Abstract
Enzyme-assisted extraction is a cost-effective, safe, and efficient method to obtain bioactives from plant materials. During this study, 10 different marine algae from Sri Lanka were individually extracted by using five commercial food-grade carbohydrases. The enzymatic and water extracts of the seaweeds were analyzed for their antioxidant and anti-inflammatory activities. The highest DPPH, hydrogen peroxide (H2O2) and intracellular H2O2 scavenging abilities were observed from the Celluclast extract of Sargassum polycystum (CSp). CSp exerted protective effects against oxidative stress-induced cell death in hydrogen peroxide-induced Chang cells and in model zebrafish. The Celluclast extract of Chnoospora minima (CCm) showed the strongest anti-inflammatory activity against lipopolysaccharide (LPS)-induced NO production in RAW 264.7 macrophages (IC50 = 44.47 µg/mL) and in model zebrafish. CCm inhibited the levels of iNOS, COX-2, PGE2, and TNF-α in LPS stimulated RAW 264.7 macrophages. Hence, CSp and CCm could be utilized in developing functional ingredients for foods, and cosmeceuticals.
Electronic supplementary material
The online version of this article (10.1007/s10068-018-0406-1) contains supplementary material, which is available to authorized users.
Keywords: Enzyme-assistant extraction, Celluclast, Marine algae, Sargassum polycystum, Chnoospora minima, Antioxidant
Introduction
Studies centered on the evaluation of natural products from marine algae have garnered much attention from the food, cosmetic and pharmaceutical industries over the last few years. A range of biologically active secondary metabolites including polyphenols, terpenoids, sulfated polysaccharides, alkaloids, polyunsaturated fatty acids, mycosporine-like amino acids, peptides and halogenated compounds have been identified from marine algae (Bhakuni and Rawat, 2006). These molecules are active against many diseases. Unlike most of the natural products isolated from terrestrial organisms, these molecules possess unique structural properties, which have extended the research perspectives from characterization to the synthesis of structural analogs (Li and Vederas, 2009). A typical method employed in the extraction of natural products is the use of organic solvents. However, new environmental-friendly extraction techniques are required for natural product research and potential industrial applications. Enzyme-assisted extraction (EAE) is an innovative technique that enables a higher extraction efficiency to be obtained. The enzymes assist in breaking the cellular matrix and cell walls, allowing the release of compounds that stay attached to the cell walls and inside the cytosol (Wijesinghe and Jeon, 2012). Hydrolytic enzymes that specifically degrade polysaccharides and proteins can be the key to producing new macromolecular substructures with potentially beneficial functionalities. In particular, marine algae contain sulfated polysaccharides, with a wide range of biological activities, which have not been reported in terrestrial plants. Recently, the enzymatic hydrolysis of sulfated algal polysaccharides has attracted special attention (Hehemann et al., 2010). EAE of algae has also been implemented for the extraction of natural products with antioxidant, anti-inflammatory, anticoagulant and antipoliferative effects (Athukorala et al., 2006; Lee et al., 2011; Samarakoon et al., 2013b).
The investigation of antioxidant effects is central to the fields of natural product research and food research, as these compounds are beneficial for physiological wellbeing, to counteract oxidative stress, and to mitigate the pathogenesis of disease conditions, such as rheumatoid arthritis, cancer, atherosclerosis, and cardiovascular and neurodegenerative diseases (Pham-Huy et al., 2008). Alternatively, these compounds can increase the shelf life of food through the reduction of lipid peroxidation. Phenolic compounds are noted for their abundance in marine algae (Goad, 1978). Anti-inflammatory activity is another interesting area of research, as inflammatory diseases have emerged as a prominent health issue in many parts of the world, exerting a considerable influence on healthcare costs. Inflammation is part of the complex stereotypical responses of the body to harmful stimuli; these inflammatory responses are mediated through a complex system of cell signaling pathways, which involves cytokines and lipid mediators (Fernando et al., 2016b). Inflammatory responses are crucial to allow an organism to counteract infection. However, chronic inflammation can result in detrimental issues to physiological well-being owing to an array of degenerative disease conditions, including inflammatory arthritis, coronary artery diseases, multiple sclerosis, cancer, obesity, atherosclerosis, migraines, interstitial cystitis, dermatitis, insulin resistance, and irritable bowel syndrome (Coussens and Werb, 2002; Hansson, 2005; Xu et al., 2003).
According to Coppejans et al. (2009), the coastal areas of Sri Lanka offer suitable habitats for the growth and distribution of a wide diversity of algae species. However, except for a few studies, the bioactive properties of components from these organisms are unexplored (Fernando et al., 2017a; 2017b; Lakmal et al., 2014; Mahendran et al., 1980; Premakumara et al., 1996). Fernando et al., (2017b) described the identification of antioxidant activities of water-soluble polysaccharides from several algae species. In another study, Fernando et al. (2017a) described the purification and anti-inflammatory effects of a fucoidan from Chnoospora minima. Mahendran et al. (1980) reported the identification of sterols from 18 Sri Lankan algae by using GC–MS. According to Lakmal et al. (2014) promising anticancer and antioxidant properties of Sri Lankan algae were observed from the methanol extracts of C. racemosa. Premakumara et al. (1996) reported the isolation of a non-steroidal contragestative agent from Gelidiella acerosa. The present study aimed to explore the biofunctional properties of the enzymatic extracts of Sri Lankan marine algae in order to examine their possible incorporation in functional food or industrial applications.
