, vitamins, and mineral were solubilized in 80% ethanol. The AIS contents ranged from 15.43 to 34.56% (Table 2) and were highly correlated with DM. The AIS contents of Daeyumi, Sincheonmi, Sinyulmi, and Sinjami (29.95–34.56%) were higher than those of Juwhangmi and Sinwhangmi (15.43–19.50%). The Dahomi showed higher hardness of cooked root, but lower AIS content than Daeyumi. The AIS of Daeyumi was related to higher starch content similar to Sinyulmi and Sincheonmi. Hernández-Carrión et al. (2011) reported that differences in AIS between raw and cooked SP implied that the starch granules within the cells of SP flesh are hydrolyzed into sugars during the heating process by amylolytic enzymes. Therefore, it was suggested that amylase activities of Daeyumi should affect the hardness of cooked SP root and AIS content.
Table 2.
Alcohol insoluble solid (AIS) content and α- and β-amylase activities of 10 different sweet potato cultivars
| Sweet potato cultivars | Moisture content | Starch content (%) | AIS content (%) | α-amylase activity | β-amylase activity |
|---|---|---|---|---|---|
| Geonhwangmi | 71.86 ± 0.91c | 11.99 ± 0.45bc | 26.30 ± 1.06c1) | 3.19 ± 0.27e | 0.85 ± 0.03c |
| Dahomi | 74.40 ± 0.04b | 10.69 ± 0.59bcd | 24.12 ± 0.48cd | 3.38 ± 0.00e | 0.67 ± 0.08cde |
| Daeyumi | 62.22 ± 0.19e | 18.57 ± 0.00a | 34.56 ± 1.69a | 1.50 ± 0.00h | 0.77 ± 0.08cd |
| Sinyulmi | 66.13 ± 1.15d | 18.34 ± 0.12a | 30.73 ± 0.07b | 3.76 ± 0.00d | 1.07 ± 0.04b |
| Sinjami | 74.47 ± 1.12b | 13.74 ± 0.26b | 29.95 ± 1.88b | 7.52 ± 0.00c | 0.58 ± 0.01de |
| Sincheonmi | 67.33 ± 0.11d | 20.05 ± 1.80a | 34.07 ± 1.82a | 1.88 ± 0.00g | 0.51 ± 0.06d |
| Sinhwangmi | 76.82 ± 1.26a | 8.78 ± 0.13d | 15.43 ± 0.46f | 8.65 ± 0.00b | 1.33 ± 0.07a |
| Yeonjami | 77.13 ± 0.33a | 12.43 ± 1.49bc | 22.79 ± 1.36d | 2.63 ± 0.00f | 0.66 ± 0.04cde |
| Juhwangmi | 72.85 ± 0.64bc | 9.76 ± 0.23cd | 19.50 ± 0.10e | 14.48 ± 0.27a | 1.33 ± 0.21a |
| Pungwonmi | 72.10 ± 2.24bc | 13.10 ± 3.44b | 21.93 ± 0.13de | 3.38 ± 0.00e | 0.52 ± 0.07e |
Values are given as mean ± SD
1)a–hValues with different superscripts in the same column are significantly different at p < 0.05 by Duncan’s multiple range test
Amylase activities of SP powder
The α-amylase and β-amylase activities of SP cultivars are presented in Table 2. The α-amylase and β-amylase activities ranged from 1.50 to 14.48 unit/g and from 0.51 to 1.33 unit/g, respectively. Alpha-amylase is a liquefying enzyme that hydrolyzes α-1,4 glucan, whereas β-amylase (EC 3.2.1.2) is an exo-enzyme that catalyzes the hydrolysis of α-(1,4) glycosidic linkages in polysaccharides and removes successive maltose units from the non-reducing ends (Hagenimana et al., 1992). The α-amylase activity of Daeyumi was the lowest (1.50 unit/g), and that of Juhwangmi was the highest (14.48 unit/g). SP root has very starchy properties when freshly harvested and the starch content of SP root gradually reduces during storage after curing procedure. During storage, the decrease in starch content corresponds to an increase in the sugar content of roots because of the reaction of α-amylase (Szyperski et al., 1986). Especially, the storage stability of orange fleshed SP roots, Juhwangmi and Sinhwangmi, was lower than others due to their high α-amylase activities. However, Daeyumi cultivar inbred to make bioethanol using starch (Lee et al., 2010a), showed lower α-amylase activities. The β-amylase activity influenced by sweet taste after cooking was the highest in Sinhwangmi and Juhwangmi. The orange-fleshed Juhwangmi and Sinhwangmi cultivars had lower starch and AIS contents, but they had been popularly consumed owing to their sweet taste and soft texture after cooking. It is suggested that the amylolytic enzymes also affect textural characteristics of SP apart from AIS and starch contents.
