Abstract
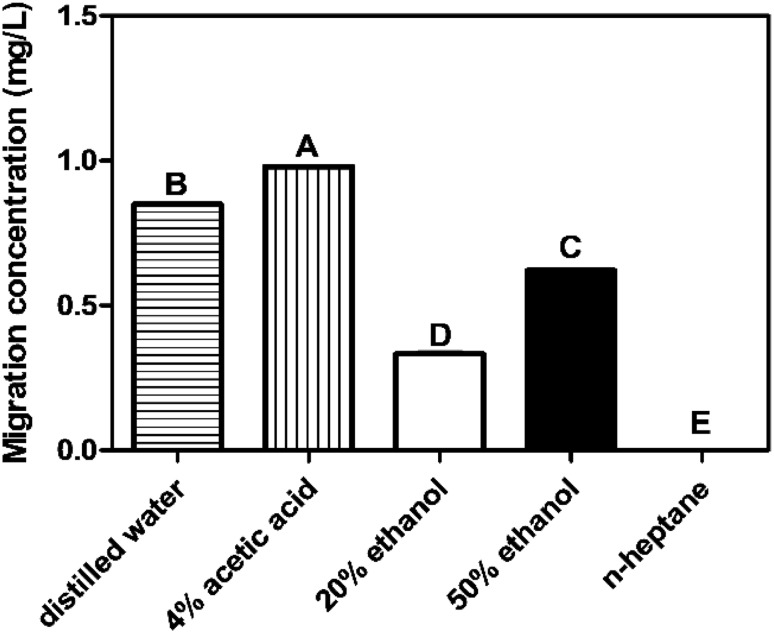
Caprolactam, used in manufacturing polyamide (PA) 6, may threaten human health. Here, PA 6 sheets were produced by using a twin-screw extruder to evaluate its safety. Caprolactam migration concentrations from the PA 6 sheets into food simulants were evaluated according to the standard migration test conditions under the Korean Food Standards Codex (KFSC). Concentrations were investigated under various food simulants (distilled water, 4% acetic acid, 20 and 50% ethanol, and heptane) and storage conditions (at 25, 60, and 95 °C). Caprolactam migration concentrations into food simulants were determined as follows: 4% acetic acid (0.982 mg/L), distilled water (0.851 mg/L), 50% ethanol (0.624 mg/L), 20% ethanol (0.328 mg/L), and n-heptane (not detected). Migrations were determined to be under the regulatory concentration (15 mg/L) according to the KFSC test conditions. Taken together, these results verified that the standard migration test conditions by KFSC were reliable to evaluate the safety of PA 6.
Keywords: Polyamide 6, Caprolactam, Migration, Food contact material, Korean Food Standards Codex regulation
Introduction
Polyamide (PA), polymer with repeating amide groups (–CO–NH–), is one of the most useful food packaging materials. It is also called nylon, which is the generic name of polyamides containing amide linkages. They are typically crystalline and produce good fibers as the backbone of polyamide is regular and symmetrical due to the amide linkages with high mechanical strength and barrier properties (Bustos et al., 2009). Several polyamides such as PA 6, 10, and 11, as well as PA 6, 6 and PA 6, 10 are used for food packaging. Among them, packaging films in the United States are mostly produced from PA 6 which is produced for flexible packaging film as a single or multilayer in the food packaging industry (Bustos et al., 2009). Also, it has been widely used for food packaging because of its thermal and fat resistance, and its aroma and gas barrier abilities (Begley et al., 1995). Therefore, it has been used for oxygen-sensitive foods and fatty foods, such as fish, cheese, sausage casings, and oven/boil-in bags for cooked meats (Félix et al., 2014; Soto-Valdez et al., 1997). PA 6 is widely used for cooking utensils such as ladles, turners, spatulas, spoons, and skimmers because of its high melting point (Heimrich et al., 2012).