Materials and methods
Viscozyme L, Celluclast 1.5L FG, AMG 300L (an exo 1,4-alpha-d-glucosidase), Termamyl 120L and Ultraflo L were obtained from Novo Co. (Novozyme Nordisk, Bagsvaerd, Denmark). Chang liver cells and RAW 264.7 macrophages were purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) was purchased from GIBCO Inc. (New York, NY, USA). Phenylthiourea (PTU), 2′,7′-2′ 7′-dichlorodihydrofluorescein diacetate (DCFH-DA), acridine orange, ethidium bromide, Hoechst 33342 and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma, Aldrich, USA. All the other chemicals used in this study were of high purity grade.
Sample collection and preparation
The algae were collected from the southern, northwestern and northeastern locations of Sri Lanka during May 2015. Samples were identified according to its morphological and anatomical characteristics referring to the guides which provide detailed taxonomical information about Sri Lankan algae (Coppejans et al., 2009; Durairatnam, 1961). Sargassum polycystum, Sargassum natans, Padina commersonii, Ahnfeltiopsis pygmaea and Gracilaria corticata were collected from the Hikkaduwa area, Cladophora herpestica Chaetomorpha antennina and Grateloupia lithophila from the Kalpitiya area while Chnoospora minima and Ulva fasciata from the Nilaveli area. All the samples were thoroughly washed using tap water to remove sand, salt, and attached epiphytes. Samples were then freeze-dried and ground to a powder.
Enzyme-assistant extraction (EAE)
Two grams of the cryodesiccated powdered algae was seperatelly suspended in 100 mL of deionized water and adjusted to the optimum pH values of the enzymes using either 1 M HCl or NaOH. Enzymes were separately introduced into each sample suspension at a concentration of 0.5% and were incubated at their optimum temperature for 24 h under continuous agitation. The optimum conditions are as follows; Viscozyme (50 °C, pH 4.5), Celluclast (50 °C, pH 4.5), AMG (60 °C, pH 4.5), Termamyl (60 °C, pH 6.0) and Ultraflo (0 °C, pH 7.0) (Wijesinghe and Jeon, 2012). The enzymes were inactivated by heating in a boiling water bath for 10 min. Finally, the hydrolysates were filtered, and the pH was re-adjusted to 7.0. In addition to EAE, each 2 g of powdered algae was extracted by using deionized water at 30 °C. Finally, all the extracts were lyophilized.
Preliminary analysis
The algae were analyzed for their proximate chemical composition according to AOAC 2005 standards (Association Of Official Analytical, 1998). The protein and the lipid content of the algae were determined using Kjeldahl and Soxhlet methods, respectively. The ash content was analyzed by dry ashing in a furnace at 550 °C for 6 h. The polysaccharide contents were determined by phenol–sulfuric acid method (DuBois et al., 1956). Each extract was analyzed for its yield, total polyphenolic content by Folin–Ciocalteu method (Chandler and Dodds, 1983), carbohydrate content by phenol–sulfuric acid method (DuBois et al., 1956) and protein content using Thermo scientific Pierce BCA protein assay kit. The mineral composition of algae was analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES) (PerkinElmer® Optima 7300 New York, NY, USA) following acid digestion (HCl/HNO3 3:1) as described by Pasławski and Migaszewski (Pasławski and Migaszewski, 2006).
Evaluation of antioxidant activities
Antioxidant activities of the extracts were analyzed each at 2 mg/mL sample concentration. Distilled water instead of sample extracts was used as the control in each colorimetric assay while maintaining necessary blanks for each sample. DPPH radical scavenging activity was measured according to Yang et al. (2011). The absorbance was measured at 517 nm using a synergy HT multi-detection microplate reader (BioTeek, Winooski, VT, USA). The hydrogen peroxide scavenging activity was determined according to the method by Siriwardhana et al. (2004). The ferrous ion chelating ability was measured according to the method described by Lee et al. (2009). Percentage antioxidant activities were calculated using the following equation:
where A0 indicates the absorbance of the control, A1 indicates the absorbance of the sample and A2 indicates the absorbance of the respective blank.
Cell culture
Cytotoxicity and intracellular ROS scavenging effects of the extracts were determined using Chang cells derived from the human liver tissue. The anti-inflammatory activity was evaluated using RAW 264.7 macrophages. Both cell lines were maintained in DMEM medium supplemented with 10% FBS and 1% antibiotics (penicillin and streptomycin). Cells were stored at 37 °C in a humidified atmosphere with 5% CO2. Cells under an exponential growth were seeded for the experiments. MTT colorimetric assay was employed for the determination of cell viability as described by Samarakoon et al. (2013a).
Intracellular ROS scavenging activity
Intracellular ROS scavenging ability of the extracts was measured using dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay as described by Yang et al. (2011). Samples were treated at 100, 50 and 25 mg/mL concentrations to a 96 well plate seeded with Chang cells (1 × 105 cells/mL) and treated with H2O2 (1 mM). After 1 h incubation, 10 µl of DCFH-DA (25 µg/mL) was treated into each well. Fluorescence was measured using a synergy HT multi-detection microplate reader (BioTeek, Winooski, VT, USA) at an excitation and an emission wavelength of 485 and 530 nm, respectively following a 10 min incubation.
