Abstract
The decontamination of spoilage-related microbes in low-alcohol red wine was performed using a serial multiple electrode pulsed electric field (PEF) treatment system. The system consisted of seven electrodes connected in series, and it has been designed to produce square-wave high-voltage pulses of 1 μs duration at various electric field strengths and frequencies for decontamination. The initial counts of aerobic bacteria, yeast and lactic acid bacteria (spoilage-associated microbes) in the wine were 5.56, 5.61 and 5.22 log CFU/mL, respectively. The pattern of decontamination of the spoilage microorganisms followed first-order kinetics and the decontamination effect increased as the field strength and frequency increases. DHz and DPEF values were inversely related to the electric field strength of the PEF treatment. The yeast exhibited relatively low DPEF-value than the aerobic and lactic acid bacteria. The lowest ZPEF-value was observed for the lactic acid bacteria (24.6 kV/cm) among the spoilage microbes.
Keywords: Low-alcohol wine, Pulsed electric field (PEF) treatment, Decontamination, DPEF-value, ZPEF-value
Introduction
The negative effects of high alcohol consumption on human health (including, but not limited to, esophageal cancer, oropharyngeal cancer, increased risk of liver diseases, and pancreatitis) and social issues have prompted the introduction of a global strategy on reducing alcohol consumption by World Health Organization (Saliba et al., 2013; WHO, 2010). Wines generally contain more than 12% alcohol by volume (ABV). However, a consumer purchasing wine with a regular (higher) alcohol content will need to drink less in order to meet a safe level of two standard drinks per day (i.e. 20 g of alcohol), as recommended by the Australian National Health and Medical Research Council (NHMRC, 2009). On the other hand, a reduced volume of wine consumption will negatively impact the wine industry profitability. To address this challenge, the adoption of wine products with lower alcohol content is a feasible solution (Saliba et al., 2013).
However, with a low alcohol content, there is an increased risk of microbial growth and subsequent spoilage of wine following malolactic fermentation. The important wine spoilage microorganisms include yeasts of the genus Dekkera/Brettanomyces and lactic acid bacteria (Cocolin et al., 2004; Puertolas et al., 2009). In addition, low-alcohol wines require longer aging periods after yeast fermentation and such conditions increase the risk of contamination. Therefore, it is necessary to apply a suitable decontamination method after fermentation stage. If microbial contamination occurs due to erroneous process operations during aging, it will lead to massive disruption in production schedule and economic damage. In order to control contamination during the aging, a continuous treatment with the preservative sulphur dioxide (sulfurous acid) has been suggested. However, there is a concern about the risk of environmental pollution by the chemical. Additionally, the World Health Organization (WHO) has recommended reducing its use because of negative health effects to consumers with a special sensitivity (Usseglio-Thomasset, 1992). Therefore, there is a need for the development of safer production methods.
Although heat is an effective sterilizer, its application for wine decontamination is undesirable since the flavor, taste and color characteristics of wine can be negatively affected by heat treatment (Mermelstein, 1998). Alternative novel non-thermal methods, such as high hydrostatic pressure and pulsed electric fields, have been proposed by different researchers for controlling spoilage without modifying the sensorial properties of wine (Garde-Cerdan et al., 2007; Mok et al., 2006). Among such innovative processing technologies, pulsed electric fields (PEF) technology is a promising one because it requires very short processing times and the treatment can be applied in continuous flow (Puertolas et al., 2010). The inactivation of vegetative cells of bacteria and yeast by PEF has been demonstrated (Alvarez et al., 2000; Saldana et al., 2009; Wouters et al., 2001). Electric field strengths in the range of 15–35 kV/cm, generated by the application of short, high-voltage pulses, and specific energies from 50 to 700 kJ/kg induce the formation of pores in microbial membranes, causing inactivation of microorganisms. Therefore, in this study, the usefulness of PEF treatment technology was tested for inactivating spoilage-related microbes in red wine with low alcohol content.
