Abstract
The major compounds of cinnamon are cinnamic acid and cinnamaldehyde, for which the conditions of microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), and reflux extraction (RE) were optimized using response surface methodology for comparing their efficiencies in terms of extraction yield, consumption of time and energy, and CO2 emission. The results indicated MAE superiority to UAE and RE owing to the highest yield of target compounds (total yield: 0.89%, cinnamic acid: 6.48 mg/100 mL, and cinnamaldehyde: 244.45 mg/100 mL) at optimum MAE conditions: 59% ethanol, 147.5 W microwave power and 3.4 min of extraction time. RE resulted in comparable yields with the highest consumption of time, energy, and solvent, and least CO2 emission. Therefore, it is concluded that MAE is the most efficient method for green extraction of cinnamic acid and cinnamaldehyde from cinnamon powder compared to UAE and RE.
Keywords: Cinnamic acid, Cinnamaldehyde, Response surface methodology, Microwave-assisted extraction, Green extraction
Introduction
Cinnamon (Cinnamomum cassia) is a common spice with a distinctive taste and smell. It is usually recovered from the inner bark of trees belonging to the genus Cinnamomum (Mathew and Abraham, 2006). Cinnamon has a long history of use in a wide range of savory and spicy foods. Cinnamon has been reported as a source of various bioactive compounds. Notable key compounds in this regard from its essential oils include cinnamic acid, cinnamaldehyde, cinnamyl acetate, salicylaldehyde, and phenylpropyl acetate (Ranasinghe et al., 2013). Among these, cinnamaldehyde is the primary component and accounts for approximately 55–75% of the total composition. Cinnamic acid exists in volatile form and is attributed to the flavor properties (resembling honey odor) of cinnamon oils (Seo et al., 2010). These two marker compounds have been reported to exhibit several health benefits owing to their high antioxidant activity (Mathew and Abraham, 2006), which may serve to lower incidence of cancer (Hamidpour et al., 2015), as well as help to combat viral infections (Askari et al., 2014). These also protect neural functions, prevent or slow cognitive decline (Frydman-Marom et al., 2011), and support the immune and digestive systems owing to their antifungal properties in the prevention of Candidiasis (an autoimmune and digestive disorder resulting from Candida albicans infection) (Pires et al., 2011).
Reflux extraction (RE) is a conventional extraction method used to extract bioactive components from plant matrices. However, thermal degradation at high temperatures can occur over a prolonged extraction time. Additionally, longer extraction time requires more energy and extraction solvent (Ameer et al., 2017c). Recently, microwave-assisted extraction (MAE) has emerged as modern green extraction method which involves the simultaneous heating of the entire sample through dipolar rotation and ionic conduction. The primary advantages of green extraction methods include improved extraction efficiency owing to shorter extraction times and significantly lower solvent requirements compared to conventional extraction (Ameer et al., 2017c; Cravotto et al., 2011). For the extraction of bioactive components from cinnamon powder, the RE method has been in use for years according to the guidelines of the Korean Herbal Pharmacopoeia (MFDS, 2013).
Optimization of experimental conditions need to be studied for improving efficiency of the extraction methods. Response surface methodology (RSM) is a sophisticated mathematical technique used for process and product optimization and involves a complex relationship between independent and response variables (Das et al., 2015). Among RSM designs, central composite design (CCD) is the most widely used approach for statistical process optimization. Along with process and product optimization, RSM provides the additional advantages of low cost and enhanced quality, accompanied by reduced total number of required experimental runs (Ameer et al., 2017b; Maeng et al., 2017).
The aim of this study was to develop an optimized and effective extraction method for target responses, such as total yield, cinnamic acid and cinnamaldehyde contents from cinnamon powder. The extraction characteristics of different extraction methods (MAE, UAE, and RE) were monitored through RSM to obtain maximum target responses. Moreover, their extraction efficiencies and properties were compared with respect to recovery of target responses, energy consumption and CO2 emission.
Materials and methods
Materials
Cinnamon (Cinnamomum cassia) powder, originating from the USA, was obtained from a local supermarket in Daegu, South Korea and uniform particle size was obtained using a sieve (40 mesh). All the reagents used in this study were of analytical grade.
Microwave-assisted extraction (MAE)
MAE was carried out according to previously reported method of Ameer et al. (2017b). Microdigest microwave extractor (Soxwave 100, Prolabo, Fontenay, France) was employed for experimentation. The microwave power was utilized in the range of 10–250 W at operational frequency of 2450 MHz. All extraction experiments were performed using the extraction conditions specified by CCD (Table 1). For each run, an accurately weighed sample (2.5 g) was added to the extraction vessel and mixed with extraction solvent (50 mL). After extraction, the vessels were cooled to room temperature and the extracts were filtered through Whatman filter paper No. 41. A total volume of 50 mL was made by adding extraction solvent and obtained extracts were stored at 4 ± 1 °C until further analysis.
Table 1.