Lipopolysaccharide (LPS)-induced NO production and cytotoxicity of RAW 264.7 macrophages
Experiments were carried out according to the method by Ko and Jeon (2015) on RAW 264.7 macrophages. Samples were treated at 200, 100 and 50 µg/mL concentrations. NO production was measured by Griess assay, and cell viability was evaluated using MTT assay.
Western blot analysis
Experiments were performed according to Lee et al. (2015). RAW 264.7 cells were treated with 25, 50 and 100 µg/mL of the Celluclast extract of C. minima (CCm). Cells were harvested using PBS, and the cell lysates were prepared using lysis buffer. Cellular proteins were collected by centrifugation (12,000 rpm) and analyzed using Thermo scientific Pierce BCA protein assay kit. Lysates were standardized and subjected to SDS-PAGE. The resolved protein bands were transferred onto polyvinylidene fluoride membranes (Bio-Rad, Irvine, CA, USA). Nonspecific binding sites on the membranes were blocked by blocking solution containing 5% nonfat dry milk in Tris-buffered saline and Tween-20. Membranes were then incubated sequentially with primary and secondary antibodies. Westar EtaC enhanced chemiluminescent substrate (Cyanagen Srl, Bologna, Italy) was used to develop the western blot signals and the image analysis was done using a fluorescence molecular imaging system (Vilber Lourmat, Paris, France).
In vivo evaluation of intracellular hydrogen peroxide scavenging and anti-inflammatory activities using zebrafish embryos
Zebrafish embryos were obtained by natural spawning (Kang et al., 2013). At 3 h post-fertilization (hpf), embryos (15/group) were transferred to 12-well plates and mounted in embryo media. After 1 h, the wells were treated with 50, 100 and 200 µg/mL of Celluclast extract of S. polycystum (CSp) for evaluating intracellular hydrogen peroxide scavenging activity and with 25, 50 and 100 µg/mL of CCm for evaluating anti-inflammatory activity. After 1 h incubation, H2O2 (10 µg/mL) or LPS (10 µg/mL) was introduced into the respective wells of the plates. After 24 h the embryos were rinsed and mounted in fresh embryo media containing 0.2 mM PTU. For evaluating viability factors experimental fish embryos and the hatched larvae were daily monitored for survival and heartbeat rate up to 5 days.
For the evaluation of ROS, NO levels, and cell death, at 3 day postfertilization (dpf) zebrafish larvae were transferred to 12 well plates and treated with different fluorescent probe dyes (Kang et al., 2013). ROS levels were detected using DCFH-DA (20 μg/mL). NO levels were detected using DAF-FM-DA solution (5 μM), and the cell death was detected using acridine orange staining (7 μg/mL). Photographs were taken using a microscopic CoolSNAP-Pro color digital camera (Olympus, Japan). The fluorescence intensities were quantified using image J program.
Statistical analysis
All the data values are expressed as the mean ± standard deviation (SD) based on at least three independent experiments. Statistical analysis for comparing the data was performed using IBM SPSS Statistics 20 software using one-way ANOVA followed by Duncan’s multiple range test (DMRT). P values less than 0.05 (P < 0.05) were considered as significant.
Results and discussion
Proximate composition
The proximate ash, protein, carbohydrate and lipid composition of the ten algae were estimated in accordance with the standard methods described in AOAC 2005. The results are provided in the Supplementary Materials (Supplementary Table 1). The highest protein and carbohydrate content was observed in U. fasciata and G. lithophila, respectively. The ash content was higher in C. herpestica and P. commersonii, which are predominantly composed of Ca2+ ions (Supplementary Table 2). These observations were related to their calcified morphology. High amounts of K, Ca, Na, and Mg were detected in all samples. Considerable levels of Arsenic (As) were detected in some algae; however, the values were within the normal levels encountered in algae (Rose et al., 2007).
Extraction yields, chemical composition, and antioxidant activities
The EAE of algae resulted in a higher yield than the water extraction process (Supplementary Table 3). In addition, the content of carbohydrates, proteins, and polyphenols, were lower after water extraction than after the EAE, which suggested that carbohydrases were more effective in yielding the above organic compounds as a result of breakage of cell walls. As given in Supplementary Table 3, the Celluclast extract of S. polycystum (CSp) at 2 mg/mL contained the strongest DPPH and hydrogen peroxide radical scavenging activities (57.23 and 39.10%, respectively). The ferrous ion chelating antioxidant power was 82.95% in the water extract of C. herpestica, which was the highest value among all the extracts. Generally, phenolic compounds and sulfated polysaccharides are among the major polar components that contribute to antioxidant activity in water-based algae extracts (Fernando et al., 2016a). Moreover, low molecular weight polysaccharides are reported to be effective as scavengers of radicals, whereas unhydrolyzed, longer polysaccharide chains can chelate the Fe2+ ions more efficiently than low molecular weight polysaccharides (Jiao et al., 2011).
Toxicity and intracellular ROS scavenging activity
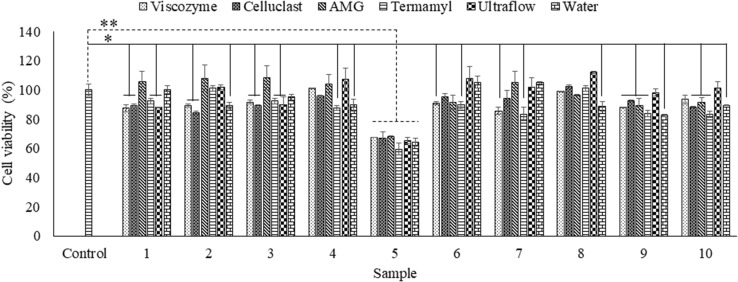
The cytotoxicity of the extracts was determined in Chang human liver cells by using an MTT assay (Fig. 1) after 24-hour incubation with a sample concentration of 100 µg/mL. As indicated in Fig. 1, all extracts, except for the extracts from sample 5 (C. herpesica), resulted in a cell viability of over 80%. The cytotoxicity results demonstrated that the algal extracts, except for C. herpestica, could be safely applied to cells at concentrations lower than 200 µg/mL for further experiments.