Caprolactam (C6H11NO), also called hexahydro-2H-azepan-2-one, is one of the most widely used chemical intermediates in the synthesis of PA 6 fibers and plastics. Caprolactam is a white, hygroscopic, and crystalline solid at ambient temperature. It is highly soluble in water and in most common organic solvents such as benzene, diethyl ether, dimethylformamide, ethanol, methanol, tetrahydrofurfuryl alcohol, and is sparingly soluble in high molecular weight aliphatic chlorinated hydrocarbons (Lide, 1997). Polymerization reactions result from low molecular weight substances producing high molecular weight substances, but the conversion yield is typically less than 100% because about 90% of the monomer is spent during the polymerization reaction (Silva et al., 2006). In other words, these monomers remain inside food packaging when the polymerization reaction is not complete (Barkby and Lawson, 1993; Begley et al., 1995). Consequently, residual monomers can migrate from the food packaging into the food. PA monomers and oligomers are the main substances in packaging materials (Abe et al., 2016). Therefore, the migratable substances should be limited, as the unreacted monomers may be absorbed by the human body and can pose health problems (Hoppe et al., 2016). Caprolactams have toxic properties in their final product that can migrate into food. According to the toxic properties of a substance, regulatory concentrations were established (Bomfim et al., 2011). The regulatory concentration of caprolactam is 15 mg/kg of food or 15 mg/L of food simulants (Ministry of Food and Drug Safety, 2016). Also, the genotoxicity of caprolactam is known to yield negative results by oral and intraperitoneal routes. Therefore, caprolactam has an acceptable daily intake (ADI) of 0.25 mg/kg body weight/day (Dupáková et al., 2010).
The objective of this study was to confirm caprolactam migration in several food simulants under different storage conditions based on the Korean Food Standards Codex (KFSC). From the results, various factors affecting migration were presented and migration behaviour in each condition was evaluated under standard test conditions. Although migration studies in plastic materials such as polyethylene, polypropylene, and polyethylene terephthalate have been widely conducted, relatively few studies were done with PA. It was the first time to study migration tests with PA in accordance with KFSC regulations.
Materials and methods
Materials
Caprolactam (99%) was purchased from Sigma Aldrich (St. Louis, MO, USA) and was used as an analytical standard. PA 6 resins were obtained from Kolon Plastics (Anyang, Korea). Ethyl alcohol (99.9%), methyl alcohol (99.9%), n-heptane (99%), and acetonitrile (99.8%) were purchased from Daejung Chemicals & Metals Co., Ltd (Siheung, Korea). Acetic acid (99.9%) was obtained from J. T. Baker (Center Valley, PA, USA). All materials were high-performance liquid chromatography (HPLC) grade.
Production of PA 6 sheets
PA 6 sheets were produced at the lab scale using a twin-screw extruder (Haake Process 11, Thermo Fisher Scientific, Karlsruhe, Germany) with a screw diameter of 11 mm. Before the production, PA 6 resins had been dried in an oven at 80 °C for 6 h as PAs are highly permeable to water vapor and easily absorb water due to the polar amide group in their polymeric structure. In the extrusion, the speed of the screw and feeder was 30 and 4 rpm, respectively. There were seven heated-barrel zones and one external heated zone for a sheet die. The die processing temperature was 250 °C. The extruder zone temperature varied from 250 °C at the feeder to 255 °C at the die. Finally, the PA 6 sheets with thickness of 0.9 ± 0.1 mm used for the migration experiments were produced.