Central composite design with experimental values of target responses from cinnamon extract by MAE method
| Exp. No.a | Extraction condition | Target responses | ||||
|---|---|---|---|---|---|---|
| Ethanol conc. (%) | Microwave power (W) | Extraction time (min) | Total yield (%) | Cinnamic acid (mg/100 mL) | Cinnamaldehyde (mg/100 mL) | |
| 1 | 25 (− 1)b | 40 (− 1) | 2 (− 1) | 0.57 ± 0.01 | 4.83 ± 0.05 | 129.09 ± 0.03 |
| 2 | 25 (− 1) | 40 (− 1) | 4 (1) | 0.66 ± 0.01 | 5.08 ± 0.03 | 129.02 ± 0.19 |
| 3 | 25 (− 1) | 120 (1) | 2 (− 1) | 0.72 ± 0.01 | 5.35 ± 0.00 | 130.95 ± 0.09 |
| 4 | 25 (− 1) | 120 (1) | 4 (1) | 0.78 ± 0.00 | 4.92 ± 0.03 | 186.46 ± 0.12 |
| 5 | 75 (1) | 40 (− 1) | 2 (− 1) | 0.73 ± 0.01 | 5.43 ± 0.00 | 210.17 ± 0.19 |
| 6 | 75 (1) | 40 (− 1) | 4 (1) | 0.75 ± 0.01 | 5.38 ± 0.02 | 204.82 ± 0.23 |
| 7 | 75 (1) | 120 (1) | 2 (− 1) | 0.84 ± 0.01 | 6.53 ± 0.02 | 235.51 ± 0.13 |
| 8 | 75 (1) | 120 (1) | 4 (1) | 0.86 ± 0.02 | 6.18 ± 0.02 | 223.90 ± 0.06 |
| 9 | 50 (0) | 80 (0) | 3 (0) | 0.81 ± 0.01 | 6.08 ± 0.02 | 207.70 ± 0.04 |
| 10 | 50 (0) | 80 (0) | 3 (0) | 0.83 ± 0.01 | 6.09 ± 0.02 | 212.37 ± 0.04 |
| 11 | 0 (− 2) | 80 (0) | 3 (0) | 0.41 ± 0.01 | 4.56 ± 0.08 | 92.41 ± 0.03 |
| 12 | 100 (2) | 80 (0) | 3 (0) | 0.65 ± 0.01 | 4.80 ± 0.06 | 203.95 ± 0.03 |
| 13 | 50 (0) | 0 (− 2) | 3 (0) | 0.68 ± 0.02 | 5.31 ± 0.02 | 200.35 ± 0.00 |
| 14 | 50 (0) | 160 (2) | 3 (0) | 0.84 ± 0.03 | 6.11 ± 0.00 | 218.45 ± 0.20 |
| 15 | 50 (0) | 80 (0) | 1 (− 2) | 0.70 ± 0.02 | 5.63 ± 0.10 | 122.56 ± 0.37 |
| 16 | 50 (0) | 80 (0) | 5 (2) | 0.86 ± 0.04 | 6.11 ± 0.02 | 217.30 ± 0.05 |
| R2 | 0.9805 | 0.8574 | 0.9154 | |||
| Morphology | Maximum | Saddle point | Saddle point | |||
| F-ratio of ethanol concentration | 48.97*** | 6.24** | 12.44*** | |||
| F-ratio of microwave power | 15.75*** | 2.91 | 0.90 | |||
| F-ratio of extraction time | 6.82** | 0.36 | 3.36* | |||
aThe number of experimental conditions by central composite design
bCoded level of independent variables
*Significant at p < 0.1; **significant at p < 0.05; ***significant at p < 0.01
Ultrasonic-assisted extraction (UAE)
The UAE procedure was performed according to the method described by Ghafoor et al. (2009). Ultrasonic cleaner (Power sonic 420, Hwashin instrument Co. Ltd., Seoul, Korea) was used for extraction experiments and all the experimental runs were performed in accordance with CCD-configuration (Table 2). After extraction, the extracts were filtered through Whatman filter paper No. 41. All obtained extracts were stored at 4 ± 1 °C until further analysis.
Table 2.
Central composite design with experimental values of target responses of from cinnamon extract by UAE method
| Exp. No.a | Extraction condition | Target responses | |||
|---|---|---|---|---|---|
| Ethanol conc. (%) | Extraction time (min) | Total yield (%) | Cinnamic acid (mg/100 mL) | Cinnamaldehyde (mg/100 mL) | |
| 1 | 75 (1)b | 40 (1) | 0.82 ± 0.02 | 6.35 ± 0.01 | 224.27 ± 0.11 |
| 2 | 75 (1) | 20 (− 1) | 0.75 ± 0.02 | 6.07 ± 0.03 | 217.16 ± 0.05 |
| 3 | 25 (− 1) | 40 (1) | 0.65 ± 0.01 | 5.31 ± 0.00 | 163.04 ± 0.02 |
| 4 | 25 (− 1) | 20 (− 1) | 0.54 ± 0.03 | 5.00 ± 0.03 | 150.09 ± 0.03 |
| 5 | 50 (0) | 30 (0) | 0.75 ± 0.01 | 6.15 ± 0.10 | 209.16 ± 0.25 |
| 6 | 50 (0) | 30 (0) | 0.75 ± 0.03 | 6.16 ± 0.09 | 209.05 ± 0.41 |
| 7 | 100 (2) | 30 (0) | 0.61 ± 0.02 | 4.49 ± 0.01 | 187.01 ± 0.03 |
| 8 | 0 (− 2) | 30 (0) | 0.31 ± 0.03 | 3.90 ± 0.02 | 94.50 ± 0.25 |
| 9 | 50 (0) | 50 (2) | 0.75 ± 0.03 | 5.72 ± 0.02 | 193.99 ± 0.09 |
| 10 | 50 (0) | 10 (− 2) | 0.74 ± 0.01 | 5.66 ± 0.06 | 196.20 ± 0.27 |
| R2 | 0.9690 | 0.9191 | 0.9786 | ||
| Morphology | Maximum | Maximum | Maximum | ||
| F-ratio of ethanol concentration | 35.90*** | 14.22** | 58.70*** | ||
| F-ratio of extraction time | 0.81 | 0.86 | 1.05 | ||
aThe number of experimental conditions by central composite design
bCoded level of independent variables
*Significant at p < 0.1; **Significant at p < 0.05; ***Significant at p < 0.01
Reflux extraction (RE)
The RE procedure was performed according to the method described by Ameer et al. (2017a) with some modifications. It was carried out using a water bath-equipped reflux extractor (C-WBS-D, Changshin Scientific Co., Seoul, Korea). All extraction experiments were performed using the extraction conditions specified by CCD (Table 3). After extraction, the vessels were cooled to room temperature and the extracts were filtered through Whatman filter paper No. 41. Afterwards, the obtained extracts were transferred to falcon tubes and stored at 4 ± 1 °C until further analysis.