Fig. 1.
Cytotoxicity of the algal extracts. The results represent the percentage viability of Chang cells 24 h after the sample treatment. The values were obtained from three independent experiments. *p < 0.05, **p < 0.001 were considered significant compared with the control
Intracellular ROS scavenging abilities of the extracts were evaluated by using the well-known DCFH-DA assay (Table 1). DCFH-DA is easily absorbed into cells and converted into non-fluorescent DCFH by cellular esterases. After oxidation by intracellular ROS and other peroxides, DCFH is converted into the highly fluorescent DCF, which can be detected by using a fluorimeter (excitation wavelength, 485 nm; emission wavelength, 530 nm) (Rastogi et al., 2010). The Celluclast extract of S. polycystum (CSp), S. natans and C. minima showed the strongest intracellular ROS scavenging effects. Overall, the Celluclast extracts exhibited better antioxidant properties than other enzymatic extracts and the DW extracts.
Table 1.
IC50 values of the algal extracts for intracellular hydrogen peroxide scavenging activity
| Sample | Viscozyme (µg/ml) | Celluclast (µg/ml) | AMG (µg/ml) | Termamyl (µg/ml) | Ultraflo (µg/ml) | DW (µg/ml) |
|---|---|---|---|---|---|---|
| 1 | 119.81 ± 3.01a | 86.19 ± 4.92a | 115.27 ± 2.99a | 114.21 ± 5.59a | 119.65 ± 0.09a | 167.14 ± 3.81b |
| 2 | 124.06 ± 3.84a | 99.65 ± 3.55b | 127.99 ± 6.69b | 127.95 ± 6.20b | 125.45 ± 0.75b | 176.05 ± 3.28b |
| 3 | 136.41 ± 6.44b | 125.27 ± 2.88c | 137.99 ± 3.03b | 138.86 ± 6.15c | 130.38 ± 3.62c | 186.42 ± 5.87c |
| 4 | 119.79 ± 0.77a | 98.07 ± 4.43b | 116.35 ± 2.20a | 124.21 ± 3.06a | 115.20 ± 2.61a | 152.99 ± 0.88a |
| 5 | > 200 | > 200 | > 200 | > 200 | > 200 | > 200 |
| 6 | 156.02 ± 1.99c | 148.51 ± 4.31d | 153.64 ± 0.66c | 160.32 ± 3.05d | 164.54 ± 6.14d | 190.18 ± 2.44c |
| 7 | > 200 | > 200 | > 200 | > 200 | > 200 | > 200 |
| 8 | 159.49 ± 5.24c | 130.33 ± 1.61c | 157.12 ± 4.99c | 160.54 ± 1.45d | 164.17 ± 3.47d | > 200 |
| 9 | > 200 | > 200 | > 200 | > 200 | > 200 | > 200 |
| 10 | 184.47 ± 6.70d | 162.40 ± 0.23e | 181.66 ± 7.00d | 173.94 ± 0.43e | 179.84 ± 7.06e | > 200 |
The numbers indicate; 1. S. polycystum; 2. S. natans; 3. P. commersonii; 4. C. minima; 5. C. herpestica; 6. C. antennina; 7. U. fasciata; 8. A. pygmaea; 9. G. corticata and 10. G. lithophila. Values are expressed as mean ± standard deviation of triplicate determinations
Values within a column indicated by different letters are significantly different (p < 0.05)
Anti-inflammatory activity of the Celluclast extract of C. minima on LPS-induced NO production
As shown in Table 2, a distinct reduction in NO production was induced by the Celluclast extract of C. minima (CCm) (IC50 = 44.47 µg/mL). In addition, all the different carbohydrase extracts of C. minima exhibited stronger anti-inflammatory activities than those from other algae. Most of the extracts possessed anti-inflammatory activities with a cell viability over 80%, except for the C. herpestica extracts, which induced cytotoxicity in (RAW cell viability < 60% at 25.00 µg/mL). As described in the previous sub-section, C. herpestica extracts were toxic to Chang cells. Given the observed cytotoxicity, none of the extracts from C. herpestica were suitable for use in further biological assays. These observations indicated that the EAE of algae achieved a higher extraction yield with better functional properties.
Table 2.