Quantification of caprolactam from PA6
For the first, the PA 6 sheets were cut into small pieces to facilitate contact area between the extracting solvent and polymer. One-gram sample of caprolactam was placed in a thimble in a siphon. Next, methyl alcohol (100 mL) was added to a round-bottom flask as extraction solvent for extraction. The round-bottom flask was placed on a magnetic stirrer and heated for 30 h. The temperature was about 85 °C, above the boiling point of methyl alcohol. The PA 6 sheet was extracted with 100 mL of methyl alcohol. Then, the methyl alcohol was added to obtain Soxhlet extraction up to 100 mL, the same volume before Soxhlet extraction. Subsequently, a portion of the extract was then filtrated with a 0.45-μm filter and injected into the HPLC. The concentrations of caprolactam were calculated as follows:
| 1 |
On the other hand, to compare the concentrations of caprolactam by PA 6 types, commercial monolayer PA 6 film and PA 6 resin which was a raw material of PA6 sheet were obtained. The PA 6 film (1 g) was cut into small pieces and the PA 6 resin was weighed to 1 g. The same methods were used for extraction as above.
Caprolactam migration test from PA6 into food simulants
Preparation of food simulants
To quantify the migration concentrations of PA 6 monomers, migration experiments were performed using various food simulants following the KFSC. According to the KFSC guidelines (Ministry of Food and Drug Safety, 2013), distilled water, 4% (v/v) acetic acid solution, 20% (v/v) and 50% (v/v) ethanol, and n-heptane were used as food simulants. Distilled water was used as the simulant for aqueous food, 4% acetic acid for acidic foods (with pH below 5), 20% ethanol for slightly alcoholic foods, 50% ethanol for highly alcoholic foods, and n-heptane for fatty foods.
KFSC test conditions
The migration experiments were performed using the total immersion method considering contact ratio between the food simulant and polymer (MFDS 2013). The PA 6 sheets were cut into 1 × 2.2 cm2 rectangular-shape with thickness of 0.9 ± 0.1 mm in recognition that migration would occur on 6 sides of the PA 6 sheets. The “PA 6 sheets” were then placed into screw-capped glass vials containing 10 mL of simulant as the KFSC guidelines. Next, glass vials containing PA 6 sheets were kept at 95, 95, 60, 60, and 25 °C, respectively. After 30 min, each sample was taken from the simulant. The compound which migrated into the simulants was subjected to HPLC analysis. All migration tests were carried out in triplicate.
HPLC analysis
The concentration of caprolactam was analysed using HPLC (1260 Infinity II LC System, Agilent Technologies, Santa Clara, CA, USA), and standard curves to evaluate the initial and post-migration concentrations were generated. The HPLC instrument was equipped with Quaternary pump (G7111A, Agilent Technologies), autosamplers (G7129A and G7165A, Agilent Technologies), and a variable wavelength detector with ultraviolet (UV) detection wavelength in 210 nm. The sample was analysed with a C18 reversed column (4.6 × 250 mm, particle size 5 μm; Zorbax Eclipse XDB-C18, Agilent Technologies) with a gradient of a mobile phase composed of distilled water and acetonitrile as in Table 1 with a flow rate of 0.5 mL/min. The injection volume was 10 µL and the solvent was filtered into HPLC vials with a syringe filter to remove any remaining solid material.
Table 1.
The high performance liquid chromatography (HPLC) mobile-phase gradient program
| Time (min) | Water (%) | Acetonitrile (%) |
|---|---|---|
| 0.0 | 85 | 15 |
| 7.5 | 80 | 20 |
| 9.0 | 75 | 25 |
| 10.0 | 70 | 30 |
| 20.0 | 70 | 30 |
| 20.1 | 85 | 15 |
| 25.0 | 85 | 15 |
Constant amounts of caprolactam were weighed and dissolved in distilled water. An external standard calibration curve for caprolactam was obtained by plotting caprolactam concentration versus peak area. The mobile phase was prepared in a gradient of 15, 20, 25, 30, 30, 15, and 15% acetonitrile in water at 0, 7.5, 9, 10, 20, 20.1, and 25 min after the sample injection (Table 1). The calibration curves had reliable linearity (0.0125–0.2 mg/L) and correlation coefficients (R2 > 0.999).