Apparent amylose content and water binding capacity
The apparent amylose contents and water binding capacities of ten different SP starches are shown in Table 3; they ranged from 18.99 to 25.57%, similar to previously reported data (Kim et al., 2013; Tian et al., 1991). The AACs of SP cultivars were significantly different by cultivars, but the trend was not correlated with textural characteristics of SP roots. The AAC of Daeyumi and Sincheonmi was the highest, but that of Yeonjami was the lowest. Generally, it is well known that the amylose content and molecular structure of starch affect pasting properties and gelatinization temperature (Baek et al. 2014; Kim et al., 2013). Nevertheless, the physicochemical and pasting properties of SP, and the molecular structure of starches purified from the SP root with same cultivars showed reverse trends (Baek et al. 2014; Han et al., 2013; Kim et al., 2013). These variations might be caused from different environment, cultivation and storage conditions (Han et al., 2013; Noda et al., 1996). The WBC of starch is measured by the extent to which water penetrates into the amorphous part of the starch granules and by the water absorption on the surface of starch granules. Sinhwangmi, and Pungwonmi starches had the highest WBC values (180.77 and 163.66%), whereas Dahomi, Sinyulmi, Yeonjami, and Sinjami starches had the lowest value (122.10, 122.06, 125.27, and 126.61%, respectively) (Table 3). It was regarded that apparent amylose content and WBC of SP starches are not related to textural characteristics of SP roots.
Table 3.
Amylose contents and water binding capacities of sweet potato starches purified from ten different cultivars
| Sweet potato cultivars | Amylose content (%) | Water binding capacity (%) |
|---|---|---|
| Geonhwangmi | 24.43 ± 1.79b1) | 158.91 ± 12.46b |
| Dahomi | 24.30 ± 0.72b | 122.10 ± 6.59d |
| Daeyumi | 25.57 ± 0.00a | 135.97 ± 3.34cd |
| Sinyulmi | 22.59 ± 0.09bcd | 122.06 ± 5.15d |
| Sinjami | 20.00 ± 1.43ef | 126.61 ± 0.71d |
| Sincheonmi | 25.25 ± 0.27a | 152.19 ± 1.91bc |
| Sinhwangmi | 23.16 ± 0.00bc | 180.77 ± 14.55a |
| Yeonjami | 18.99 ± 0.18f | 125.27 ± 3.50d |
| Juhwangmi | 22.22 ± 0.63cd | 160.96 ± 10.59b |
| Pungwonmi | 20.89 ± 0.54de | 163.66 ± 6.39ab |
Values are given as mean ± SD
1)a–e Means in the same column with different letters are significantly different (p < 0.05) by Duncan’s multiple range test
Pasting properties
The pasting behaviors of the raw SP powders and starches are shown in Table 4. The initial pasting temperatures of SP powders and starches ranged from 68.6 to 79.1 °C and from 71.9 to 80.9 °C, respectively. All paste viscosities of SP powders were much lower than those of SP starches. Peak viscosity indicates the highest viscosity reached during gelatinization of starch and is a RVA profile index representing the maximum value reached by viscosity during the heating phase. It is a commonly used extent of granules swelling during the gelatinization phase (Liang and King, 2003). Compared to the peak viscosity of SP starches with same cultivars, peak viscosity of SP powders showed very low values, 1.3–23.1% of peak viscosity of starches. These phenomena might be caused by the α- and β-amylase activities of the SP powders prepared using a freeze-dryer. These amylolytic enzymes were going to activate at optimal temperature and hydrolyze when starch was gelatinized. Juhwangmi and Sinhwangmi powders showed the lowest peak viscosities, which might be caused by the higher α- and β-amylase activities of their powders (Table 2). The peak viscosity of Daeyumi powder was the highest (1185.0 cP), which could be attributed to the lowest α-amylase activity and lower β-amylase activity (Table 2). Unlike the pasting viscosities of SP powders, the pasting viscosities of starches did not have any relationship with the texture types of SP cultivars. Peak and final viscosities of Sinyumi SP powders also showed higher values; however, the α- and β-amylase activities were higher than others. From the above results, it is discovered that the pasting viscosity of SP powders must not only affect amylolytic enzyme activities. Because the amylose content, crystallinity, and molecular structure of SP starches might not affect the textural characters of cooked SPs (Baek et al. 2014; Han et al., 2013; Kim et al., 2013), it is concluded that the role of the chemical constituents should be considered except starch.