IC50 values of the algal extracts against LPS induced NO production in RAW 264.7 cells as a measurement of anti-inflammatory activity
| Sample | Viscozyme (µg/ml) | Celluclast (µg/ml) | AMG (µg/ml) | Termamyl (µg/ml) | Ultraflo (µg/ml) | DW (µg/ml) |
|---|---|---|---|---|---|---|
| 1 | 53.74 ± 2.18a | 50.28 ± 2.55a | 63.80 ± 0.46b | 74.30 ± 1.61c | 73.49 ± 3.65b | 86.61 ± 2.87b |
| 2 | 83.28 ± 1.85c | 58.81 ± 3.35b | 78.45 ± 2.15d | 71.24 ± 3.36c | 77.44 ± 1.56c | 129.14 ± 0.89c |
| 3 | 80.34 ± 4.03c | 66.00 ± 0.18c | 71.10 ± 0.46c | 64.71 ± 1.37b | 69.94 ± 2.10b | 128.27 ± 1.38c |
| 4 | 50.97 ± 3.87a | 44.47 ± 0.99a | 59.38 ± 1.77a | 54.82 ± 0.61a | 55.07 ± 2.61a | 66.56 ± 0.96a |
| 5 | 129.99 ± 3.21g | 124.96 ± 1.61g | 113.09 ± 0.41g | 156.95 ± 1.64f | 109.79 ± 1.58e | > 200 |
| 6 | 60.40 ± 0.36b | 49.55 ± 4.21a | 65.90 ± 2.35b | 53.63 ± 2.00a | 54.63 ± 3.32a | 148.13 ± 3.08d |
| 7 | > 200 | > 200 | > 200 | > 200 | > 200 | > 200 |
| 8 | 99.19 ± 2.15d | 116.59 ± 4.09f | 83.61 ± 2.77e | 60.27 ± 2.81b | 99.98 ± 2.64d | > 200 |
| 9 | 108.87 ± 1.59e | 109.82 ± 3.88e | 103.83 ± 0.10f | 117.23 ± 1.41e | 103.22 ± 2.94d | 131.35 ± 0.42c |
| 10 | 119.41 ± 1.45f | 102.12 ± 2.50d | 111.66 ± 2.91g | 111.54 ± 4.17d | 138.34 ± 2.17f | 164.44 ± 2.55e |
The numbers indicate; 1. S. polycystum; 2. S. natans; 3. P. commersonii; 4. C. minima; 5. C. herpestica; 6. C. antennina; 7. U. fasciata; 8. A. pygmaea; 9. G. corticata and 10. G. lithophila. Values are expressed as mean ± standard deviation of triplicate determinations
Values within a column indicated by different letters are significantly different (p < 0.05)
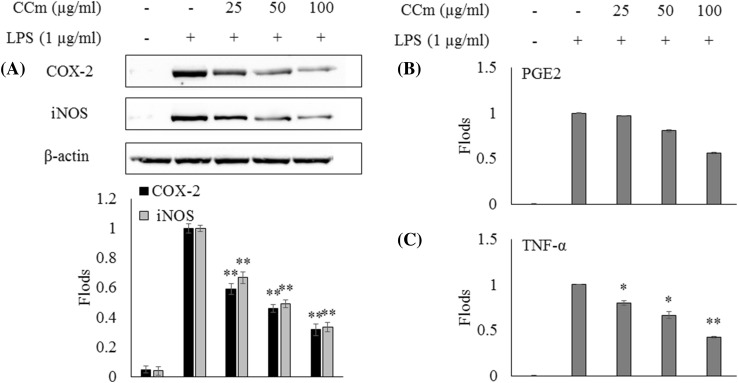
Effect of CCm upon LPS-induced iNOS, COX-2, PGE2 and TNF-α protein expression in LPS-induced RAW cells
Inflammatory responses are mediated by a complex system of signaling pathways. iNOS, COX-2, PGE2, and TNF-α are some of the key cell signaling mediators that regulate the production of NO and prostaglandins, which, in turn, regulate an array of cellular responses (Sanjeewa et al., 2016). As shown in Fig. 2, LPS treatment significantly increased cytokine production compared with the control. However, the treatment of CCm effectively suppressed the expression levels of iNOS, COX-2, PGE2, and TNF-α in a dose-dependent manner. Further, the observed reduction of NO production in RAW 264.7 macrophages could be attributed to the downregulation of iNOS expression. The Celluclast extracts of all algae had higher anti-inflammatory activity than the DW extract, as EAE is a more efficient DW extraction to obtain bioactives from algae. The suppression of the expression of iNOS, COX-2, PGE2 and proinflammatory cytokines, TNF-α, IL-β, and IL-6, demonstrate the ability of CCm to reduce detrimental inflammatory responses.
Fig. 2.