Materials and methods
Wine preparation
The wine was made from red grapes purchased from a local grape market. After washing the grapes, they were treated with potassium metabisulfite (K2S2O5) at a level of 200 ppm. After 3 h, the grapes were pressed manually and the juices were separated by filtration through gauze. The sugar content of the filtered juice was measured and the amount of sugar was adjusted so that the final sugar content was 24°Bx. Then, yeast starter culture (Saccharomyces cerevisiae, grown at 30 °C for 12 h) was added at a level of 0.5% and fermented at 25 °C for 2 weeks. After fermentation, the mixture was filtered with a 70-μm mesh nylon filter and transferred to a glass bottle and aged at 15 °C for 6 weeks. After aging, the mixture was again filtered with a 70-μm mesh nylon filter, and the resultant filtrate was used in the present experiment. The alcohol content of the wine aged at 15 °C for 6 weeks was 8.5%. The counts of aerobic bacteria were 5.56 log CFU/mL, and the counts of yeast and lactic acid bacteria were 5.61 and 5.22 log CFU/mL, respectively.
Pulse electric field (PEF) processing system and wine treatment
The PEF processing system includes a pulse generator (Model JP-PGT50, Jaepae Hi-Tec Co., Inchon, Korea), a power supply (Model JP-PS2550, Jaepae Hi-Tec Co., Korea), and a treatment chamber. The pulse generator consisted of a pulse forming network (PFN) and a switch capable of momentarily generating high voltage electricity. The pulse generation network consisted of a capacitor (1800 pF/each) that stores electrical energy supplied from the power supply and determines the rising time, and a discharge delay inductor (1–20 μH) that adjusts the pulse length and falling time. A square-wave pulse was generated. The charging method was the resonance type, and a hot cathode discharge tube (50 kV, 2500 A) was used as a switch to instantaneously discharge the high voltage. The capacitor was cooled using a cooling device to offset the heat generated during discharging.
The power supply unit was able to boost the input voltage of 220 V AC through a high voltage transformer and rectify it to generate a maximum 50 kV DC power, and the maximum allowable power was 50 kW. The treatment vessel was constructed so that 7 cells having electrode holes of 2 mm diameter were connected in series to have a total treatment volume of 0.175 mL. Observations of the waveforms formed in the processing vessel and the electric field strength were performed using an oscilloscope (Model 9300, Switzerland) and an ammeter was installed to measure the current.
Microbial decontamination of the sample by the PEF was carried out by generating a square-wave pulse with a pulse width of 1 μs, with an electric field intensity of 20–50 kV/cm and a frequency of 500–1500 Hz. The operation mode was distinctly different from the continuous recirculation method, used a single pass method in which the sample was returned to the reservoir after the PEF treatment. The wine was poured into the multi-electrode PEF device at a flow rate of 1 mL/s.
Measurement of survival counts
The survival counts of spoilage microorganisms in the low-alcohol red wine after PEF treatment was measured by the pour plate method (Harrigan, 1998). Total aerobic bacteria and lactic acid bacteria were determined at 37 °C using plate count agar (Difco, Becton Dickinson and Co., Sparks, MD, USA) and Rogosa SL agar media, respectively. Yeast counts were determined on malt extract agar following incubation at 30 °C for 48 h.
DPEF-value and DHz value
DPEF-value is calculated as the inverse of the slope using a graph based on the frequency and the processing time for each field strength. DHz value is the frequency (Hz) required to reduce the number of microorganisms of treating wine to 1/10.
ZPEF-value
The ZPEF-value for PEF inactivation corresponds to the z-value of the heat sterilization theory, calculated from the slope of the straight line obtained by plotting the log (DPEF-value) for the field strength (Álvarez et al., 2003). In other words, ZPEF-value was defined as the increase in electric field strength required to shorten the DPEF-value to 1/10.