Table 4.
Pasting properties of ten different sweet potato powders and starches
| SP cultivars | Sweet potato powders | Sweet potato starches | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial pasting temp. (°C) | Viscosity (cP) | Initial pasting temp. (°C) | Viscosity (cP) | |||||||
| Peak | Final | Breakdown | Setback | Peak | Final | Breakdown | Setback | |||
| Geonhwangmi | 77.5 ± 0.0c1) | 201.5 ± 2.1f | 32.5 ± 2.1g | 174.0 ± 2.9d | 5.0 ± 1.4g | 79.3 ± 0.0b | 5314.0 ± 223.4de | 3379.5 ± 55.9b | 2608.5 ± 324.6de | 1028.0 ± 8.5a |
| Dahomi | 76.6 ± 0.0d | 273.0 ± 1.4e | 69.5 ± 0.7e | 222.0 ± 1.4c | 18.5 ± 0.7e | 80.9 ± 0.1a | 5017.0 ± 5.7e | 3627.5 ± 10.6a | 2410.0 ± 1.4e | 1020.5 ± 14.8a |
| Daeyumi | 78.3 ± 0.0b | 1185.0 ± 8.5a | 1522.5 ± 13.5a | 125.5 ± 0.7e | 463.0 ± 4.2a | 80.1 ± 0.0ab | 5128.0 ± 7.0de | 3225.0 ± 28.3c | 2814.0 ± 14.1d | 911.0 ± 7.0bc |
| Sinyulmi | 79.1 ± 0.1a | 941.0 ± 11.3b | 1249.5 ± 7.7b | 81.5 ± 2.1g | 391.6 ± 3.6b | 76.8 ± 0.0c | 5693.5 ± 374.0bc | 3143.5 ± 169.0c | 3502.5 ± 202.9b | 952.5 ± 2.1b |
| Sinjami | 75.0 ± 0.0e | 275.5 ± 9.2e | 56.8 ± 5.9ef | 231.5 ± 6.4b | 15.5 ± 0.7ef | 77.6 ± 0.1c | 5399.5 ± 24.8cd | 3488.5 ± 12.0b | 2811.0 ± 17.0d | 900.0 ± 4.2c |
| Sincheonmi | 76.6 ± 0.0d | 869.0 ± 2.8c | 395.0 ± 5.7d | 592.0 ± 4.2a | 118.0 ± 4.2d | 80.4 ± 0.6ab | 5278.0 ± 19.8de | 3391.0 ± 24.0b | 2806.5 ± 0.8d | 919.5 ± 3.5bc |
| Sinhwangmi | 75.0 ± 0.0e | 136.0 ± 1.4g | 46.0 ± 1.4fg | 102.0 ± 0.0f | 12.0 ± 0.0f | 77.5 ± 1.2c | 5865.5 ± 31.8b | 3645.0 ± 5.6a | 3118.5 ± 17.7c | 898.0 ± 43.9c |
| Yeonjami | 79.0 ± 0.0a | 494.5 ± 2.1d | 531.0 ± 2.9c | 120.5 ± 2.1e | 157.0 ± 2.8c | 80.8 ± 0.0a | 5176.5 ± 27.6de | 3206.0 ± 45.2c | 2695.0 ± 35.4de | 724.5 ± 37.4d |
| Juhwangmi | 73.4 ± 0.0f | 77.5 ± 0.7h | 35.5 ± 0.7g | 48.0 ± 1.4h | 6.0 ± 1.4g | 76.8 ± 1.2c | 5886.5 ± 105.4b | 3660.5 ± 10.6a | 3279.0 ± 111.7bc | 1053.0 ± 4.2a |
| Pungwonmi | 68.6 ± 0.1g | 201.5 ± 2.1f | 32.5 ± 2.1g | 174.0 ± 2.9d | 5.0 ± 1.4g | 71.9 ± 0.0d | 6656.0 ± 5.7a | 3745.5 ± 37.4a | 3917.0 ± 32.5a | 1006.5 ± 10.6a |
Each value represents mean ± SD
1)a–j means in the same column with different letters are significantly different (p < 0.05) by Duncan’s multiple range test
Correlation analysis among characteristics of sweet potatoes
Pearson’s correlation coefficient is a statistical measure of the strength of a linear relationship between paired data. It is known as the best method to measure the association between variables of interest because it is based on covariance. It gives information about the magnitude of an association, or correlation, as well as the direction of the relationship. The textural characters (i.e., hardness of raw and cooked SP, and hardness ratio of cooked to raw SP), and moisture, starch and AIS contents of SP, α- and β-amylase