The effects of the CCm on LPS-induced iNOS and COX-2 protein expression and pro-inflammatory cytokine production in RAW 264.7 cells. Expression analysis of (A) iNOS and COX-2 levels using western blot, (B) PGE2 and (C) TNF-α levels using Elisa kits. The results were obtained from three independent experiments and presented as the means. The error bars indicate the standard deviations. *p < 0.05, **p < 0.001 were considered significant compared with the control
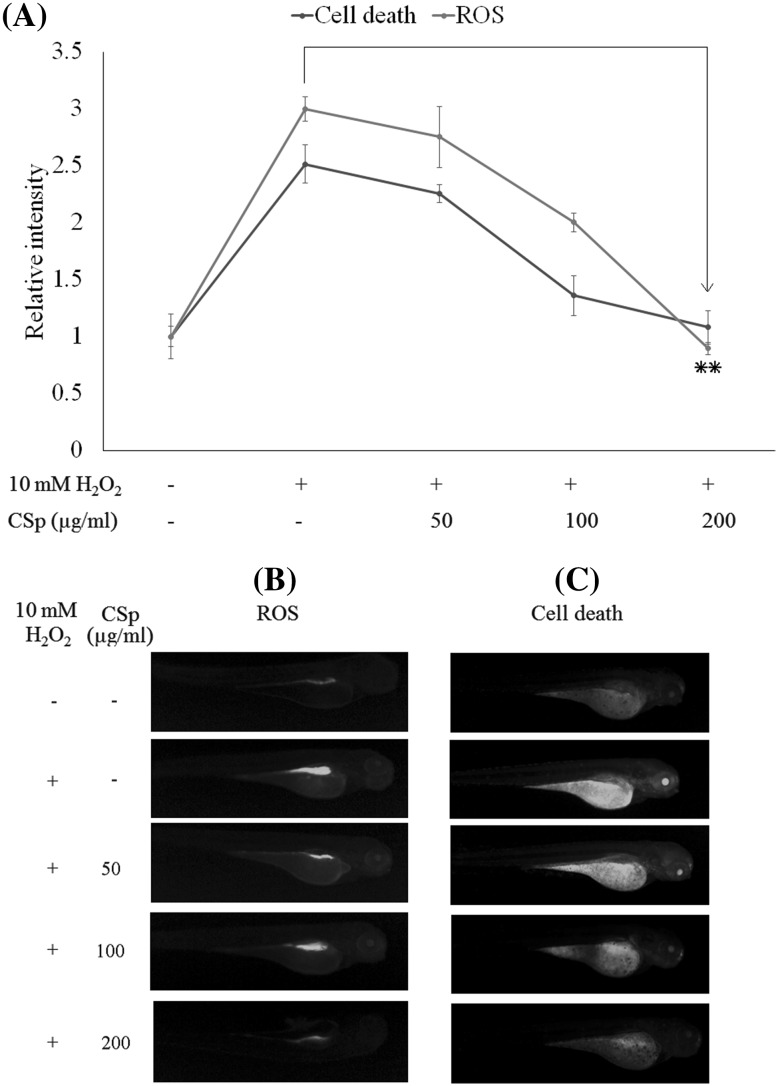
Protective effect of CSp against LPS-induced oxidative stress and cell death in zebrafish
The survival rate for the zebrafish treated with 10 mM H2O2 at 5 dpf was 58.6%, whereas the treatment with CSp (200 µg/mL) increased survival up to 93.0%. The heart beating rate at 2 dpf was increased to 112.2% in zebrafish treated with 10 mM H2O2, with an average of 160 beats/min. The treatment with CSp (200 µg/mL) reduced the rate to the normal levels (100%), with an average of 143 beats/min. Further, the cell death of zebrafish induced by oxidative stress was observed by using acridine orange staining (Fig. 3). The decrease in fluorescence intensities after the treatment with CSp (200 µg/mL) effectively reduced the cell death caused by H2O2-induced oxidative stress to normal levels. Intracellular ROS levels were simultaneously analyzed by using the DCFH-DA staining method (Fig. 3). Again, CSp (200 µg/mL) effectively reduced the intracellular ROS levels to those similar to the control. These results provided convincing evidence of the efficacy of these extracts in animal models, suggesting their possible use in humans.
Fig. 3.
The in vivo evaluation of the protective effect of CSp against hydrogen peroxide-induced oxidative stress and cell death in zebrafish. (A) Relative fluorescence intensities of ROS levels and cell death. (B) Microscopic fluorescence images of ROS levels in zebrafish larvae (stained with DCF-DA). (C) Microscopic fluorescence images of cell death in zebrafish larvae (stained with acridine orange). At 3 hpf, embryos were mounted in embryo media containing 1.00 mL of 0.2 mM PTU. After 1 h embryos were treated with 50, 100, and 200 µg/mL of CSp. After a further 1 h, 10 µg/mL H2O2 was introduced to the embryos. At 3 dpf, the zebrafish larvae were examined by using fluorescence staining methods. The results were obtained from three independent experiments. *p < 0.05, **p < 0.001 were considered significant compared with the control
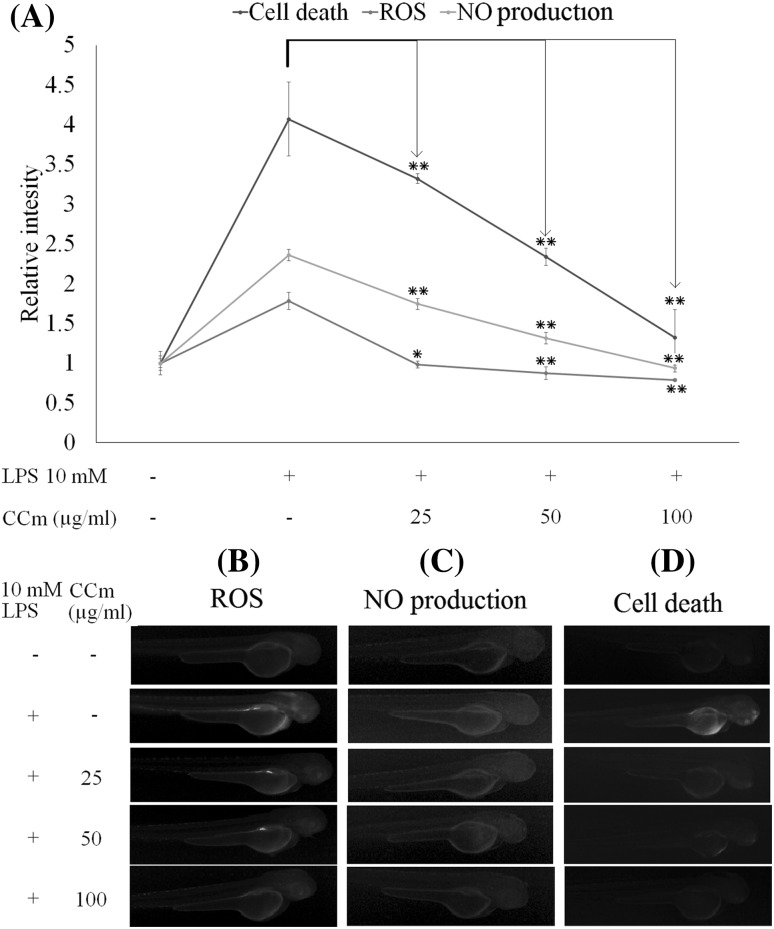
Anti-inflammatory activity of CCm against LPS-induced NO production, oxidative stress and cell death in zebrafish
The heart rate was markedly increased after LPS treatment in zebrafish, by up to 113.4% (161 beats/min) of the control value (140 beats/min). CCm (100 µg/mL) treatment effectively reduced it to the normal level (142 beats/min). The survival rate of the LPS-treated zebrafish at 5 dpf was 51.7%, but the treatment of CCm at 25, 50, and 100 µg/mL successfully increased the survival rate up to 59.2, 76.5, and 93.1%, which represented a dose-dependent response. As shown in Fig. 4, LPS treatment increased cell death, ROS production, and NO production in zebrafish at 3 dpf, which was effectively ameliorated by 100 µg/mL CCm and returned to levels similar to the control sample. The high fluorescence intensity of the LPS-treated positive control group of zebrafish indicated increased NO production. Treatment with CCm effectively reduced the NO levels, demonstrating the anti-inflammatory effects.
Fig. 4.
In vivo evaluation of the protective effect of CCm against LPS-induced NO production, oxidative stress and cell death in zebrafish. (A) Relative fluorescence intensities of ROS levels NO production and cell death. (B) Microscopic fluorescence images of ROS levels in zebrafish larvae (stained with DCF-DA). (C) Microscopic fluorescence images of NO levels in zebrafish larvae (stained with DAF-FM-DA). (D) Microscopic fluorescence images of cell death in zebrafish larvae (stained with acridine orange). At 3 hpf, the embryos were mounted in embryo media containing 1.00 mL of 0.2 mM PTU. After 1 h, embryos were treated with 25, 50, and 100 µg/mL of CCm. After a further 1 h, 10 µg/mL LPS was introduced to the embryos. At 3 dpf the zebrafish larvae were examined by using fluorescence staining methods. The results were obtained from three independent experiments. *p < 0.05, **p < 0.001 were considered significant compared with the control
