Abstract
The objective of this study was to evaluate probiotic effects of two Lactobacillus plantarum strains (GBL16 and 17) isolated from black raspberry. Results revealed that the number of GBL16 was gradually decreased as bile salt concentration was increased from 0.3 to 1%. However, GBL17 did not show any difference when GBL17 was applied to 1% bile salt, and it indicates that GBL17 is more tolerant to bile salt than GBL16. GBL17 exhibited higher heat resistance and adhesion ability to Caco-2 cells than GBL16. Regarding gut microbiome, no significant change in the number of total bacteria in intestines of mice after treatment with GBLs was determined. However, the combination of GBL16 and GBL17 significantly increased the number of total bacteria in intestines of mice after they were orally administered. Therefore, the results suggest that both GBL16 and 17 strains could be one of major probiotics that can improve human gut health.
Keywords: Black raspberry, Lactobacillus plantarum, Bile salt, Gut, Probiotic
Introduction
In gastrointestinal (GI) tract, more than 1000 species of microorganisms with count of about 1014 live. These microorganisms are not only absolute components of the GI tract, but also related to functions of the GI system (Hooper and Macpherson, 2010). Normally, in GI tract of young people, there are more than 10 billion Bifidobacterium per gram. However, the number of beneficial bacteria such as Bifidobacterium is gradually decreased when people get old or diseased. In contrast, the number of Clostridium, a harmful bacterium, is only about 1000 cells per gram in the GI tract at an early age. Its harmful effects such as generation of decay products are increased with age (Homma, 1998). Therefore, beneficial bacteria including Bifidobacterium and Lactobacillus in the GI tract might compete with harmful bacteria for growth, resulting in human health and longevity.
Enteric microbiota inside the intestine affects human health in various ways. For healthy individuals, enteric microbiota not only digests food ingredients to supply nutrients and energy for the host, but also maintains intestinal homeostasis and balance of the immune system (Hooper and Macpherson, 2010; Hooper et al., 2012). Thus, both number and diversity of intestinal microorganisms play an important role in maintaining human health. Probiotic is derived from the Greek word “for life” (Randey et al., 2015). It refers to a single or complex strain of living microorganisms that can improve the distribution of intestinal microorganisms upon ingestion, thereby providing a beneficial effect on the host (Randey et al., 2015). Many studies have evaluated the functionality of probiotics for human health (Hooper et al., 2012; Randey et al., 2015). The most frequently used microorganisms as probiotics are Bifidobacterium genus and Lactobacillus genus (Randey et al., 2015). Among them, lactic acid bacteria (LAB) such as Lactobacillus have been traditionally used in a variety of fermented foods and adopted as a good source of probiotic bacterium. LABs are Generally Recognized as Safe (GRAS).
Berries such as black raspberry (Rubus occidentalis) hold a large amount of free sugar and minerals such as phosphorus, iron, and potassium (Nile and Park, 2014). They also contain abundant nutrients such as organic acid and vitamin C (Lee et al., 2013; Nile and Park, 2014). Black raspberry has been shown to possess anti-cancer, anti-inflammatory, antioxidant, and antibacterial effects (Lee et al., 2014; Nile and Park, 2014). Its effect on lipid metabolism has also been studied with human and animal cell lines (Chen et al., 2006). Although studies have clearly defined how black raspberry influences human health, little information is available on Lactobacillus strains isolated from black raspberry. Since black raspberry has been used to improve sexual dysfunction in aged men of Korea (Lee et al., 2014), Lactobacillus plantarum derived from black raspberry might potential effect on male adults. Two strains of Lactobacillus plantarum (GBL16 and GBL17) have been isolated from black raspberry (Ryu et al., 2015). The objective of this study was to characterize the two Lactobacillus plantarum strains as probiotics and their influence on gut microbiome of BALB/c mice.
Materials and methods
Bacterial strains and culture conditions
Lactobacillus plantarum GBL16 (Accession No. KCCM11621p) and 17 (Accession No. KCCM11622p) used in this study were isolated from black raspberry and identified by Berry & Biofood Research Institute, Korea (Ryu et al., 2015). Commercial L. plantarum (KCTC13093) and L. brevis (KCCM40399) were purchased from Korean Collection for Type Cultures (KCTC, Jeongeup, Korea) or Korea Culture Center of Microorganisms (KCCM, Seoul, Korea). Frozen cultures stored at − 80 °C were revived in 10 mL of sterile MRS (Difco, Detroit, MI, USA) broth at 30 °C for 24 h. Working culture at stationary phase was prepared by incubating each bacterium in 10 mL of MRS at 30 °C for 24 h with two consecutive transfers.