activities, peak viscosity of SP powder, AAC and WBC of SP starch of ten different Korean SP cultivars had correlation coefficient among themselves, as shown in Table 5. The hardness of cooked SP correlated positively with AIS content (0.59), but negatively with WBC of SP starch (− 0.58). The hardness ratio of cooked/raw SP correlated positively with the hardness of cooked SP (0.91), AIS content (0.75), and starch content (0.64) (p < 0.01). But that correlated negatively with moisture content (− 0.49), α-amylase activity (− 0.48), and WBC (− 0.47) (p < 0.05). Generally, a coefficient of about ± 0.7 or more is regarded as indicating fairly strong correlation, and in the region of ± 0.9, it indicates very strong correlation. In the region of ± 0.5 the correlation is moderate, and in the range − 0.3 to + 0.3, it is weak (Leighton et al., 2010). Although the interaction between starch and β-amylase activity was an important factors to affect textural character during cooking of SP root, no relation was found.
Table 5.
Pearson correlation coefficients of characteristics of sweet potato cultivars, flesh, raw powders, and starches
| Samples | Moisture content | Starch content | Apparent amylose content | WBC | AIS | α-Amylase activity | β-Amylase activity | SPpowder/peak | Raw/hardness | Cooked/hardness | (Cooked/raw hardness) * 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture content | – | − 0.74** | − 0.61** | 0.20 | − 0.78** | 0.40 | − 0.16 | − 0.83** | 0.62** | − 0.27 | − 0.49* |
| Starch content | – | 0.28 | − 0.32 | 0.83** | − 0.56* | − 0.02 | 0.83** | − 0.58** | 0.42 | 0.64** | |
| Apparent amylose content | – | 0.20 | 0.37 | − 0.25 | − 0.27 | 0.39 | − 0.44 | 0.26 | 0.44 | ||
| WBC | – | − 0.51* | 0.38 | 0.25 | − 0.44 | 0.12 | − 0.58** | − 0.47* | |||
| AIS | – | − 0.58** | 0.08 | 0.81** | − 0.54* | 0.59** | 0.75** | ||||
| α-Amylase activity | – | 0.61** | − 0.60** | 0.08 | − 0.42 | − 0.48* | |||||
| β-Amylase activity | – | − 0.14 | − 0.03 | − 0.02 | − 0.13 | ||||||
| SPpowder/peak | – | − 0.66** | 0.33 | 0.56* | |||||||
| Raw/hardness | – | − 0.06 | − 0.43 | ||||||||
| Cooked/hardness | – | 0.91** | |||||||||
| (Cooked/raw hardness) * 100 | – |
*p < 0.05
**p < 0.01
AIS means alcohol insoluble solid
WBC means water binding capacity
The textural characters of cooked SP root were predicted to contribute to the hardness of cooked SP root or hardness ratio of cooked/raw SP. It could be confirmed that hardness and hardness ratio are able to determine the textural types of SP root, such as meanly, intermediate, and waxy. However, while AIS and starch contents influence the textural character, amylose content and pasting properties of starch do not have any relationship with the texture types of SP root as described in the report of Walter et al.’s (1975).