Results and discussion
Critical electric field strength (Ec) of spoilage microbes
The critical electric field intensity (Ec), which is defined as the minimum electric field intensity required for microbial inactivation, was lowest for the yeast (14.83 kV/cm) and it was 18.64 kV/cm for the aerobic bacteria. Whereas, the highest Ec value of 19.46 kV/cm was noted for the lactic acid bacteria. This variation in PEF sensitivity of the microorganisms could be due to the differences in cell size since the relatively large-sized cells (yeast) inactivated at the lowest electric field intensity. It is known that the critical electric field strength value depends on microbial characteristics, especially on cell size and shape, and the characteristics of growing medium (Pagan et al., 2005).
Microbial inactivation in wine by PEF treatment
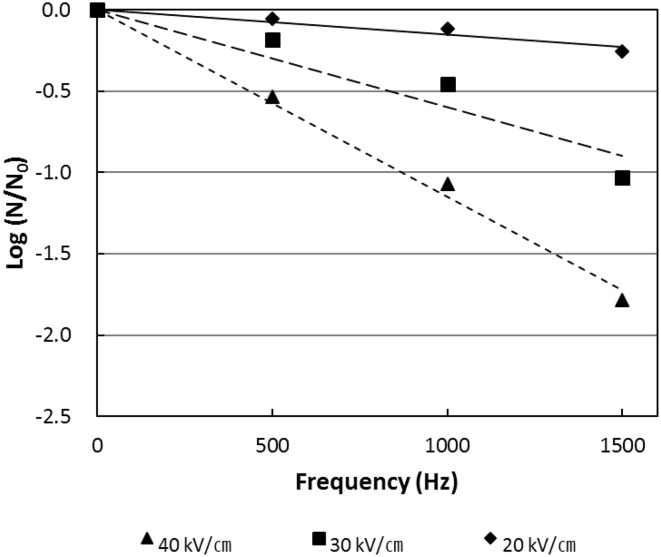
The initial counts of aerobic bacteria, yeast, and lactic acid bacteria were 5.56, 5.61, and 5.22 log CFU/mL, respectively. In general, the viability of microorganisms decreases linearly with the increase of treatment time, and in this study the survival rate decreased in proportion to the field strength. At 30 and 40 kV/cm, the mortality rate was significantly increased with the increase of frequency from 500 to 1500 Hz, whereas the mortality rate was relatively lower at 20 kV/cm even with increased frequency (Fig. 1).
Fig. 1.
Inactivation of total aerobes in red wine by PEF treatment at different electric field strengths and frequencies
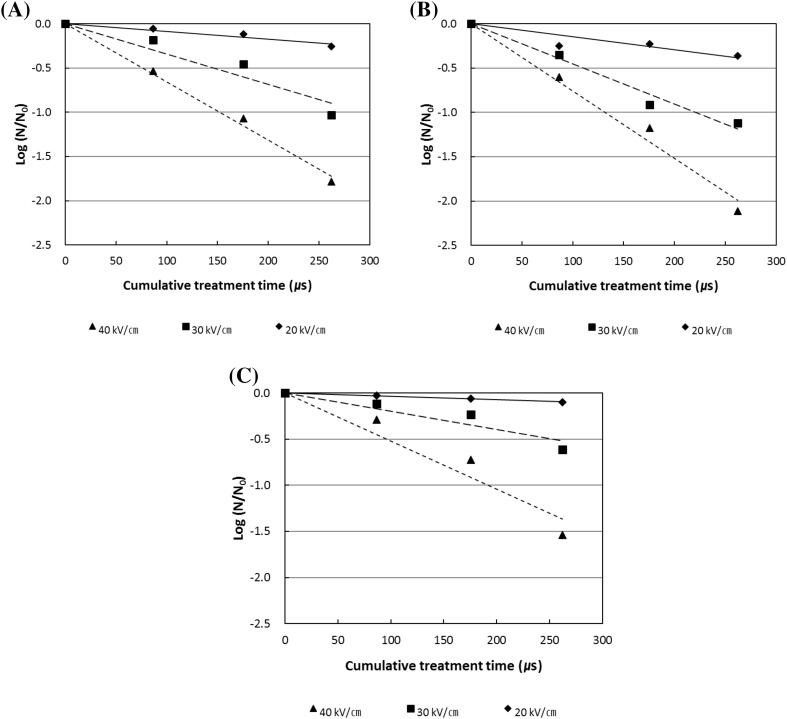
In the case of aerobic bacteria, the inactivation rate increased with increasing electric field intensity of the PEF treatment from 20 to 40 kV/cm. About 1.8 log reduction in the initial counts of the aerobic bacteria was noted using the PEF treatment with electric field intensity of 40 kV/cm within a cumulative treatment time of 270 μs (Fig. 2). In the case of yeast, the inactivation rate was higher than that of the aerobic bacteria and lactic acid bacteria after PEF treatment. Even at low electric field intensity of 20 kV/cm, the death rate was about twice that of the aerobic bacteria and 4 times greater than the lactic acid bacteria. About 2 log reduction in the viable yeast counts was noted upon the PEF treatment with electric field intensity of 40 kV/cm within a cumulative treatment time of 270 μs. The lactic acid bacteria (Fig. 2) also showed a linear log (s) decrease with increase of the treatment time, but a gentle slope was observed among the microorganisms present in the wine.
Fig. 2.
Changes in the counts of total aerobes (A), yeast (B) and lactic acid bacteria (C) in low-alcohol red wine with electric field strength and treatment time during PEF treatment