Bacterial growth and pH measurement
To monitor bacterial growth in MRS broth at 30 °C for 24 h, optical density was measured at wavelength of 600 nm using a spectrophotometer (BioTek Int. Kor. Ltd., Seoul, Korea). The pH of the inoculated MRS broth during 24 h of growth was measured using a pH meter (S20, Mettler Toledo, Schwerzenbach, Switzerland).
Acid and bile juice tolerance test
For acid tolerance test, two different MRS neutral broths were prepared by adding 1 N NaOH or 1 N HCl [pH 6.8 (MRS broth pH, control) and 2.5 (stomach pH)] (Clark et al., 1993). Individual L. plantarum GBL16 or GBL17 was inoculated into the broth followed by incubation at 30 °C for 3 h. After incubation, 0.1 mL of culture was plated onto MRS agar medium. All plates were incubated at 30 °C for 24–48 h. Colony numbers on the plate were manually counted and expressed in colony forming units (CFU/mL). KCTC13093 was used as a positive control for acid tolerance test.
Tolerance of L. plantarum GBL16 and GBL17 to bile juice was determined using published method of Park et al. (1996). Briefly, MRS broth was supplemented with bile salt at concentration of 0, 0.3, 0.6, or 1%. One mL of bacterial culture was inoculated into 9 mL of the broth to reach a final concentration of approximately 109 CFU/mL and cultured at 30 °C for 24 h. After culture, colony numbers were enumerated and expressed as CFU/mL.
Heat tolerance test
For heat tolerance test, each strain cultured in MRS broth at 30 °C for 24 h was incubated at 30, 60, or 75 °C for 30 min and the number of viable cells was counted and expressed as CFU/mL (Jung et al., 2009).
Adhesion ability of L. plantarum to Caco-2 cells
Caco-2 cell, a human small intestinal epithelial cell line, was purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). Caco-2 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Welgene, Gyeonsan, Korea) supplemented with 10% Fetal Bovine Serum (FBS, Millipore, Billerica, USA), 100 U/mL penicillin (Gibco, Grand island, NY, USA), and 100 µg/mL streptomycin (Gibco, Grand island, NY, USA) at 37 °C in 5% CO2 incubator (Memmert, Schwabach, Germany). Caco-2 cells were plated into a 24-well cell culture plate at density of 1 × 104 cells/mL. After that, the medium was replaced with fresh DMEM medium without antibiotics or FBS after washing twice with sterilized phosphate buffered saline (PBS). GBL16 or GBL17 strain of L. plantarum was cultured in 5 mL of MRS broth at 37 °C overnight and 1 mL of each strain was centrifuged. The resultant pellet was washed twice with 1 mL of PBS and the pellet was suspended in 1 mL DMEM without antibiotics or FBS. About 109 CFU/mL of each strain in DMEM was inoculated into each well containing Caco-2 cells and cultured for 2 h. After washing wells with PBS three times, bacterial cells were collected by treating wells with 0.05% Triton X-100 (Sigma, St. Louis, MO, USA). Washed cells in each well were then serially diluted and plated onto MRS agar followed by incubation at 37 °C for 48 h. Colony number on the plate was enumerated as described previously. KCCM40399 was used as a control in this experiment. Cell adhesion rate was calculated by the following equation:
Animal experiment
Seven-week-old male BALB/c mice were purchased from Samtaco (Samtako Inc., Osan, Korea) and maintained at 22 ± 2 °C with 50 ± 5% of humidity. These mice with an average body weight of around 22 g were randomly divided into five different groups (n = 10 per group): (1) normal control (CTL), (2) positive control (POS, commercially available probiotics), (3) L. plantarum GBL16 (GBL16), (4) L. plantarum GBL17 (GBL17), and (5) L. plantarum GBL16 and GBL17 complex treated group (GBL16 + GBL17). A dose of 1010 CFU/mL was orally administered at the same time every day for 4 weeks. During the experimental period, body weight, drinking water, and diet intake were measured at the same time once a week. Weight gain was calculated based on final weight and initial weight:
At the end of the experiment, blood, feces, and small intestine of mice were collected from experimental sacrificed animal for colony counts and hematologic changes.
For feces collection, each experimental animal cage was replaced with alcohol disinfected cage on the day before the end of the experiment. Feces were collected over 4 h after replacing animal cages. These feces were diluted 50 times with PBS and 1 mL of the diluent was added to AC medium (3M, St. Paul, MN, USA).
On the last day of the experiment, the small intestine of each experimental animal was extracted in the direction from the duodenum to the total length in both directions. The small intestine was washed with PBS to collect bacterial cells followed by serial dilution with PBS. These diluents were plated onto suitable medium and cultured. Colony numbers were then counted to obtain total bacteria and lactic acid bacterial counts.