Table 3.
Central composite design with experimental values of target responses of from cinnamon extract by RE method
| Exp. No.a | Extraction condition | Target responses | ||||
|---|---|---|---|---|---|---|
| Ethanol conc. (%) | Extraction temp. (oC) | Extraction time (min) | Total yield (%) | Cinnamic acid (mg/100 mL) | Cinnamaldehyde (mg/100 mL) | |
| 1 | 25 (− 1)b | 60 (− 1) | 1.5 (− 1) | 0.65 ± 0.01 | 5.30 ± 0.03 | 129.06 ± 0.07 |
| 2 | 25 (− 1) | 60 (− 1) | 3.5 (1) | 0.68 ± 0.01 | 5.42 ± 0.05 | 134.64 ± 0.05 |
| 3 | 25 (− 1) | 80 (1) | 1.5 (− 1) | 0.79 ± 0.01 | 5.43 ± 0.02 | 131.44 ± 0.11 |
| 4 | 25 (− 1) | 80 (1) | 3.5 (1) | 0.75 ± 0.01 | 5.26 ± 0.03 | 117.53 ± 0.00 |
| 5 | 75 (1) | 60 (− 1) | 1.5 (− 1) | 0.83 ± 0.00 | 5.86 ± 0.02 | 223.44 ± 0.11 |
| 6 | 75 (1) | 60 (− 1) | 3.5 (1) | 0.84 ± 0.03 | 6.15 ± 0.01 | 228.05 ± 0.10 |
| 7 | 75 (1) | 80 (1) | 1.5 (− 1) | 0.94 ± 0.04 | 6.24 ± 0.00 | 232.64 ± 0.13 |
| 8 | 75 (1) | 80 (1) | 3.5 (1) | 0.98 ± 0.02 | 6.25 ± 0.02 | 232.46 ± 0.26 |
| 9 | 50 (0) | 70 (0) | 2.5 (0) | 0.89 ± 0.02 | 6.23 ± 0.05 | 221.43 ± 0.09 |
| 10 | 50 (0) | 70 (0) | 2.5 (0) | 0.89 ± 0.02 | 6.24 ± 0.06 | 220.11 ± 0.03 |
| 11 | 0 (− 2) | 70 (0) | 2.5 (0) | 0.41 ± 0.01 | 4.93 ± 0.00 | 96.65 ± 0.03 |
| 12 | 100 (2) | 70 (0) | 2.5 (0) | 0.73 ± 0.01 | 6.04 ± 0.01 | 225.67 ± 0.05 |
| 13 | 50 (0) | 50 (− 2) | 2.5 (0) | 0.80 ± 0.05 | 5.89 ± 0.01 | 217.91 ± 0.17 |
| 14 | 50 (0) | 90 (2) | 2.5 (0) | 0.93 ± 0.01 | 6.09 ± 0.02 | 219.36 ± 0.25 |
| 15 | 50 (0) | 70 (0) | 0.5 (− 2) | 0.87 ± 0.03 | 6.16 ± 0.00 | 233.11 ± 0.05 |
| 16 | 50 (0) | 70 (0) | 4.5 (2) | 0.90 ± 0.02 | 6.07 ± 0.04 | 214.16 ± 0.07 |
| R2 | 0.9857 | 0.9112 | 0.8819 | |||
| Morphology | Maximum | Maximum | Saddle point | |||
| F-ratio of ethanol concentration | 76.72*** | 14.33* | 10.51*** | |||
| F-ratio of extraction temperature | 11.73*** | 1.08 | 0.06 | |||
| F-ratio of extraction time | 0.46 | 0.43 | 0.07 | |||
aThe number of experimental conditions by central composite design
bCoded level of independent variables
*Significant at p < 0.1; **significant at p < 0.05; ***significant at p < 0.01
Experimental design
Based on preliminary experiments, independent process variables for MAE, UAE and RE were selected. In total, 16 experimental runs were performed based on the CCD for MAE and RE (Tables 1, 2) and 10 runs were performed for UAE (Table 3). The dependent variables (Yn) were total (Y1), cinnamic acid (Y2), and cinnamaldehyde (Y3) yields. The results were used for multiple linear regression (MLR) analysis and SAS software (ver. 8.0, SAS Institute Inc., Cary, NC, USA) was used to analyze the experimental results to obtain the regression equation (Eq. 1) as given below:
| 1 |
In this equation, Yn denotes the response variable and X1, X2, and X3 denote the independent MAE process variables. B0 represents the constant term and bn is the regression coefficient for various terms, including the intercept, linear, quadratic, and cross product terms. In addition, predicted model equations were modified to generate four-dimensional (4D) response surfaces to elucidate the interaction effects using the Mathematica 7.0 program. (Wolfram Research, Champaign, IL, USA) and three-dimensional (3D) response surfaces were generated using SAS software (SAS Institute, 1990).
Based on the model equations, canonical analysis was used to analyze the maximum and minimum points. In case of presence of a saddle point (representing relative minimum or relative maximum), ridge analysis was used to determine the optimum point within the region of interest.
Determination of total yield, cinnamic acid and cinnamaldehyde contents
The total extract yield of cinnamon extracts from all extraction methods was determined using a standard method reported in the Korean food code with some modifications. An aliquot of 5 mL of extract was transferred into an aluminum dish and dried at 105 °C until all solvent was removed (MFDS, 2013). Cinnamic acid and cinnamaldehyde were analyzed according to a previously reported method using high performance liquid chromatography (HPLC) (Agilent 1260, Agilent, Santa Clara, CA, USA) (Seo et al., 2010). The sample was filtered through a 0.45 µm membrane filter and an injection volume of 20 µL was used for the analysis. To separate the cinnamic acid and cinnamaldehyde components using HPLC, a Zorbax eclipse plus C18 column (4.6 × 150 mm, 5 μm) (Agilent technologies, Santa Clara, CA, USA) was used at a temperature of 40 °C and a flow rate of 1.0 mL/min. The mobile phase comprised of 1% acetic acid (A) and 1% acetic acid with acetonitrile (B) using the following gradient flow: A:B = 95:5 (0 min), A:B = 30:70 (40 min). A photodiode array detector (Agilent technologies, Santa Clara, CA, USA) was used for analysis at set wavelength of 280 nm.