Numerous studies demonstrated the effectiveness of PEF treatment in inactivation of pathogens in liquid foods such as fruit juices (Buckow et al., 2013). The susceptibility of Listeria innocua in orange juice to PEF processing was reported by McDonald et al. (2000). In that study, treatments at 30 kV/cm for only 5 μs (42 °C) or 12 μs (50 °C) resulted in 3.5 and 6.0-log10 inactivation of the L. innocua, respectively. In sour cherry juice, Listeria monocytogenes was inactivated by 3-log10 CFU/mL upon application of PEF of 27 kV/cm for 131 μs at approximately 20 °C (Altuntas et al., 2011). PEF or high voltage electrical discharge (HVED) was used as an alternative to SO2 to control and inhibit microbial growth in sweet white must fermentation (Delsart et al., 2015). Their results showed that PEF (4–20 kV/cm; 0.25–6 ms) and HVED (40 kV/cm; 1 or 4 ms) can inactivate total yeasts and non-Saccharomyces yeasts. Additionally, the PEF treatment was found to be more suitable than the HVED for sweet wines since the former induced no browning issue.
PEF induces microbial cell inactivation in a unique way; the treatment selectively removes membrane barriers in cells without causing any visible cell damage (Somolinos et al., 2008). The inactivation of microorganisms by PEF treatment occurs due to the irreversible structural changes in their cell membrane, resulting in pore formation and subsequent loss of cellular constituents (Castro et al., 1993). As ethanol has the ability to alter the fluidity of microbial membranes, it may have played some role in the inactivation of the wine microorganisms of this study along with the PEF treatment in a synergistic manner, as shown in an earlier study (Heinz and Knorr, 2000).
Kinetics of microbial inactivation by PEF treatment
The PEF decontamination of the wine contaminants followed first-order kinetics. Therefore, the DHz value representing the frequency (Hz) required to reduce the number of microorganisms of treating wine to 1/10 was calculated and shown in Table 1. On comparing the DHz values of the microorganisms according to the applied electric field strength, it was observed that the aerobic bacteria required 7092 Hz at 20 kV/cm, 1702 Hz at 30 kV/cm, and 877 Hz at 40 kV/cm. When compared based on the type of microorganism, the lactic acid bacteria exhibited the highest DHz value, followed by the aerobic bacteria and yeast (lowest value). The value decreased with increasing the electric field strength. The yeast showed a DHz value of 3617 Hz at 20 kV/cm, but rapidly decreased to 1240 Hz at 30 kV/cm and to 894 Hz at 40 kV/cm, indicating that the yeast was relatively more sensitive to the PEF inactivation effect.