Before sacrifice, all mice were fasted for 12 h and blood samples were collected after sacrifice. After blood was taken, serum was separated by centrifugation at 3500 rpm for 15 min. Activities of glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), and triglyceride (TG) level in the serum were analyzed using Asan pharmaceutical kits (Asan Pharm., Hwaseong, Gyeonggi, Korea) following the manufacturer’s instructions. All animal experiment and management were carried out after obtaining approval from the Ethical Committee of Experimental Animals in the Efficacy Evaluation Center of the Berry & Biofood Research Institute (Animal Experiment Approval No.: BBRI-IACUC-16005).
Statistical analysis
All experiments were performed independently in duplicates with triplicate samples except for the animal experiment described above. All data are expressed as mean ± standard error. Mean values were statistically analyzed by analysis of variance (ANOVA) at p < 0.05 with Duncan’s multiple range test using SPSS (version 12.0, SPSS Inc, IBM Co., Armonk, NY, USA).
Results and discussion
Growth characteristics of L. plantarum GBL16 and GBL17
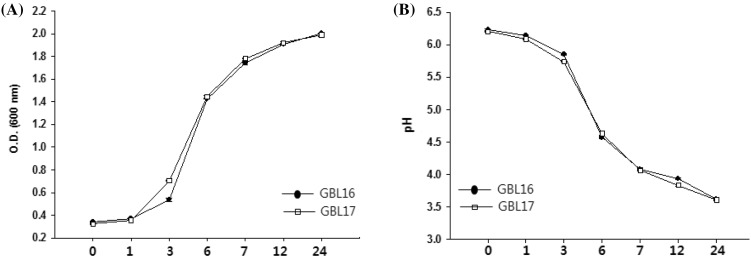
The growth of L. plantarum GBL16 and GBL17 isolated from black raspberry was observed during the incubation in MRS broth at 30 °C for 24 h to find when they would reach the stationary phase. As shown in Fig. 1(A), there were no significant difference in the growth pattern between GBL16 and GBL17 strains. Both strains reached the stationary phase after 24 h of growth. The pH of inoculated medium gradually decreased as cell grew, reaching pH 3.6 after 24 h, similar to the result of a previous study by Ryu et al. (2015) showing that L. plantarum GBL17 had the maximum proliferation after 24 h of incubation and the pH of medium decreased to pH 3.8. Other studies have shown that L. plantarum AF1 has the maximum growth proliferation within 20 h under similar condition (Yang and Chang, 2008). These results indicate that optimal growth of both GBL16 and GBL17 strains could be obtained by culturing them at 30 °C for 24 h with inoculation ratio to MRS broth at 5%. Thus, the following experiments were conducted with cells grown under these conditions.
Fig. 1.
Growth curve (A) and pH change (B) of L. plantarum GBL16 and 17
Acid and bile juice resistance of L. plantarum GBL16 and GBL17
To act as a probiotic in human body, lactic acid bacteria (LAB) need to be physiologically stable in the digestion system. In general, LAB are taken through the oral cavity, passing through the stomach where the pH is around 1.8–2.5 (Clark et al., 1993; Marteau et al. 1997) and reaching the final target site through the duodenum in which bile is present. Thus, L. plantarum GBL16 and GBL17 strains as probiotics should be resistant to acid conditions such as gastric juice (Chou and Weimer, 1999; Saarela et al., 2000) and bile juice (Dunne et al., 2001). To determine whether both strains could survive in the stomach and duodenum, they were exposed to pH 2.5 for 3 h and various concentrations of bile salts for 24 h to simulate human digestion system. As shown in Table 1, populations of both GBL strains decreased by about 0.1 log CFU/mL in pH 2.5 for 3 h. There was no significant difference in the reduction of population between L. plantarum GBL16 and GBL17. However, reduction of 0.26 log CFU/mL was found for commercial L. plantarum (p > 0.05). Mishra and Prasad (2005) have demonstrated that it is crucial to show a viable count of more than 104 CFU/mL at pH 2 to exert probiotic function in the human body. Drahomira et al. (2006) have also reported that over 90% of L. plantarum need to survive at pH 3 to be used as a probiotic. Thus, both L. plantarum GBL16 and GBL17 strains might be able to survive in the GI tract.
Table 1.