Principal component analysis
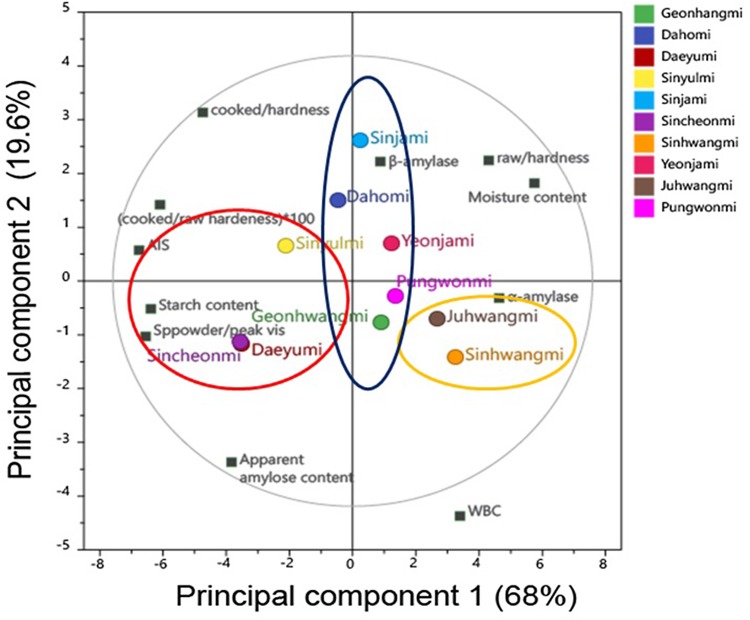
The principal component analysis (PCA) plot, which was analyzed to investigate the relationship between the texture types of SP root and chemical constituents and their properties of SP root, is shown in Fig. 1. The loading and score plots for components 1 and 2 of PCA results were combined. The first (PC1) and the second (PC2) principal components are interpreted for variance of data when multidimensional data are projected as one-dimensional data.
Fig. 1.
Principal component analysis plot of chemical constituent and physicochemical properties affected textural characters and ten Korean sweet potato (SP) cultivars. Cooked/hardness, hardness of cooked SP root; raw/hardness, hardness of raw SP root; cooked/raw hardness, hardness ratio of cooked SP root to raw SP root; AIS, alcohol insoluble solid; Sppowder/peak vis, peak viscosity of SP powder; β-amylase, β-amylase activity; α-amylase, α-amylase activity; WBC, water binding capacity of SP starch. The three types of SP cultivars were represented ellipse
The first two principal components showed 87.6% of the total variance. The first principal component (PC1) accounted for 68% of the total variation in the data. PCA was applied to identify certain factors (texture characters, chemical constituents and their properties) that differentiate between the SP cultivars. The findings from PCA indicated that the main differences were in PC1 of the different cultivars (Leighton et al., 2010). It was characterized by AIS, starch, amylose contents, peak viscosity of SP powder, hardness of cooked SP root, and hardness ratio of cooked/raw SP root with a negative loading while moisture content, α- and β-amylase activities, and hardness of raw SP root displayed a positive loading. As shown in Fig. 1, the PC1 (x-axis) showed Sincheonmi, Daeyumi, Sinyulmi, and Dahomi (left of the graph) contrasted with Sinjami, Geonhwangmi, Yeonjami, Pungwonmi, Juhwangmi, and Sinhwangmi (right of the graph).
For the second principal component (PC2), α-amylase activity, starch content, peak viscosity of SP powder, and AAC showed a negative loading. PC2 (y-axis) showed that Pungwonmi, Juhwangmi, Geonhwangmi, Sincheonmi, Daeyumi, and Sinhwangmi contrasted with Sinyulmi, Yeonjami, Dahomi, Sinjami. From the above results, in PC1, it was discovered that the ten Korean SP cultivars could be classified into three types: mealy (Sincheomi, Daeyumi, and Sinyumi), intermediate Dahomi, Sinjami, Geonhwami, Yeonjami, and Pungwonmi), and waxy (Juhwangmi and Sinhwangmi) types. The intermediate cultivars are assorted into the slightly mealy type (Dahomi and Sinjami) and slightly waxy type (Geonhwangmi, Yeonjami and Pungwonmi).