Energy consumption and CO2 emissions
Energy consumption and CO2 emissions were calculated using a previously reported method (Ameer et al., 2017a). Equation 2 was used to calculate energy consumption (Tonne of Oil Equivalent: TOE). The fuel calorific value used in Eq. 2 is found in the Republic of Korea Energy Act (MTIE, 2011). CO2 emissions (Tonnes CO2: TCO2) were calculated by multiplying the greenhouse gas emission factor (0.4585 TCO2/MWh) as mentioned in the guidelines of the Korea Power Exchange (KPX, 2017) by the consumption of electric power (kWh).
| 2 |
Prediction of optimal ranges of extraction conditions
Prediction of the optimal ranges of extraction conditions was carried out by superimposing the response surfaces for all target responses. Random points were selected within the optimum ranges and these randomly selected points were further used for polynomial regression analysis to determine the optimum extraction conditions (Kim et al., 2012).
Statistical analysis
Statistical analysis was performed using Microsoft Office (ver. 2016, USA), Mathematica 7.0 program (Wolfram Research, Champaign, IL, USA), and SAS software (ver. 8.0, SAS Institute Inc., Cary, NC, USA).
Results and discussion
Effects of MAE conditions
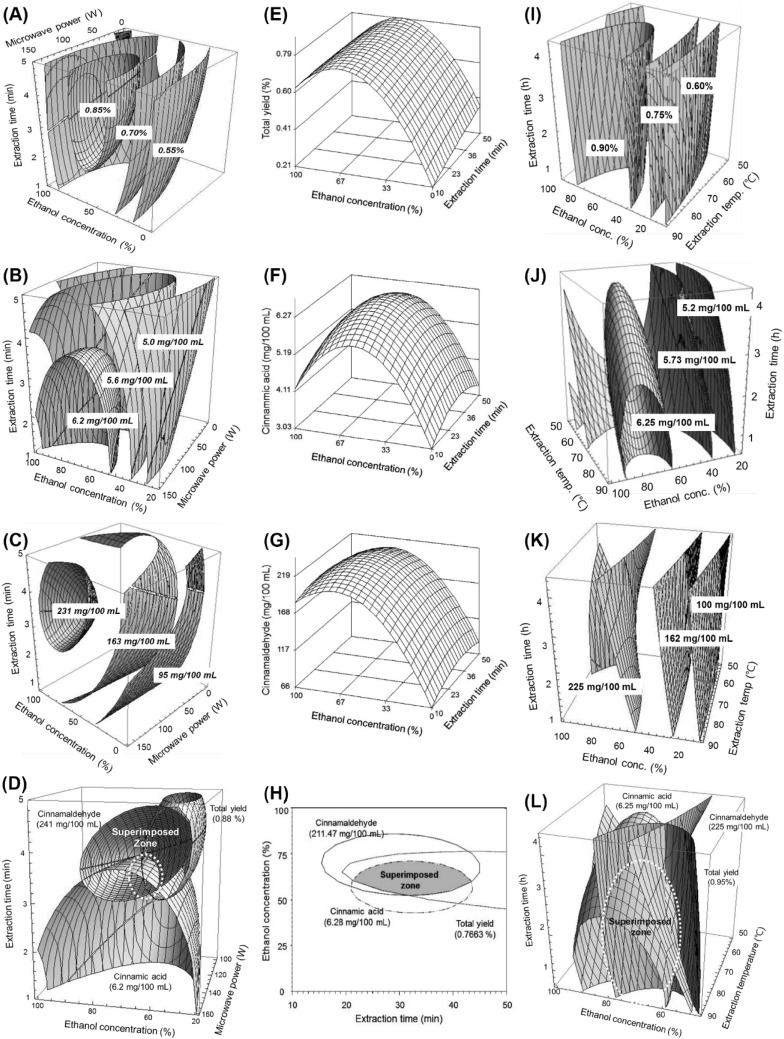
MAE parameters were varied over different ranges: X1, ethanol concentration (0–100%); X2, microwave power (0–160 W); and X3, extraction time (1–5 min). These independent variables and their corresponding levels were chosen based on the results of preliminary experiments. The results of MAE experiments performed under different conditions are shown in Table 1. The results from the CCD-matrix for the three target responses were subjected to MLR analysis. Model validity was confirmed with the coefficient of determination (R2) values, which were provided by regression equations shown in Table 1. The fitted model showed relatively high R2 values for total yield (0.9805), cinnamic acid yield (0.8574), and cinnamaldehyde yield (0.9154). Run No. 7 yielded the maximum total extract yield. Whereas the response surface plot for total extract yield showed the maximum predicted peak at constant values of 0.55, 0.70, and 0.85% (2A). After optimization, the model predicted a maximum yield of 0.89% at the following extraction conditions: X1 = 55.67%, X2 = 139.32 W, and X3 = 4.16 min. The experimental yield value of 0.86% was similar to the model-predicted value (0.89%). The most significant effect was observed for the ethanol concentration followed by microwave power and extraction time. As is evident from the 4D response surface plot shown in Fig. 1, increases in both microwave power and extraction time led to corresponding linear increases gradually in total extract yield from cinnamon powder. Similarly, the total yield of soluble extracts and saponins were extracted by MAE from ginseng extracts using 90% methanol as the extraction solvent, a power output in the range of 75–300 W, and different irradiation time intervals (20, 30, and 40 s), and the efficiency of MAE was compared with those of conventional RE (Kwon et al., 2003). The authors reported a maximum total soluble extract yield of 30.43% at the optimum MAE conditions of 300 W microwave power and an irradiation time of 30 s. MAE resulted in a relatively higher extraction yield compared with time-consuming (12 h) conventional RE. Moreover, the authors reported that MAE was feasible as an alternative green extraction method for extracting bioactive components from ginseng over a shorter extraction time with reduced energy consumption.