Acid and bile salt tolerance of L. plantarum GBL16, L. plantarum GBL17 and a commercial L. plantarum (KCTC13093)
| Strains | Acid tolerance1 | Bile salt tolerance2 | ||||
|---|---|---|---|---|---|---|
| Control (pH 6.8) | pH 2.5 | Control (0%) |
0.3% | 0.6% | 1% | |
| GBL16 | 9.82 ± 0.02b | 9.74 ± 0.03b | 9.81 ± 0.03c,z | 9.16 ± 0.18b,y | 7.76 ± 0.12a,x | 7.77 ± 0.00a,x |
| GBL17 | 9.86 ± 0.03b | 9.75 ± 0.01b | 9.72 ± 0.01b,y | 8.34 ± 0.04a,x | 8.30 ± 0.02b,x | 8.31 ± 0.01b,x |
| KCTC13093 | 9.45 ± 0.08a | 9.19 ± 0.01a | 9.36 ± 0.01a,z | 8.03 ± 0.02a,y | 7.77 ± 0.05a,x | 7.78 ± 0.04a,x |
1Acid tolerance results are shown in viable count (log CFU/mL) of each isolate at pH 6.8 and pH 2.5 for 3 h
2Bile salt tolerance results are shown in viable count (log CFU/mL) of each isolate grown in MRS media containing 0 and 1% bile extract at 30 °C for 24 h
a,b,cMeans within a column followed by different letters are significantly different (p < 0.05) according to Duncan’s multiple range test
x,y,zMeans within a column followed by different letters are significantly different (p < 0.05) according to Duncan’s multiple range test
LAB also need to be resistant to higher concentrations of bile salt than that of intestinal bile (0.3%) to exhibit desired functionality as probiotics (Hyronimus et al., 2000). Thus, viability of both GBL strains in bile salts at 0.3–1% was determined. Results are shown in Table 1. When bile salt concentration was increased from 0.3 to 1%, population of L. plantarum GBL16 was reduced by 0.7–2.1 log CFU/mL while that of GBL17 was reduced by 0.7–1.4 log CFU/mL, similar to the reduction in population of commercial L. plantarum (KCCM40399) which was decreased by 1.3–2.4 log CFU/mL with increasing of bile salt concentrations. This is consistent with observations of previous studies in which acid tolerant GBL17 shows relatively good resistance to bile salts (Cho et al., 2013; Lee et al., 2012).
Heat resistance of L. plantarum GBL16 and GBL17
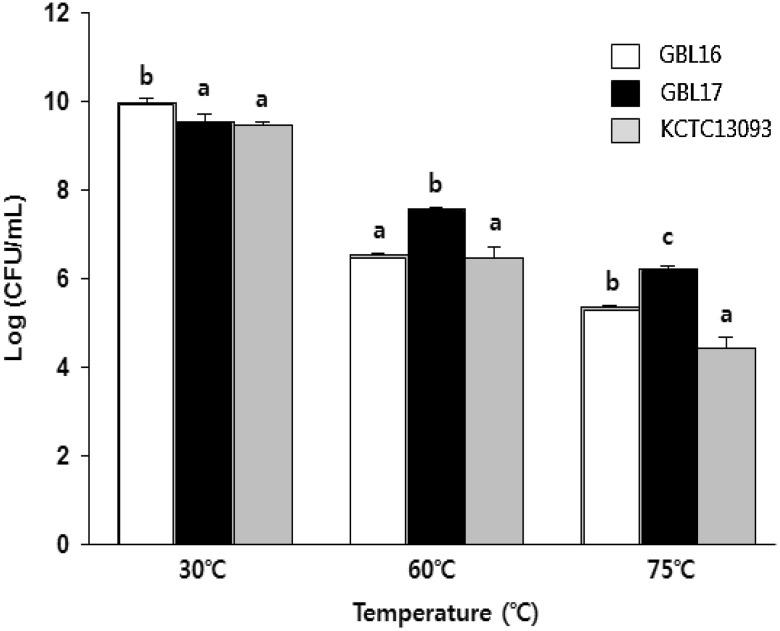
Resistance of probiotics to heat is important since they should survive during the spray drying process of formulation (Gouesbet et al., 2002). Thus, this study compared heat resistance of L. plantarum GBL16 and GBL17 with commercial L. plantarum strain (KCTC13093) to determine whether these black raspberry isolates might show better survival at high temperatures than the commercial strain. More than 9.5 log CFU/mL survived for all three strains tested when they were exposed to 30 °C for 30 min, although the survivability of L. plantarum GBL16 was better than the other two strains (Fig. 2). After treatment at 60 and 75 °C for 30 min, about 79.6 and 65.3% of GBL17 populations survived, respectively. For L. plantarum GBL16, 65.6 and 53.9% survived 30 min treatment at 60 and 75 °C, respectively. For the commercial strain, 68.5 and 47.0% survived, respectively. These results are better or similar to results of published studies (Byun et al., 2000; Lee et al., 2016). Both Byun et al. (2000) and Lee et al. (2016) reported that, when L. plantarum strains were exposed to 80 °C for 15 min and 55 °C for 2 h, about 16.0 and 71.2% of populations survived, respectively. These results indicate that both black raspberry isolates have similar or better heat resistance during spray drying than the commercial strain.
Fig. 2.