Table 1.
Effect of electric field strength on DHz-value of spoilage microorganisms in low- alcohol red wine. (unit: Hz)
| Microorganism | Electric field strength (kV/cm) | ||
|---|---|---|---|
| 20 | 30 | 40 | |
| Total aerobes | 7092 | 1702 | 877 |
| Yeast | 3617 | 1240 | 894 |
| Lactic acid bacteria | 18,975 | 2873 | 1313 |
It has been shown that large cells are more sensitive to lower field strengths than small ones. This could be due to a preferential damage of larger cells (Ben Ammar et al., 2011). Yeasts are more sensitive to electric fields than Gram-positive bacteria due to their larger size (Mohammed and Eissa, 2012).
PEF inactivation rate constant
Table 2 shows the PEF lethal rate constants for each microorganism calculated using the linear regression equation. At the 20 and 30 kV/cm electric field strengths, the rate constant was lowered in the order of yeast, aerobic bacteria, and lactic acid bacteria. At an electric field strength of 40 kV/cm, the rate constant of the yeast and aerobic bacteria was 0.015 μs−1, whereas for the lactic acid bacteria it was 0.010 μs−1.
Table 2.
PEF inactivation rate constant (k) of spoilage microorganisms in low-alcohol red wine. (unit: μs−1)
| Microorganism | Electric field strength (kV/cm) | ||
|---|---|---|---|
| 20 | 30 | 40 | |
| Total aerobes | 1.86 × 10−3 | 7.73 × 10−3 | 0.015 |
| Yeast | 3.73 × 10−3 | 0.011 | 0.015 |
| Lactic acid bacteria | 9.51 × 10−4 | 4.64 × 10−3 | 0.010 |
DPEF-value of microorganisms
DPEF-values are useful for the field application of PEF decontamination process, and the DPEF-values obtained in this study are shown in Table 3. The electric fields of 20 kV/cm, with a 1241 μs pulse duration, were able to reduce the aerobic bacterial counts to 1/10. As the electric field intensity increased, the DPEF-value became smaller. At the 40 kV/cm electric field strength, it took 156 μs for l log reduction. The DPEF-value of the yeast was lower than that of the aerobic bacteria, indicating that the yeast was more sensitive to the PEF inactivation. The lactic acid bacteria showed the highest DPEF-value among the microorganisms.
Table 3.
DPEF-value of spoilage microorganisms in low-alcohol red wine. (unit: μs)
| Microorganism | Electric field strength (kV/cm) | ||
|---|---|---|---|
| 20 | 30 | 40 | |
| Total aerobes | 1241 | 298 | 156 |
| Yeast | 617 | 217 | 156 |
| Lactic acid bacteria | 2422 | 496 | 225 |
ZPEF-value of microorganisms
When the log (DPEF) was plotted against the field strength, ZPEF-value was obtained, which is the increase in the field intensity needed to shorten the DPEF-value to 1/10. It was calculated by taking the reciprocal of the slope of the plotted line, and the values are shown in Table 4. The ZPEF-values of the lactic acid bacteria were the lowest, 24.6 kV/cm, whereas these values for the aerobic bacteria and yeast were 33.2 kV/cm and 46.8 kV/cm, respectively. From these results, it can be concluded that the PEF inactivation of the lactic acid bacteria was highly dependent on the electric field strength, whereas the inactivation of the aerobic bacteria and yeast was relatively less dependent. These results suggest that it is desirable to increase the electric field strength in order to inactivate the lactic acid bacteria using the PEF when associating the result with the above rate constant.
Table 4.