Fig. 1.
Four dimensional response surfaces at optimum MAE conditions for total yield (A), cinnamic acid (B), cinnamaldehyde (C) and superimposed response surfaces at optimum MAE conditions (D); three dimensional response surfaces at optimum UAE conditions for total yield (E), cinnamic acid (F) cinnamaldehyde (G) and superimposed response surfaces at optimum UAE conditions (H); four dimensional response surfaces at optimum RE conditions for total yield (I), cinnamic acid (J), cinnamaldehyde (K) and superimposed response surfaces at optimum RE conditions (L)
The effects of MAE parameters on cinnamic acid and cinnamaldehyde yields were also analyzed and the results are tabulated in Table 1. The R2 values obtained from the regression analysis were 0.8574 (p < 0.1) and 0.9154 (p < 0.01) for cinnamic acid and cinnamaldehyde, respectively. In case of morphology of predicted peak points, both cinnamic acid and cinnamaldehyde exhibited saddle points. Therefore, ridge analysis was further performed to determine the maximum predicted values. Run 7 yielded the maximum yields of cinnamic acid (6.53 mg/100 mL) and cinnamaldehyde (235.51 mg/100 mL). After ridge analysis, the maximum predicted yield of cinnamic acid was 6.48 mg/100 mL at X1 = 66.18%, X2 = 145.94 W, and X3 = 2.07 min. Conversely, the maximum predicted cinnamaldehyde yield was 244.45 mg/100 mL under following extraction conditions: X1 = 65.12%, X2 = 150.34 W, and X3 = 3.74 min. The most significant factor affecting cinnamic acid was ethanol concentration, whereas microwave power and extraction time were less significant. In the case of cinnamaldehyde yield, ethanol concentration was the most significant (p < 0.01) factor followed by extraction time (p < 0.1) which was less significant. 4D response surface plots were plotted to elucidate the interaction effects of the independent MAE process variables. Surface plots demonstrated that the peak point for cinnamic acid occurred when X1 was in the range of 45–100%, X2 = 110 W, and X3 = 3.6 min (Fig. 1B). However, the peak point for cinnamaldehyde occurred when X1 was in the range of 35–95%, X2 = 105 W, and X3 was more than 2.6 min (Fig. 1C). 4D response surfaces were superimposed to predict the optimum MAE conditions, which were as follows: X1 = 59–63%, X2 = 135–160 W, and X3 = 3.2–3.6 min (Fig. 1D). The predicted optimum conditions were X1 = 59%, X2 = 148 W, and X3 = 3.4 min (Table 4). Similar to our results, MAE of the target compounds in Stevia rebaudiana (Bertoni) leaves was optimized by RSM and artificial neural network (ANN) modeling. The authors reported an optimum total extract yield of 7.7%, stevioside yield of 20 mg/g, and rebaudioside-A yield of 15 mg/g from MAE-derived S. rebaudiana (Bertoni) extracts under optimum extraction conditions of 4 min of extraction time, 75% ethanol concentration, and 160 W of microwave power (Ameer et al., 2017b). Similar findings were reported regarding the optimum MAE of functional compounds from wild grapewine (Vitis coignetiae) (Kim et al., 2012).
Table 4.
CCD-based optimum conditions of each extraction method for maximum target responses
| Extraction methods | Target responses | ||||
|---|---|---|---|---|---|
| Independent variables | Optimum conditions | Yield (%) | Cinnamic acid (mg/100 mL) | Cinnamaldehyde yield (mg/100 mL) | |
| Microwave-assisted extraction (MAE) | |||||
| X1 | Ethanol concentration (%) | 59.13 | 0.90 ± 0.02 | 6.13 ± 0.08 | 226.26 ± 1.56 |
| X2 | Microwave power (W) | 147.59 | |||
| X3 | Extraction time (min) | 3.41 | |||
| Ultrasound-assisted extraction (UAE) | |||||
| X1 | Ethanol concentration (%) | 55.34 | 0.76 ± 0.01 | 5.67 ± 0.04 | 205.26 ± 0.03 |
| X2 | Extraction time (min) | 33.12 | |||
| Reflux extraction (RE) | |||||
| X1 | Ethanol concentration (%) | 63.56 | 0.94 ± 0.02 | 6.93 ± 0.21 | 229.60 ± 0.06 |
| X2 | Extraction temperature (°C) | 77.62 | |||
| X3 | Extraction time (h) | 2.25 | |||
Effects of UAE conditions
UAE experiments were performed in accordance with the CCD using two independent variables that were varied over specific ranges based on the results of preliminary experiments: ethanol concentration, X1 = 0–100% and extraction time, X2 = 10–50 min. A total of 10 experimental runs were carried out and the results are depicted in Table 2. All the response values were subjected to MLR analysis for model fitting. High R2 values were observed for all three target responses: total yield (0.9690, p < 0.01), cinnamic acid (0.9192, p < 0.1), and cinnamaldehyde yield (0.9786, p < 0.01). Run 1 yielded the maximum target responses (Table 2). 3D response surface plots indicated the maximum points for the corresponding peaks (Fig. 1E–G). In the case of total yield, the maximum predicted yield of 0.80% occurred at X1 = 58.90% and X2 = 59.36 min. The total yield of cinnamon extract was most affected by ethanol concentration followed by extraction time. In the case of cinnamic acid, the maximum predicted yield of 6.28 mg/100 mL was obtained under the following UAE parameters: X1 = 51.90% and X2 = 32.33 min. Ethanol concentration was more influential than extraction time. The maximum predicted yield of cinnamaldehyde was 211.15 mg/100 mL under the UAE parameters of X1 = 69.06% and X2 = 30.27 min. Superimposition of the surface plots revealed the range of optimum extraction conditions, which were ethanol concentration between 51.5% and 58% and extraction time in the range of 25.9 to 39.9 min, whereas the optimum extraction conditions were X1 = 55% and X2 = 33 min. Similar to our results, UAE of antioxidant compounds from germinated chickpeas was optimized using RSM and extraction efficiency was compared with conventional solvent extraction (CSE). The authors reported that the extraction yield of antioxidant compounds was higher using UAE compared to that using CSE. Additionally, UAE could be used as a green extraction method with significant potential for extraction of bioactive components from plant matrices (Hayta and İşçimen, 2017).