Heat tolerance of L. plantarum GBL16 and 17 at 30, 60, and 75 °C for 30 min. Means with different letters (a, b) are significantly different (p < 0.05) according to Duncan’s multiple range test
Intestinal adhesiveness of L. plantarum GBL16 and GBL17 to Caco-2 cells
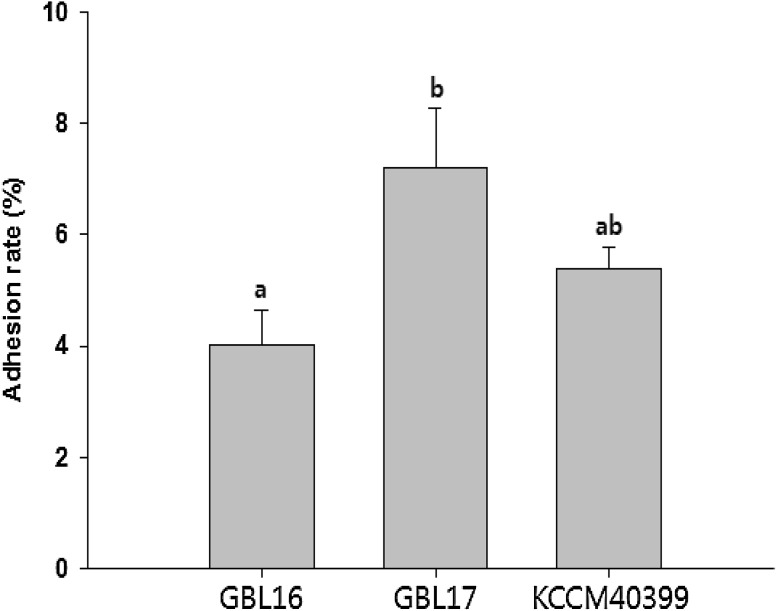
Intestinal adhesion property is known to be important factor for LAB to show their efficacy as a probiotic. To date, some studies on intestinal adhesion properties of LAB have been conducted mainly based on their binding abilities to Caco-2 or HT-29 human intestinal epithelial cells (Chauviere et al., 1992; Coconnier et al., 1992; Ouwehand et al., 2001). It is known that the binding of LAB to human intestinal mucosa is caused by the binding between lectin protein on the surface of LAB and mucin, a protein polysaccharide constituting the mucosal layer (Matsumura et al., 1999). It has been reported that L. brevis has excellent adhesion to Caco-2 cells (Ramos et al., 2013). Thus, adhesion ability of L. plantarum GBL16 or GBL17 was compared to that of L. brevis. Adhesion rates of L. brevis, L. plantarum GBL16, GBL 17 to Caco-2 cells were 5.4 ± 0.4, 7.2 ± 1.1 and 4.0 ± 0.6%, respectively (Fig. 2). The adhesion ability of L. plantarum GBL17 was significantly higher than that of GBL16. However, the adhesion ability was not significantly different between GBL17 and L. brevis. Similarly, Lee et al. (2016) have reported that intestinal adhesion properties of L. plantarum and L. mesenteroides isolated from Kimchi are as high as 7.0%. On the other hand, adhesion abilities of L. plantarum BSM-2 and TJH-1 isolated from Makkolli are 4.0 and 4.6%, respectively (Jung and Kim, 2015), which are lower than that of GBL17 but similar to that of GBL16. These results indicate that GBL16 and GBL17 have sufficient ability to adhere to human intestinal epithelial cells, suggesting their potential as probiotics.
Changes in microbial community in the gastrointestinal tract and feces by administration of L. plantarum GBL16 and GBL17
It is well known that LAB as probiotics is influenced by total composition of intestinal microorganisms and interaction between ingested LAB and intestinal cells. Thus, changes in the intestinal microbiota, weight gain, feed intake and water intake of mice after oral administration of L. plantarum GBL16 and GBL17 for 4 weeks were observed using BALB/c mouse model. Body weight, feed intake, or water intake during the test period was not significantly different among groups (Table 2). There was no significant difference in total bacterial count in the intestine among commercial probiotic, L. plantarum GBL17, and the combination of GBL16 and GBL17 groups (Table 2). However, total counts of GBL17 were significantly higher than those of the commercial control and GBL16 groups. Among these groups, only GBL17 and the combination of GBL16 and GBL17 combination group showed a difference when LAB of small intestine was enumerated after 4 weeks of GBL administration. Surprisingly, 2.96 log CFU/mL of GBL16 remained in the small intestine. Such LAB numbers remained in small intestine were proportionally high compared to those in other groups (intestinal LAB/total bacteria). Although GBL17 had strong adhesion ability to small intestine as seen in Fig. 3, GBL16 seemed to have higher competition effects, reducing the number of other bacteria. All treatment groups significantly increased LAB in feces compared to the control group, indicating that GBL16 and/or GBL17 could be easily eliminated or adhered onto small intestine. Some studies have reported the loss of LAB when LAB are supplied to mouse (Kim et al., 2010; Nguyen et al., 2007). However, both Nguyen et al. (2007) and Kim et al. (2010) have insisted that LABs or Bifidobacteria in feces are significantly increased after administration of L. plantarum PH04 (5 × 108 CFU/mL) or Bifidobacterium longum SPM1205 mixed preparation (3.0 × 1011 CFU/g) to mouse.