ZPEF-value of spoilage microorganisms in low-alcohol red wine
| Microorganism | ZPEF-value (kV/cm) |
|---|---|
| Total aerobes | 33.2 |
| Yeast | 46.8 |
| Lactic acid bacteria | 24.6 |
In conclusion, based on the initial observed critical electric field intensities (Ec) of the wine spoilage microorganisms, the electric field intensities in the range of 20–50 kV/cm and frequencies from 500 to 1500 Hz were chosen for the PEF treatment. The detected spoilage microorganisms include aerobic bacteria, yeast, and lactic acid bacteria, and the yeast were found to be more susceptible to the PEF treatment. The first-order reaction kinetics can be used to describe the PEF treatment-induced inactivation of the wine microbial contaminants. In conclusion, the PEF treatment could be considered for controlling common spoilage microbes in high quality low-alcohol wine production and is expected to be applied to the development of various low-alcohol products.
Acknowledgements
We did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors for this work.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Altuntas J, Evrendilek GA, Sangun MK, Zhang HQ. Effects of pulsed electric field processing on the quality and microbial inactivation of sour cherry juice. Int. J. Food Sci. Technol. 2011;45:899–905. doi: 10.1111/j.1365-2621.2010.02213.x. [DOI] [Google Scholar]
- Álvarez I, Mañas P, Condón S, Raso J. Resistance variation of Salmonella enterica serovars to pulsed electric fields treatments. J. Food Sci. 2003;68:2316–2320. doi: 10.1111/j.1365-2621.2003.tb05765.x. [DOI] [Google Scholar]
- Alvarez I, Raso J, Palop A, Sala FJ. Influence of factors on the inactivation of Salmonella Senftenberg by pulsed electric fields. Int. J. Food Microbiol. 2000;55:143–146. doi: 10.1016/S0168-1605(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Ben Ammar J, Lanoisellé JL, Lebovka NI, Van Hecke E, Vorobiev E. Impact of a pulsed electric field on damage of plant tissues: effects of cell size and tissue electrical conductivity. J. Food Sci. 2011;76:E90–E97. doi: 10.1111/j.1750-3841.2010.01893.x. [DOI] [PubMed] [Google Scholar]
- Buckow R, Ng S, Toepfl S. Pulsed electric field processing of orange juice: a review on microbial, enzymatic, nutritional, and sensory quality and stability. Compr. Rev. Food Sci. F. 2013;12:455–467. doi: 10.1111/1541-4337.12026. [DOI] [PubMed] [Google Scholar]
- Castro AJ, Barbosa-Canovas GV, Swanson BG. Microbial inactivation of foods by pulsed electric field. J. Food Process Pres. 1993;17:47–73. doi: 10.1111/j.1745-4549.1993.tb00225.x. [DOI] [Google Scholar]
- Cocolin L, Rantsiou K, Iacumin L, Zironi R, Comi G. Molecular detection and identification of Brettanomyces/Dekkera bruxellensis and Brettanomyces/Dekkera anomalus in spoiled wines. Appl. Environ. Microb. 2004;70:1347–1355. doi: 10.1128/AEM.70.3.1347-1355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsart C, Grimi N, Boussetta N, Sertier CM, Ghidossi R, Peuchot MM, Vorobiev E. Comparison of the effect of pulsed electric field or high voltage electrical discharge for the control of sweet white must fermentation process with the conventional addition of sulfur dioxide. Food Res. Int. 2015;77:718–724. doi: 10.1016/j.foodres.2015.04.017. [DOI] [Google Scholar]