Effects of RE conditions
RE is the conventional method used for the extraction of bioactive components from cinnamon powder. RE was carried out according to the conditions specified by the CCD-matrix and total 16 runs were performed. The results are shown in Table 3. Ethanol concentration (X1), extraction temperature (X2), and extraction time (X3) were the independent variables and varied over the following ranges: X1, 0–100%; X2, 50–90 °C; and X3, 0.5–4.5 h. Target response values from all 16 runs were subjected to MLR analysis. The model equations provided R2-values for the three target responses from RE as follows: total yield (0.9857, p < 0.01), cinnamic acid yield (0.9112, p < 0.05), and cinnamaldehyde yield (0.8819, p < 0.05). With respect to the morphology of the peaks, total and cinnamic acid yields exhibited maximum points, whereas cinnamaldehyde yield demonstrated a saddle point. Ethanol concentration and extraction temperature more significantly influenced the RE process than extraction time. A 4D response surface plot (Fig. 1I) showed the maximum peak point for total yield at constant values of 0.90, 0.75, and 0.60%. In case of cinnamic acid, the response surface plot showed the peak point at constant values of 5.2 mg/100 mL, 5.73 mg/100 mL, and 6.25 mg/100 mL (Fig. 1J). These conditions resulted in a yield similar to that of the experimental yield of cinnamic acid (6.24 mg/100 mL) obtained from run 7. Moreover, the relatively high R2 value (0.9112) of the model suggested model adequacy. Conversely, the 4D response surface plot for cinnamaldehyde showed a saddle point at the constant values of 100 mg/100 mL, 162 mg/100 mL and 225 mg/100 mL (Fig. 1K) and this required further use of ridge analysis to determine the maximum point. The maximum predicted yield of cinnamaldehyde was 245.16 mg/100 mL and was achieved at RE conditions of X1 = 81.09%, X2 = 81.73 °C, and X3 = 1.46 h. This maximum predicted yield was similar to the experimentally-obtained yield (235.51 mg/100 mL) from run 7 as shown in Table 3. The most influential independent variable was ethanol concentration, whereas extraction time and extraction temperature were less influential. Superimposition of the 4D response surface plots indicated the ranges of the optimum extraction conditions as follows: ethanol concentration, 59–67%; extraction temperature, 69–86 °C; and extraction time, 0.8–3.7 h (Fig. 1L). In support of our results, RE of total phenolic content, total flavonoid content, and antioxidant activities of Pandan (Pandanus amaryllifolius Roxb.) were optimized using RSM with a methanol concentration of 40–80%, extraction temperature of 40–70 °C, and liquid-to-solid ratio of 20–40 mL/g (Ghasemzadeh and Jaafar, 2014). Moreover, in another study, RE of total, stevioside, and rebaudioside-A yields from stevia leaf powder was optimized using RSM. The authors reported improvement in RE with maximum responses at optimum RE conditions of 100% ethanol concentration, 55 °C extraction temperature, and 60 min extraction time (Ameer et al., 2017a).
Comparison of extraction efficiency among extraction methods
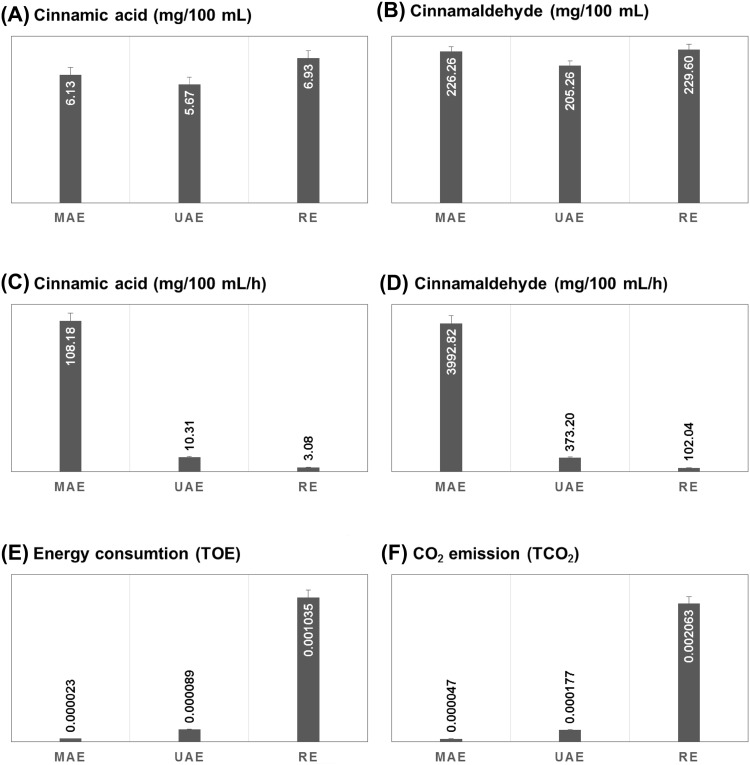
The extraction methods were compared for their efficiencies with respect to recovery of cinnamic acid and cinnamaldehyde (Fig. 2) obtained from cinnamon extracts. In case of cinnamic acid (Fig. 2A), the results indicated that RE rendered the highest yields (6.93 mg/100 mL) compared to those obtained using MAE (6.13 mg/100 mL) and UAE (5.67 mg/100 mL). Even though RE, as the conventional method, led to slightly higher yields compared to MAE, however, time consumption and solvent requirement were considerably higher in case of RE. Moreover, extraction efficiency was also compared with respect to the quantity of cinnamic acid obtained per h from all extraction methods, as shown in Fig. 2C. Among all extraction methods, MAE yielded the highest recovery of cinnamic acid (108.18 mg/100 mL/h), followed by UAE (10.31 mg/100 mL/h) and RE (3.08 mg/100 mL/h). Similarly, the obtained cinnamaldehyde yield was used as a parameter to compare the efficiency of different extraction methods. As shown in Fig. 2B, comparable yields of cinnamaldehyde were obtained from both MAE (226.26 mg/100 mL) and RE (229.60 mg/100 mL), whereas UAE resulted in the lowest yield (205.26 mg/100 mL). MAE was the most efficient method when extraction methods were compared with respect to cinnamaldehyde yield/h. MAE ranked first with a cinnamaldehyde yield of 3992.82 mg/100 mL/h, followed by UAE (373.20 mg/100 mL/h) and RE (102.04 mg/100 mL/h). Comparatively, MAE had significantly higher values of cinnamic acid and cinnamaldehyde yield/h and was found to be the most efficient extraction method compared to UAE and RE.