Table 2.
Weight gain, feed intake, water intake, and counts in intestinal tract and feces of the experimental BALB/c mouse groups after administration of lactic acid bacteria
| Items | CTL | POS | GBL16 | GBL17 | GBL16 + 17 |
|---|---|---|---|---|---|
| Initial weight (g) | 23.69 ± 0.42 | 24.00 ± 0.36 | 23.90 ± 0.24 | 24.10 ± 0.30 | 24.16 ± 0.40 |
| Final weight (g) | 24.94 ± 0.50 | 25.31 ± 0.30 | 25.20 ± 0.36 | 25.79 ± 0.44 | 25.01 ± 0.31 |
| Weight gain (g/day) | 0.42 ± 0.08 | 0.44 ± 0.13 | 0.43 ± 0.11 | 0.56 ± 0.09 | 0.28 ± 0.11 |
| Feed intake (g/day) | 2.66 ± 0.20 | 2.56 ± 0.20 | 2.53 ± 0.22 | 2.73 ± 0.22 | 2.59 ± 0.29 |
| Water intake (ml/day) | 2.93 ± 0.29 | 3.00 ± 0.32 | 2.91 ± 0.26 | 2.95 ± 0.26 | 2.95 ± 0.35 |
| Total bacteria (intestines) | 2.82 ± 0.25a | 8.94 ± 0.38b | 2.98 ± 0.17a | 8.34 ± 0.17b | 9.26 ± 0.38b |
| LAB (intestines) | 2.81 ± 0.36a,b | 3.37 ± 0.19a,b | 2.96 ± 0.33a,b | 2.57 ± 0.96a | 3.5 ± 0.66b |
| LAB (Feces) | 4.77 ± 0.07a | 6.08 ± 0.08b | 5.99 ± 0.10b | 6.09 ± 0.04b | 6.38 ± 0.11c |
CTL, control group; POS, positive control group (1010 CFU/mL); GBL16, L. plantarum GBL16 group (1010 CFU/mL); GBL17, L. plantarum GBL17 group (1010 CFU/mL); GBL16 + 17, L. plantarum GBL16 and 17 combination group (1010 CFU/mL)
a,bMeans within a row followed by different letters are significantly different (p < 0.05) according to Duncan’s multiple range test
Fig. 3.
Adhesion rate (%) of L. plantarum GBL16 and 17 to Caco-2 cells. Means with different letters (a, b) are significantly different (p < 0.05) according to Duncan’s multiple range test
Serological changes in vivo by administration of L. plantarum GBL16 and GBL17
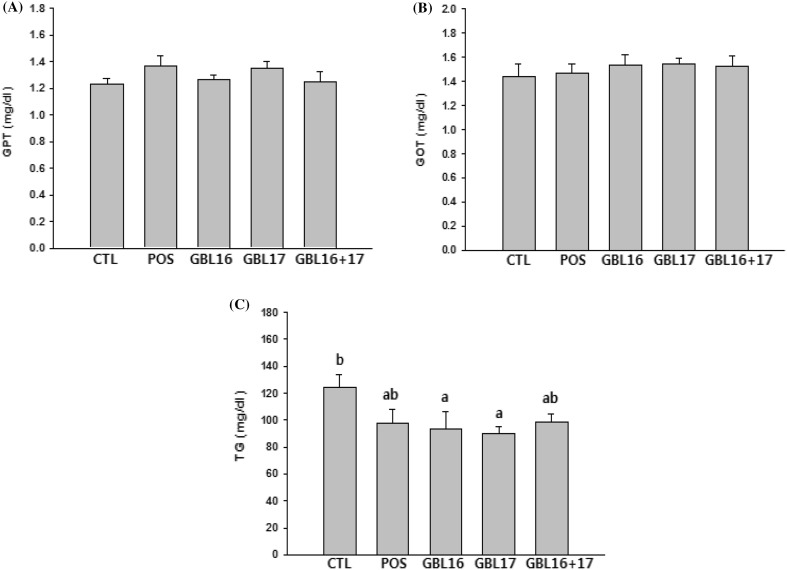
To investigate the biotoxicity of L. plantarum GBL16 and GBL17, activities of glutamic pyruvic transaminase (GPT) and glutamic oxaloacetic transaminase (GOT) reflecting hepatic necrosis and hepatocyte hypertrophy were measured in this study. Results showed that there were no significant differences in GPT or GOT activities among groups (Fig. 4A, B). Thus, administration of L. plantarum GBL16 and GBL17 might not be toxic to human health. Fermentation using beneficial microorganisms to improve human health has recently received increased attention due to increase of functional ingredients and physiological activity of natural products (Ko, 2013). Furthermore, some previous studies have demonstrated that intestinal microorganisms are highly related to obesity. For example, when L. plantarum PH04 (5 × 108 CFU/mL) was administered for 14 days, there was 10% reduction in triglyceride in the serum of high cholesterol mice (Abd El-Gawad et al., 2005). It has also been reported that 35% of yogurts with Bifidobacterium added to albino cholesterol rats can result in 51.2% reduction in triglyceride (Rolfe, 2000). Thus, the amount of triglyceride (TG) in mice was measured in this study after administration of L. plantarum GBL16 and GBL17 (Fig. 4C). The level of TG in the control group was 124.2 ± 9.9 mg/dL. However, administration of L. plantarum GBL16 and GBL17 decreased the level of TG by 31.2 and 34.3 mg/dL, respectively, although no significant reduction in TG level was observed after administration of commercial probiotic or the combination of GBL16 and GBL17.