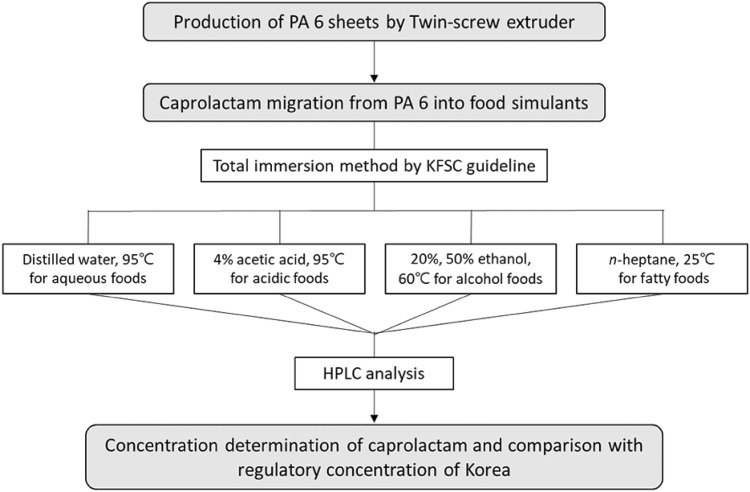
A schematic diagram was presented to help understand the overall concept of experimental design (Fig. 1).
Fig. 1.
Flow diagram of analysing concentrations of caprolactam from polyamide 6 sheets. PA 6, polyamide 6; KFSC, Korean Food Standards Codex; HPLC, high-performance liquid chromatography
Statistical analysis
All values were expressed as mean ± standard deviation. Data analysis was carried out using the Statistical Package for the Social Sciences (SPSS) software version 23.0 (IBM, North Castle, New York, USA). Mean differences were determined by analysis of variance (ANOVA) with Tukey B post hoc comparison. Significant differences were defined as p < 0.05.
Results and discussion
Initial concentration of caprolactam
Initial migrant concentration in the plastic is another factor affecting migration behaviors of a chemical from a polymer into a foodstuff (Maia et al., 2016). Caprolactam concentration present in PA 6 sheets was measured using soxhlet extraction. The concentration of the methyl alcohol extract (dissolving caprolactam) in PA 6 sheets was determined to be 2218.22 ± 64.43 mg caprolactam/kg PA 6 sheet. High concentration of caprolactam was shown in the PA 6 sheets since the lab-scale extruder had short polymerization sections owing to less polymerization process. Additionally, only one cycle of extrusion was performed on each PA 6 sheet. Moreover, post-processing was not implemented after synthesis in this study. It is known that 90% of the monomer is spent during the polymerization reaction of caprolactam, then post-processing such as vacuum or filtration is performed to remove the residual caprolactam (Bustos et al., 2009). However, small amount of caprolactams remain in the polymer material after post-processing. Therefore, caprolactam concentrations in the PA 6 sheets produced without post-processing could be high.
Meanwhile, the results from comparison with a commercial sample showed that caprolactam in PA 6 sheets was much higher than in two other types of PA 6 tested. When the same method of soxhlet extraction except the type of PA 6 were applied, concentrations of caprolactam in 1 kg of PA 6 resin, which is pre-formed in sheets, were determined to be 1935.96 ± 389.19 mg/kg. An increase of caprolactam in PA 6 was discovered by thermal treatment, the extrusion process of PA 6 sheets from granulate. Thermal treatment, including the process of melting polymers, promotes an increase in caprolactam concentrations by caprolactam reformation (Heimrich et al., 2015). The increase in caprolactam concentration can be explained by the influence of thermal processing, like the extrusion of PA 6 sheets. Therefore, to evaluate packaging safety on migration, the concentration of potential migrants in PA 6 has to be ascertained after thermal treatment including extrusion. To compare with commercial sample, PA 6 monolayer film was selected. The concentration of caprolactam in PA 6 monolayer film was 239.34 ± 29.71 mg/kg. The concentration from commercial PA 6 film was 10 times lower than the concentration from lab scale prepared PA 6 sheets.