Fig. 2.
Comparison of cinnamic acid yield (A), cinnamaldehyde yield (B), cinnamic acid yield per h (C) cinnamaldehyde yield per h (D), energy consumption (E), and CO2 emission (F) from microwave-assisted extraction (MAE), ultrasonic-assisted extraction (UAE) and reflux extraction (RE)
All extraction methods (MAE, UAE, and RE) were also compared for the energy consumed and CO2 emitted from each extraction method. As depicted in Fig. 2E, MAE was the eco-friendliest method with the lowest energy consumption (0.000023 TOE), followed by UAE (0.000089 TOE) and RE (0.001035 TOE). A similar tendency was observed in case of CO2 emission. MAE led to comparable yields of target compounds (cinnamic acid and cinnamaldehyde) with significantly reduced emission (0.000047 TCO2) compared with RE. In contrast, UAE ranked second with a CO2 emission of 0.000177 TCO2, whereas RE resulted in a significantly higher CO2 emission (0.002063 TCO2). The results suggested that MAE offered several advantages as the eco-friendliest and best method for extracting bioactive compounds from cinnamon powder. Second was UAE, which resulted in a lower extraction efficiency than MAE and a reduced resource consumption and CO2 emission. RE, as the conventional method, rendered yields comparable to MAE, but this method was resource-intensive owing to longer extraction times and higher solvent and energy consumption, accompanied by a higher CO2 emission. Therefore, we concluded that MAE was the most efficient green method for extracting bioactive components from cinnamon powder. Similar findings have been reported by Martino et al. (2006) who evaluated the effects and efficiencies of different extraction methods, including MAE, UAE, and Soxhlet extraction (SE) for the recovery of coumarin, o-coumaric acid, and melilotic acid from Melilotus officinalis. MAE was the most efficient and fastest method for extracting phytochemicals over a relatively shorter time (10 min) and resulted in the highest yields compared to UAE (60 min) and SE (120 min). In another report, MAE of flavonoids from Radix Astragali was compared with UAE, RE, and SE. The authors reported that MAE was the most efficient extraction method because it resulted in the highest percent yield of flavonoids over a shorter extraction time compared to UAE and other conventional methods (RE and SE). Moreover, MAE did not cause any degradation of flavonoid compounds and the authors implied that it could be used as an alternative to UAE, SE and RE (Xiao et al., 2008). Similarly, ultrasound and microwave-assisted extraction (UMAE) and UAE for extracting lycopene from tomatoes were optimized using RSM and compared for their extraction efficiencies. The results indicated that UMAE performed well with higher efficiency and exhibited significant potential for extracting lycopene compared to UAE by overcoming the inherent limitations of UAE.
In conclusion, cinnamon has a long history of use as a spice and condiment in daily life and various published reports have confirmed the health-beneficial properties of its bioactive compounds in cinnamon that exist in the form of essential oils, phenolic compounds, flavonoids, cinnamic acid and cinnamaldehyde. The extraction characteristics of MAE, UAE and RE were monitored through RSM by optimizing the extraction conditions of each extraction method for obtaining maximum target responses: total yield (Y1), cinnamic acid (Y2) and cinnamaldehyde (Y3) yields. The extraction methods were compared for their efficiencies in terms of obtained target responses, energy consumption and CO2 emission. MAE yielded the maximum target responses at the optimum extraction conditions of 59% ethanol concentration, 147.5 W microwave power, and 3.4 min of extraction time. UAE resulted in maximum yields at optimum conditions of 55% ethanol concentration and 33 min of extraction time. RE yielded total extract, cinnamic acid and cinnamaldehyde contents comparable to MAE at its optimum conditions of 63% ethanol concentration, 77.5 °C extraction temperature, and 2.25 h of extraction time. MAE rendered the highest yields with the least consumption of time, energy, and solvent and resulted in the lowest CO2 emission compared to UAE and RE. While, RE was the most time-consuming and laborious method with the least efficiency. Therefore, it was concluded that MAE is the most efficient green method for obtaining target components from cinnamon extract to be used for further analytical and food processing purposes. This work demonstrated the feasibility of MAE for phytochemical extraction from plant matrices as well as cinnamon on large scale. Moreover, successful application of RSM has opened future research avenues for optimization of bioactive components from other plants of medicinal and pharmaceutical significance.