Fig. 4.
Effects of L. plantarum GBL16 and 17 on serum glutamic pyruvic transaminase (GPT, A) and glutamic oxaloacetic transaminase (GOT, B) activities and triglyceride (TG, C) level in BALB/c mice. CTL, Control group; POS, Positive control group (1010 CFU/mL); GBL16, L. plantarum GBL16 group (1010 CFU/mL); GBL17, L. plantarum GBL17 group (1010 CFU/mL); GBL16 + 17, L. plantarum GBL16 and 17 combination group (1010 CFU/mL). Means with different letters (a, b) are significantly different (p < 0.05) according to Duncan’s multiple range test
This is the first study that shows the potential of L. plantarum GBL16 and GBL17 isolated from black raspberry as probiotics. Although the acid, bile salt, and heat tolerances and adhesion ability of GBL16 and 17 were similar to those of the commercial strain, the combined administration of GBL16 and GBL17 significantly improved the number of LAB in intestines and feces of BALB/c mice. Therefore, GBL16 and GBL17 have potential as probiotics for improving human health. However, LAB in its final habitat differs from species to species. For example, Lactobacillus species are mainly present in the small intestine whereas Bifidobacterium species are present in the colon. Further study is needed to elucidate the best combination of GBL16 and GBL17 strains with different habitat types to generate optimal methods for ingesting LABs that are beneficial to human health.
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Export Promotion Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116072-3), Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest related to this study to disclose.
References
- Abd El-Gawad IA, El-Sayed EM, Hafez SA, El-Zeini HM, Saleh FA. The hypocholesterolaemic effect of milk yoghurt and soy-yoghurt containing Bifidobacteria in rats fed on a cholesterol-enriched diet. Int. Dairy J. 2005;15:37–44. doi: 10.1016/j.idairyj.2004.06.001. [DOI] [Google Scholar]
- Byun JW, Kim GT, Bae HS, Baek YJ, Lee YK. In vitro selection of lactic acid bacteria for probiotic use in pigs. Korea J. Vet. Res. 2000;40:701–706. [Google Scholar]
- Chauviere G, Coconnier MH, Kerneis S, Fourniat J, Servin AL. Adhesion of human Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J. Gen. Microbiol. 1992;138:1689–1696. doi: 10.1099/00221287-138-8-1689. [DOI] [PubMed] [Google Scholar]
- Chen T, Hwang HJ, Rose ME, Nines RG, Stoner GD. Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis: down regulation of cyclooxygenase-2, inducible nitric oxide synthase, and c-jun. Cancer Res. 2006;66:2853–2859. doi: 10.1158/0008-5472.CAN-05-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YH, Hong SM, Kim CH. Isolation and characterization of lactic acid bacteria from kimchi, Korean traditional fermented food to apply into fermented dairy products. Korean J. Food Sci. An. 2013;33:75–82. doi: 10.5851/kosfa.2013.33.1.75. [DOI] [Google Scholar]
- Chou LS, Weimer B. Isolation and characterization of acid- and bile-tolerant isolates from strains of Lactobacillus acidophilus. J. Dairy Sci. 1999;82:23–31. doi: 10.3168/jds.S0022-0302(99)75204-5. [DOI] [PubMed] [Google Scholar]
- Clark PA, Cotton LN, Martin JH. Selection of bifidobacteria for use as dietary adjuncts in cultured dairy foods: II-Tolerance to simulated pH of human stomachs. Cult. Dairy Prod. J. 1993;28:11–14. [Google Scholar]
- Coconnier MH, Klaenhammer TR, Kerneis S, Bernet MF, Servin AL. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl. Environ. Microbiol. 1992;58:2034–2039. doi: 10.1128/aem.58.6.2034-2039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drahomira L, Jolana K, Maria G, Gabriel G, Andrea S, Zlatica K. In vitro testing of selected probiotic characteristics of Lactobacillus plantarum and Bifidobacterium longum. J. Food Nutr. Res. 2006;45:77–83. [Google Scholar]