Acknowledgements
This research was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ011332)” Rural Development Administration, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- AACCI. Approved Methods of the American Association of Cereal Chemists, 11th Ed., AACC International St. Paul, MN, USA, (2012)
- Baek HR, Kim HR, Kim KM, Kim JS, Han GJ, Moon TW. Characterization of Korean sweet potato starches: physicochemical, pasting, and digestion properties. Korean J. Food Sci. Technol. 2014;46:135–142. doi: 10.9721/KJFST.2014.46.2.135. [DOI] [Google Scholar]
- Garcia AM, Walter WM., Jr Physicochemical characterization of starch from Peruvian sweetpotato selections. Starch/Stärke. 1998;50:331–337. doi: 10.1002/(SICI)1521-379X(199808)50:8<331::AID-STAR331>3.0.CO;2-J. [DOI] [Google Scholar]
- Hagenimana V, Vezina LP, Simard RE. Distribution of amylases within sweet potato (Ipomoea batatas L.) root tissue. J. Agr. Food Chem. 1992;40:1777–1783. doi: 10.1021/jf00022a010. [DOI] [Google Scholar]
- Han SK, Song YS, Lee HU, Ahn SH, Yang JW, Lee JS, Chung MN, Suh SJ, Park KH. Difference of starch characteristics of sweetpotato (Ipomoea batatas (L.) Lam) by cultivated regions. Korean. J. Food Sci. Technol. 2013;45:682–692. [Google Scholar]
- Hernández-Carrión T, Ortiz CE, Montalvo-Zapata R, Rivera LE. Sugars and alcohol insoluble solids assessment for sweet potato cultivars recommended for Puerto Rico. J. Agric Univ P.R. 2011;95:223–231. [Google Scholar]
- Kathleen DG. Alcohol-insoluble-solids and dry-matter contents in the assessment of quality and maturity in French beans. J. Horticultural Sci. 1970;45:163–174. doi: 10.1080/00221589.1970.11514342. [DOI] [Google Scholar]
- Kim JM, Park SJ, Lee CS, Ren C, Kim SS, Shin M. Functional properties of different Korean sweet potato varieties. Food Sci. Biotechnol. 2011;20:1501–1507. doi: 10.1007/s10068-011-0208-1. [DOI] [Google Scholar]
- Kim JE, Ren CS, Shin M. Physicochemical properties of starch isolated from eight different varieties of Korean sweet potatoes. Starch/Stärke. 2013;65:923–930. doi: 10.1002/star.201200217. [DOI] [Google Scholar]
- Kitahara K, Nakamura Y, Otani M, Hamada T, Nakayachi O, Takahata Y. Carbohydrate components in sweetpotato storage roots: their diversities and genetic improvement. Breeding Sci. 2017;67:62–72. doi: 10.1270/jsbbs.16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YC, Wang SY, Gao HY, Nguyen KM, Nguyen CH, Shih MC, Lin KH. Physicochemical properties of starches and expression and activity of starch biosynthesis-related genes in sweet potatoes. Food Chem. 2016;199:556–564. doi: 10.1016/j.foodchem.2015.12.053. [DOI] [PubMed] [Google Scholar]
- Lee HU, Chung MN, Lee JS, Song YS, Han SK, Kim JM, Ahn SH, Nam SS, Kim HS, Suh SJ, Park KG. A new sweetpotato variety ‘Dahomi’ for table use. Korean J. Breed Sci. 2015;47:324–329. doi: 10.9787/KJBS.2015.47.3.324. [DOI] [Google Scholar]
- Lee HU, Chung MN, Lee JS, Ahn SH, Kim JM, Han SK, Yang JW, Choi KH, Choi IH, Nam SS. A new sweetpotato variety ‘Geonhwangmi’ for table use. Korean J. Breed Sci. 2016;48:355–361. doi: 10.9787/KJBS.2016.48.3.355. [DOI] [Google Scholar]
- Lee JS, Ahn YS, Chung MN, Kim HS, Jeong KH, Bang JK, Song YS, Shim HK, Han SK, Suh SJ. A new sweetpotato cultivar for use of bioethanol ‘Daeyumi’. Korean J. Breed Sci. 2010;42:674–678. [Google Scholar]
- Lee JS, Ahn YS, Chung MN, Kim HS, Jeong KH, Bang JK, Song YS, Shim HK, Han SK, Suh SJ. A new purple sweetpotato cultivar for table use ‘Yeonjami’. Korean J. Breed Sci. 2010;42:679–683. [Google Scholar]