- Garde-Cerdan T, Marselles-Fontanet AR, Arias-Gil M, MartinBelloso O, Ancin-Azpilicueta C. Influence of SO2 on the consumption of nitrogen compounds through alcoholic fermentation of must sterilized by pulsed electric fields. Food Chem. 2007;103:771–777. doi: 10.1016/j.foodchem.2006.09.018. [DOI] [Google Scholar]
- Harrigan WF. Laboratory methods in food microbiology. 3. San Diego, California: Academic Press; 1998. pp. 53–58. [Google Scholar]
- Heinz V, Knorr D. Effect of pH, ethanol addition and high hydrostatic pressure on the inactivation of Bacillus subtilis by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2000;1(2):151–159. doi: 10.1016/S1466-8564(00)00013-8. [DOI] [Google Scholar]
- McDonald CJ, Lloyd SW, Vitale MA, Petersson K, Innings F. Effect of pulsed electric fields on microorganisms in orange juice using electric field strengths of 30 and 50 kV/cm. J. Food Sci. 2000;65:984–989. doi: 10.1111/j.1365-2621.2000.tb09404.x. [DOI] [Google Scholar]
- Mermelstein NH. Beer and wine making. Food Technol. 52(4): 84, 86, 88 (1998)
- Mohammed MEA, Eissa AHA. Pulsed electric fields for food processing technology. pp. 275–305. In: Structure and Function of Food Engineering. Eissa AHA (ed). InTech Publisher, Rijeka, Croatia (2012)
- Mok C, Song K, Park Y, Lim S, Ruan R, Chen P. High hydrostatic pressure pasteurization of red wine. J. Food Sci. 2006;71:M265–M269. doi: 10.1111/j.1750-3841.2006.00145.x. [DOI] [Google Scholar]
- NHMRC. Australian guidelines to reduce health risks from drinking alcohol. Canberra, Australia: National Health and Medical Research Council (2009)
- Pagan R, Condon S, Raso J. Microbial inactivation by pulsed electric fields. Pp. 45-68. In: Novel Food Processing Technologies. Barbosa-Canovas GV, Tapia MS, Cano MP (eds). CRC Press (2005)
- Puertolas E, Lopez N, Condon S, Alvarez I, Raso J. Potential applications of PEF to improve red wine quality. Trends Food Sci. Tech. 2010;21:247–255. doi: 10.1016/j.tifs.2010.02.002. [DOI] [Google Scholar]
- Puertolas E, Lopez N, Condon S, Raso J, Alvarez I. Pulsed electric fields inactivation of wine spoilage yeast and bacteria. Int. J. Food Microbiol. 2009;130:49–55. doi: 10.1016/j.ijfoodmicro.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Saldana G, Puertolas E, Lopez N, Garcia D, Alvarez I, Raso J. Comparing the PEF resistance and occurrence of sublethal injury on different strains of Escherichia coli, Salmonella Typhimurium, Listeria monocytogenes and Staphylococcus aureus in media of pH 4 and 7. Innov. Food Sci. Emerg. Technol. 2009;10:160–165. doi: 10.1016/j.ifset.2008.11.003. [DOI] [Google Scholar]
- Saliba AJ, Ovington LA, Moran CC. Consumer demand for low-alcohol wine in an Australian sample. Int. J. Wine Res. 2013;5:1–8. doi: 10.2147/IJWR.S41448. [DOI] [Google Scholar]
- Somolinos M, Mañas P, Condón S, Pagán R, García D. Recovery of Saccharomyces cerevisiae sublethally injured cells after pulsed electric fields. Int. J. Food Microbiol. 2008;125:352–356. doi: 10.1016/j.ijfoodmicro.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Usseglio-Thomasset L. Properties and use of sulphur dioxide. Food Addit. Contam. 1992;9:399–404. doi: 10.1080/02652039209374090. [DOI] [PubMed] [Google Scholar]
- WHO. Global strategy to reduce the harmful use of alcohol. Geneva, Switzerland: World Health Organization (2010)
- Wouters PC, Alvarez I, Raso J. Critical factors determining inactivation kinetics by pulsed electric field food processing. Trends Food Sci. Tech. 2001;12:112–121. doi: 10.1016/S0924-2244(01)00067-X. [DOI] [Google Scholar]