Acknowledgements
This research was supported by Kyungpook National University Bokhyeon Research Fund, 2015.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ameer K, Bae SW, Jo Y, Chung N, Gao Y, Kwon JH. Optimization and modeling for heat reflux extraction of total yield, stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni) leaves. Sep. Sci. Technol. 2017;52:1193–1205. doi: 10.1080/01496395.2017.1285313. [DOI] [Google Scholar]
- Ameer K, Bae SW, Jo Y, Lee HG, Ameer A, Kwon JH. Optimization of microwave-assisted extraction of total extract, stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni) leaves using response surface methodology (RSM) and artificial neural network (ANN) modelling. Food Chem. 2017;229:198–207. doi: 10.1016/j.foodchem.2017.01.121. [DOI] [PubMed] [Google Scholar]
- Ameer K, Shahbaz HM, Kwon JH. Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Compr. Rev. Food Sci. F. 2017;16:295–315. doi: 10.1111/1541-4337.12253. [DOI] [PubMed] [Google Scholar]
- Askari F, Rashidkhani B, Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr. Res. 2014;34:143–148. doi: 10.1016/j.nutres.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Cravotto G, Binello A, Orio L. Green extraction techniques. Agro Food Ind. Hi-tech. 2011;22:57–59. [Google Scholar]
- Das A, Golder AK, Das C. Enhanced extraction of rebaudioside-A: experimental, response surface optimization and prediction using artificial neural network. Ind. Crop. Prod. 2015;65:415–421. doi: 10.1016/j.indcrop.2014.11.006. [DOI] [Google Scholar]
- Frydman-Marom A, Levin A, Farfara D, Benromano T, Scherzer-Attali R, Peled S, Vassar R, Segal D, Gazit E, Frenkel D, Ovadia M. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011;6:e16564–e16564. doi: 10.1371/journal.pone.0016564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafoor K, Choi YH, Jeon JY, Jo IH. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009;57:4988–4994. doi: 10.1021/jf9001439. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh A, Jaafar HZE. Optimization of reflux conditions for total flavonoid and total phenolic extraction and enhanced antioxidant capacity in Pandan (Pandanus amaryllifolius Roxb.) using response surface methodology. Sci. World J. 2014;2014:1–10. doi: 10.1155/2014/523120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidpour R, Hamidpour M, Hamidpour S, Shahlari M. Cinnamon from the selection of traditional applications to its novel effects on the inhibition of angiogenesis in cancer cells and prevention of Alzheimer’s disease, and a series of functions such as antioxidant, anticholesterol, antidiabetes, antibacterial, antifungal, nematicidal, acaracidal, and repellent activities. J. Tradit. Complem. Med. 2015;5:66–70. doi: 10.1016/j.jtcme.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayta M, İşçimen EM. Optimization of ultrasound-assisted antioxidant compounds extraction from germinated chickpea using response surface methodology. LWT-Food Sci. Technol. 2017;77:208–216. doi: 10.1016/j.lwt.2016.11.037. [DOI] [Google Scholar]
- Kim HK, Do JR, Lim TS, Akram K, Yoon SR, Kwon JH. Optimisation of microwave-assisted extraction for functional properties of Vitis coignetiae extract by response surface methodology. J. Sci. Food Agric. 2012;92:1780–1785. doi: 10.1002/jsfa.5546. [DOI] [PubMed] [Google Scholar]
- Korea Power Exchange (KPX). http://www.kpx.or.kr/Korean/htdocs/popup/pop_1224.html. Accessed Oct. 2, 2017.
- Kwon JH, Belanger JM, Pare JJ, Yaylayan VA. Application of the microwave-assisted process (MAP™) to the fast extraction of ginseng saponins. Food Res. Int. 2003;36:491–498. doi: 10.1016/S0963-9969(02)00197-7. [DOI] [Google Scholar]
- Maeng JH, Shahbaz HM, Ameer K, Jo Y, Kwon JH. Optimization of microwave-assisted extraction of bioactive compounds from Coriolus versicolor mushroom using response surface methodology. J. Food Process Eng. 2017;40:e12421. doi: 10.1111/jfpe.12421. [DOI] [Google Scholar]
- Martino E, Ramaiola I, Urbano M, Bracco F, Collina S. Microwave-assisted extraction of coumarin and related compounds from Melilotus officinalis (L.) Pallas as an alternative to Soxhlet and ultrasound-assisted extraction. J. Chromatogr. A. 2006;1125:147–151. doi: 10.1016/j.chroma.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Mathew S, Abraham TE. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts through various in vitro models. Food Chem. 2006;94:520–528. doi: 10.1016/j.foodchem.2004.11.043. [DOI] [Google Scholar]
- Ministry of Food and Drug Safety (MFDS). The Korean Pharmacopoeia. 10th ed. The Society of Korean Official Compendium for Public Health, The Yakup Shinmoon Inc., Seoul, Korea. p. 84 (2013)
- Ministry of Trade, Industry and Energy (MTIE). Implementing regulations in energy law, Sejong, Republic of Korea (2011)
- Pires RH, Montanari LB, Martins CHG, Zaia JE, Almeida AMF, Matsumoto MT, Mendes-Giannini MJS. Anticandidal efficacy of cinnamon oil against planktonic and biofilm cultures of Candida parapsilosis and Candida orthopsilosis. Mycopathologia. 2011;172:453–464. doi: 10.1007/s11046-011-9448-0. [DOI] [PubMed] [Google Scholar]
- Ranasinghe P, Pigera S, Premakumara GAS, Galappaththy P, Constantine GR, Katulanda P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complem. Altern. M. 2013;13:275. doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute, Inc. SAS User’s Guide. Cary, NC, USA: Statistical Analysis Systems Institute; 1990. [Google Scholar]
- Seo CS, Kim JH, Shin HK. Simultaneous determination of albiflorin, cinnamaldehyde, cinnamic acid, daidzin, glycyrrhizin, liquiritin, paeoniflorin and puerarin in galgeun-tang by HPLC-PDA. J. Korean Orien. Med. 2010;31:8–15. [Google Scholar]
- Xiao W, Han L, Shi B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep. Purif. Technol. 2008;62:614–618. doi: 10.1016/j.seppur.2008.03.025. [DOI] [Google Scholar]