Evaluation of caprolactam migration into food simulants
Migration experiments were performed using standardized methods followed by the ministry of food and drug safety (MFDS, 2013). The order of high migration into food simulants was as follows: 4% acetic acid (0.98 mg/L), distilled water (0.85 mg/L), 50% ethanol (0.62 mg/L), 20% ethanol (0.33 mg/L), and n-heptane (not detected) (Fig. 2). Overall, these concentrations did not exceed the KFSC migrant regulations and were under the caprolactam regulatory concentration of 15 mg/L (MFDS 2016). Also, according to the results, the migration concentration of caprolactam strongly depended on the contact conditions (simulant type and storage temperature) as the polymer was in contact with the simulants.
Fig. 2.
Evaluation of caprolactam migration concentrations according to the Korea Food Standards Codex (KFSC) conditions. The caprolactam migration concentrations in square with horizontal fill, distilled water; square with vertical fill, 4% acetic acid; open square 20% ethanol; filled square 50% ethanol
Caprolactam migrations into 4% acetic acid were higher than in the aqueous or fatty food simulants. In a previous study, 3% acetic acid was considered to be an aggressive simulant for PA due to the polymer type and the chemical characteristics (Bustos et al., 2009). Also, the ethanolic simulants can easily migrate caprolactams. This can be explained by the high affinity of ethanol to PA 6, which causes swelling of the polymer and facilitates migration of the caprolactam into the food simulants. This might lead high migration rates since storage at high temperatures in polar simulants can result in significant absorption and swelling with a potential to increase the diffusion coefficient. This can be explained by the easy swelling of alcoholic beverages, where migration concentrations were high in 10% ethanol, as the diffusion of migrants occurs at a higher rate in the swollen matrix (Stoffers et al., 2005). Caprolactam was also migrated into distilled water, and it could be explained by the high affinity of water to PA 6 due to the polar amide group in its polymeric structure (Heimrich et al., 2015). While, no migration was even detected into n-heptane after 1 h at 25 °C. This can be explained by low solubility of the polar substance such as the caprolactam in a hydrophobic medium. The fatty food simulant was the most resistant among the tested simulants. From the results, it can be concluded that caprolactam migrations are different depending on the food simulant type and the storage temperature.
Taken together, PA 6 sheets were produced using a co-rotating twin-screw extruder and the caprolactam migration concentrations were examined under the KFSC test conditions. Especially, migrations were determined not to exceed the KFSC caprolactam migrant regulatory concentration of 15 mg/L under the various food simulants and temperature conditions. Consequently, this study provides information regarding the migration study of PA 6 in food contact materials.
Acknowledgements
This research was supported by a Grant 15162MFDS043 from Ministry of Food and Drug Safety in 2015–2017 and also supported by a Basic Science Research Program of National Research Foundation (NRF) of Korea, funded by the Ministry of Education (NRF-2017R1D1A1B03029743).