In summary, EAE is a safe and inexpensive method to obtain bioactives from plant material compared with conventional methods. The enzymatic extracts of the ten different Sri Lankan marine algae yielded a higher polyphenolic content with notable antioxidant and anti-inflammatory activities in the Celluclast extracts of S. polycystum and C. minima both in in vitro and in vivo zebrafish model systems. These results suggested that enzymatic extraction using Celluclast was an efficient method of obtaining bioactives from algae. Further, this study showed the potential of EAE in the industrial manufacture of algae-based functional ingredients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was a part of the project titled “Development of overseas marine bio-resources and a system for their utilization”, funded by the Ministry of Oceans and Fisheries, Korea. A special thank goes to the Department of Wildlife Conservation, Sri Lanka for granting the permission to collect the algae.
References
- Association Of Official Analytical Chemists . Official methods of analysis of AOAC International. Rockville: AOAC International; 1998. [Google Scholar]
- Athukorala Y, Jung WK, Vasanthan T, Jeon YJ. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr Polym. 2006;66:184–191. doi: 10.1016/j.carbpol.2006.03.002. [DOI] [Google Scholar]
- Bhakuni DS, Rawat DS. Bioactive marine natural products. Berlin: Springer; 2006. [Google Scholar]
- Chandler SF, Dodds JH. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of Solanum laciniatum. Plant Cell Rep. 1983;2:205–208. doi: 10.1007/BF00270105. [DOI] [PubMed] [Google Scholar]
- Coppejans E, Leliaert F, Dargent O, Gunasekara R, De Clerck O. Sri Lankan seaweeds: methodologies and field guide to the dominant species. Abc Taxa. 2009;6:265. [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Durairatnam M. Contribution to the study of the marine algae of Ceylon. Fisheries Research Station. 1961;10:1–181. [Google Scholar]
- Fernando IPS, Kim M, Son KT, Jeong Y, Jeon YJ. Antioxidant Activity of Marine Algal Polyphenolic Compounds: a Mechanistic Approach. J Med Food. 2016;19:1–14. doi: 10.1089/jmf.2016.3706. [DOI] [PubMed] [Google Scholar]
- Fernando IPS, Nah JW, Jeon YJ. Potential anti-inflammatory natural products from marine algae. Environ Toxicol Pharmacol. 2016;48:22–30. doi: 10.1016/j.etap.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Fernando IPS, Sanjeewa KKA, Samarakoon KW, Lee WW, Kim H-S, Kang N, Ranasinghe P, Lee HS, Jeon YJ. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int J Biol Macromol. 2017;104:1185–1193. doi: 10.1016/j.ijbiomac.2017.07.031. [DOI] [PubMed] [Google Scholar]
- Fernando IPS, Sanjeewa KKA, Samarakoon KW, Lee WW, Kim HS, Kim EA, Gunasekara UKDSS, Abeytunga DTU, Nanayakkara C, de Silva ED, Lee H-S, Jeon Y-J. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. ALGAE. 2017;32:75–86. doi: 10.4490/algae.2017.32.12.1. [DOI] [Google Scholar]
- GOAD L.J. Marine Natural Products. 1978. The Sterols of Marine Invertebrates: Composition, Biosynthesis, and Metabolites; pp. 75–172. [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. New Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- Jiao G, Yu G, Zhang J, Ewart HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9:196–223. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MC, Cha SH, Wijesinghe WAJP, Kang SM, Lee SH, Kim EA, Song CB, Jeon YJ. Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem. 2013;138:950–955. doi: 10.1016/j.foodchem.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Ko SC, Jeon YJ. Anti-inflammatory effect of enzymatic hydrolysates from Styela clava flesh tissue in lipopolysaccharide-stimulated RAW 264.7 macrophages and in vivo zebrafish model. Nutr. Res. Pract. 2015;9:219–226. doi: 10.4162/nrp.2015.9.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakmal C, Chaminda H, Samarakoon KW, Lee W, Lee J-H, Abeytunga D, Lee H-S, Jeon Y-J. Anticancer and antioxidant effects of selected Sri Lankan marine algae. J Natl Sci Found Sri Lanka. 2014;42:315–323. doi: 10.4038/jnsfsr.v42i4.7730. [DOI] [Google Scholar]