- Leighton CS, Schonfeldt HC, Kruger R. Quantitative descriptive sensory analysis of five different cultivars of sweet potato to determine sensory and textural profiles. J Sensory Studies. 2010;25:2–18. doi: 10.1111/j.1745-459X.2008.00188.x. [DOI] [Google Scholar]
- Liang X, King JM. Pasting and crystalline property differences of commercial and isolated rice starch with added amino acids. J. Food Sci. 2003;68:832–838. doi: 10.1111/j.1365-2621.2003.tb08251.x. [DOI] [Google Scholar]
- Medcalf DR, Gilles KA. Wheat starches. I. Comparison of physicochemical properties. Cereal Chem. 1965;42:558–568. [Google Scholar]
- Nakamura Y, Takada AO, Kuranouchi T, Katayama K. Disintegration of steamed root tissues of sweet potato and its relation to texture and the contents of starch, calcium and pectic substances. J. Jpn. Soc. Food Sci. Technol. 2015;62:555–562. doi: 10.3136/nskkk.62.555. [DOI] [Google Scholar]
- NICS. http://www.nics.go.kr/api/breed.do?m=100000128&homepageSeCode=nics. Accessed 4 September 2017
- Noda T, Takahata Y, Nagata T, Shibuya N. Chemical composition of cell wall material from sweet potato starch residue. Starch/Starke. 1995;46:232–236. doi: 10.1002/star.19940460607. [DOI] [Google Scholar]
- Noda T, Takahata Y, Sata T, Ikoma H, Mochida H. Physicochemical properties of starches from purple and orange fleshed sweet potato roots at two levels of fertilizer. Starch/Stärke. 1996;48:395–399. doi: 10.1002/star.19960481103. [DOI] [Google Scholar]
- Park SJ, Choe EO, Kim JI, Shin M. Physicochemical properties of mung bean starches in different Korean varieties and their gel textures. Food Sci. Biotechnol. 2012;21:1359–1365. doi: 10.1007/s10068-012-0179-x. [DOI] [Google Scholar]
- Rao VNM, Hamann DD, Humphries EG. Mechanical testing as a measure of kinesthetic quality of raw and baked sweetpotatoes. Trans ASAE. 1974;17:1187–1190. doi: 10.13031/2013.37058. [DOI] [Google Scholar]
- Shin MS, Ahn SY. Textural properties of dry and moist type sweet potatoes. J. Korean Agr. Chem. Soc. 1987;30:315–322. [Google Scholar]
- Sato A, Truong VD, Johanningsmeier SD, Reynolds R, Pecota KV, Yencho GC. Chemical constituents of sweetpotato genotypes in relation to textural characteristics of processed French fries. J. Food Sci. 2017;83:60–73. doi: 10.1111/1750-3841.13978. [DOI] [PubMed] [Google Scholar]
- Szyperski RJ, Hamann DD, Walter WW. Controlled alpha amylase process for improve the sweet potato puree. J Food Sci 51: 360–363, 377 (1986)
- Takahata Y, Noda T, Sato T. Changes in carbohydrates and enzyme activities of sweet potato lines during storage. J Agr. Food Chem. 1995;43:1923–1928. doi: 10.1021/jf00055a031. [DOI] [Google Scholar]
- Teow CC, Truong V, McFeeters RF, Thompson RL, Pecoto KV, Yencho GC. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007;103:829–838. doi: 10.1016/j.foodchem.2006.09.033. [DOI] [Google Scholar]
- Tian SJ, Rickard JE, Blanshard JMV. Physicochemical properties of sweet potato starch. J. Sci. Food Agr. 1991;57:459–491. doi: 10.1002/jsfa.2740570402. [DOI] [Google Scholar]
- Walter WM, Jr, Purcell AE, Nelson AM. Effects of amylolytic enzymes on moistness and carbohydrate changes of baked sweet potato cultivars. J. Food Sci. 1975;40:793–796. doi: 10.1111/j.1365-2621.1975.tb00558.x. [DOI] [Google Scholar]
- Walter WM, Jr, Truong VD, Wiesenborn DP, Carvajal P. Rheological and physicochemical properties of starches from moist and dry-type sweetpotatoes. J. Agr. Food Chem. 2000;48:2937–2942. doi: 10.1021/jf990963l. [DOI] [PubMed] [Google Scholar]
- Williams PC, Kuzina FD, Hlynka I. A rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970;47:411–421. [Google Scholar]