References
- Abe Y, Mutsuga M, Ohno H, Kawamura Y, Akiyama H. Isolation and quantification of polyamide cyclic oligomers in kitchen utensils and their migration into various food simulants. PLoS One. 2016;11:e0159547. doi: 10.1371/journal.pone.0159547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkby C, Lawson G. Analysis of migrants from nylon 6 packaging films into boiling water. Food Addit. Contam. 1993;10:541–5531. doi: 10.1080/02652039309374177. [DOI] [PubMed] [Google Scholar]
- Begley TH, Gay ML, Hollifield HC. Determination of migrants in and migration from nylon food packaging. Food Addit. Contam. 1995;12:671–676. doi: 10.1080/02652039509374355. [DOI] [PubMed] [Google Scholar]
- Bomfim MV, Zamith HP, Abrantes SM. Migration of ɛ-caprolactam residues in packaging intended for contact with fatty foods. Food Control. 2011;22:681–684. doi: 10.1016/j.foodcont.2010.09.017. [DOI] [Google Scholar]
- Bustos J, Sendón R, Sánchez JJ, Paseiro P, Cirugeda ME. Migration of ε-caprolactam from nylon cooking utensils: validation of a liquid chromatography-ultraviolet detection method. Eur. Food Res. Technol. 2009;230:303–313. doi: 10.1007/s00217-009-1171-4. [DOI] [Google Scholar]
- Dupáková Z, Dobiáš J, Votavová L, Klaudisová K, Voldrich M. Ocurrence of extractable ink residuals in packaging materials used in the Czech Republic. Food Addit. Contam. A. 2010;27:97–106. doi: 10.1080/02652030903225765. [DOI] [PubMed] [Google Scholar]
- Félix JS, Manzoli JE, Padula M, Monteiro M. Evaluation of different conditions of contact for caprolactam migration from multilayer polyamide films into food simulants. Packag. Technol. Sci. 2014;27:457–466. doi: 10.1002/pts.2046. [DOI] [Google Scholar]
- Heimrich M, Boensch M, Nickl H, Simat T. Cyclic oligomers in polyamide for food contact material: quantification by HPLC-CLND and single-substance calibration. Food Addit. Contam. A. 2012;29:846–860. doi: 10.1080/19440049.2011.649496. [DOI] [PubMed] [Google Scholar]
- Heimrich M, Nickl H, Bönsch M, Simat TJ. Migration of cyclic monomer and oligomers from polyamide 6 and 66 food contact materials into food and food simulants: direct food contact. Packag. Technol. Sci. 2015;28:123–139. doi: 10.1002/pts.2094. [DOI] [Google Scholar]
- Hoppe M, de Voogt P, Franz R. Identification and quantification of oligomers as potential migrants in plastics food contact materials with a focus in polycondensates–A review. Trends Food Sci. Technol. 2016;50:118–130. doi: 10.1016/j.tifs.2016.01.018. [DOI] [Google Scholar]
- Lide DR. CRC Handbook of Chemistry and Physics. 78. Baca Raton, FL, USA: CRC Press; 1997. pp. 3–16. [Google Scholar]
- Maia J, Rodríguez-Bernaldo de Quirós A, Sendón R, Cruz JM, Seiler A, Franz R, Simoneau C, Castle L, Driffield M, Mercea P. Determination of key diffusion and partition parameters and their use in migration modelling of benzophenone from low-density polyethylene (LDPE) into different foodstuffs. Food Addit. Contam. A. 2016;33:715–724. doi: 10.1080/19440049.2016.1156165. [DOI] [PubMed] [Google Scholar]
- Ministry of Food and Drug Safety (MFDS). Study of food simulants for migration test of utensils, containers and packaging for food products. Available from: http://www.ndsl.kr/ndsl/search/detail/report/reportSearchResultDetail.do?cn=TRKO201400011730. Accessed Nov. 2013.
- Ministry of Food and Drug Safety (MFDS). Study on re-evaluation of standards and specifications for food packaging. Available from: http://www.foodsafetykorea.go.kr/foodcode/05_01.jsp. Accessed Jul. 2016.
- Silva A. Sanches, García R. Sendón, Cooper I., Franz R., Losada P. Paseiro. Compilation of analytical methods and guidelines for the determination of selected model migrants from plastic packaging. Trends in Food Science & Technology. 2006;17(10):535–546. doi: 10.1016/j.tifs.2006.04.009. [DOI] [Google Scholar]
- Soto-Valdez H, Gramshaw J, Vandenburg H. Determination of potential migrants present in Nylon ‘microwave and roasting bags’ and migration into olive oil. Food Addit. Contam. 1997;14:309–318. doi: 10.1080/02652039709374529. [DOI] [PubMed] [Google Scholar]
- Stoffers NH, Dekker M, Linssen JP, Störmer A, Franz R, van Boekel MA. Modelling of simultaneous two-sided migration into water and olive oil from nylon food packaging. Eur. Food Res. Technol. 2005;220:156–162. doi: 10.1007/s00217-004-1010-6. [DOI] [Google Scholar]