- Lee SH, Kang SM, Sok CH, Hong JT, Oh JY, Jeon YJ. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae. 2015;30:163–170. [Google Scholar]
- Lee SH, Kim AD, Kang MC, Lee JB, Jeon YJ. Potential antioxidant activities of enzymatic digests from fresh water microalgae. Pediastrum duplex and Dactylococcopsis fascicularis. Algae. 2009;24:169–177. [Google Scholar]
- Lee WW, Ahn G, Wijesinghe W, Yang X, Ko CI, Kang MC, Lee BJ, Jeon YJ. Enzyme-assisted extraction of Ecklonia cava fermented with Lactobacillus brevis and isolation of an anti-inflammatory polysaccharide. Algae. 2011;26:343–350. doi: 10.4490/algae.2011.26.4.343. [DOI] [Google Scholar]
- Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- Mahendran M, Sirisena D, Morisaki M, Ikekawa S, Sivapalan A. Sterols of some Sri Lankan marine algae. J Natl Sci Counc Sri Lanka. 1980;8:69–74. [Google Scholar]
- Pasławski P, Migaszewski Z. The quality of element determinations in plant materials by instrumental methods. Pol. J. Environ. Stud. 2006;15:154–164. [Google Scholar]
- Pham-Huy LA, He H, Pham-Huy C. Free Radicals. Antioxidants in Disease and Health. IJBS. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- Premakumara GAS, Ratnasooriya WD, Tillekeratne LMV. Isolation of a non-steroidal contragestative agent from Sri Lankan marine red alge. Gelidiella acerosa. Contraception. 1996;54:379–383. doi: 10.1016/S0010-7824(96)00198-9. [DOI] [PubMed] [Google Scholar]
- Rastogi RP, Singh SP, Hader DP, Sinha RP. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2’,7’-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Res Commun. 2010;397:603–607. doi: 10.1016/j.bbrc.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Rose M, Lewis J, Langford N, Baxter M, Origgi S, Barber M, MacBain H, Thomas K. Arsenic in seaweed-Forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45:1263–1267. doi: 10.1016/j.fct.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Samarakoon KW, Ko JY, Rahman SM, Lee JH, Kang MC, Kwon ON, Lee JB, Jeon YJ. In vitro studies of anti-inflammatory and anticancer activities of organic solvent extracts from cultured marine microalgae. Algae. 2013;28:111–119. doi: 10.4490/algae.2013.28.1.111. [DOI] [Google Scholar]
- Samarakoon KW, Kwon ON, Ko JY, Lee JH, Kang MC, Kim D, Lee JB, Lee JS, Jeon YJ. Purification and identification of novel angiotensin-I converting enzyme (ACE) inhibitory peptides from cultured marine microalgae (Nannochloropsis oculata) protein hydrolysate. J Appl Phycol. 2013;25:1595–1606. doi: 10.1007/s10811-013-9994-6. [DOI] [Google Scholar]
- Sanjeewa KKA, Fernando IPS, Kim EA, Ahn G, Jee Y, Jeon YJ. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr Res Pract. 2016;10:3–10. doi: 10.4162/nrp.2016.10.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriwardhana N, Jeon YJ, Kim SH, Ha JH, Heo SJ, Lee KW. Enzymatic hydrolysis for effective extraction of antioxidative compounds from Hizikia fusiformis. Algae. 2004;19:59–68. doi: 10.4490/ALGAE.2004.19.1.059. [DOI] [Google Scholar]
- Wijesinghe WAJP, Jeon YJ. Enzyme-assistant extraction (EAE) of bioactive components: a useful approach for recovery of industrially important metabolites from seaweeds: A review. Fitoterapia. 2012;83:6–12. doi: 10.1016/j.fitote.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Kang MC, Lee KW, Kang SM, Lee WW, Jeon YJ. Antioxidant activity and cell protective effect of loliolide isolated from Sargassum ringgoldianum subsp. coreanum. Algae. 2011;26:201–208. doi: 10.4490/algae.2011.26.2.201. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.