- Dunne C, O’Mahony L, Murphy L, Thornton G, Morrissey D, O’Halloran S, Feeney M, Flynn S, Fitzgerald G, Daly C, Kiely B, O’Sullivan GC, Shanahan F, Collins JK. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J. Clin. Nutr. 2001;73:386S–392S. doi: 10.1093/ajcn/73.2.386s. [DOI] [PubMed] [Google Scholar]
- Gouesbet G, Jan G, Boyaval P. Two-dimensional electrophoresis study of Lactobacillus delbrueckii subsp. bulgaricus thermotolerance. Appl. Environ. Microbiol. 2002;68:1055–1063. doi: 10.1128/AEM.68.3.1055-1063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma N. Bifidobacteria as a resistance factor in human beings. Bifidobact. Microfl. 1998;7:35–43. doi: 10.12938/bifidus1982.7.1_35. [DOI] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Sci. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- Hyronimus B, Marrec CL, Sassi AH, Deschamps A. Acid and bile tolerance of spore- forming lactic acid bacteria. Int. J. Food Microbiol. 2000;61:193–197. doi: 10.1016/S0168-1605(00)00366-4. [DOI] [PubMed] [Google Scholar]
- Jung SE, Kim SH. Probiotic properties of lactic acid bacteria isolated from commercial raw Makgeoli. Korean J. Food Sci. Technol. 2015;47:45–50. [Google Scholar]
- Jung MY, Park YH, Kim HS, Poo HY, Chang YH. Probiotic property of Lactobacillus pentosus Miny-148 isolated from human feces. Kor. J. Microbiol. 2009;45:177–184. [Google Scholar]
- Kim JR, Lee DK, Baek EH, An HM, Yang HJ, Kim HJ, Choi KS, Yun ME, Jung YJ, Oh PJ, Chung MJ, Ha NJ. Efficacy of mixture of lactic acid bacteria (LAB) and Bifidobacteria supplement in the management of constipation; Demonstration of functionality in animal and clinical trials. Korean J. Microbiol. 2010;46:52–62. [Google Scholar]
- Ko JS. The intestinal microbiota and human disease. Korean J. Gastroenterol. 2013;62:85–91. doi: 10.4166/kjg.2013.62.2.85. [DOI] [PubMed] [Google Scholar]
- Lee KH, Bong YJ, Lee HA, Kim HY, Park KY. Probiotic effects of Lactobacillus plantarum and Leuconostoc mesenteroides isolated from kimchi. J Korean Soc. Food Sci. Nutr. 2016;45:12–19. doi: 10.3746/jkfn.2016.45.1.012. [DOI] [Google Scholar]
- Lee SJ, Choi HR, Lee J-H, Kwon JW, Lee HK, Jeong JT, Lee T-B. Effects of unripe black raspberry extracts on prostate cancer cell line and rat model of benign prostatic hyperplasia. J. Korean Soc. Food Sci. Nutr. 2014;43:507–515. doi: 10.3746/jkfn.2014.43.4.507. [DOI] [Google Scholar]
- Lee Y, Kim JC, Hwang KT, Kim D-H, Min JC. Quality characteristics of black raspberry wine fermented with different yeasts. J. Korean Soc. Food Sci. Nutr. 2013;42:784–791. doi: 10.3746/jkfn.2013.42.5.784. [DOI] [Google Scholar]
- Lee SG, Lee KW, Park TH, Park JY, Han NS, Kim JH. Proteomic analysis of proteins increased or reduced by ethanol of Lactobacillus plantarum ST4 isolated from Makgeolli, traditional Korean rice wine. J. Microbiol. Biotechnol. 2012;22:516–525. doi: 10.4014/jmb.1109.09012. [DOI] [PubMed] [Google Scholar]
- Marteau P, Minekus M, Havenaar R, Huis In’t Veld JHJ. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J. Dairy Sci. 1997;80:1031–1037. doi: 10.3168/jds.S0022-0302(97)76027-2. [DOI] [PubMed] [Google Scholar]
- Matsumura A, Saito T, Arakuni M, Kitazawa H, Kawau Y, Itoh T. New binding assay and preparative trial of cell-surface lectin from Lactobacillus acidophilus group lactic acid bacteria. J. Dairy Sci. 1999;82:2525–2529. doi: 10.3168/jds.S0022-0302(99)75505-0. [DOI] [PubMed] [Google Scholar]
- Mishra V, Prasad DN. Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int. J. Food Microbiol. 2005;103:109–115. doi: 10.1016/j.ijfoodmicro.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Kang JH, Lee MS. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. 2007;113:358–361. doi: 10.1016/j.ijfoodmicro.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Nile SH, Park SW. Edible berries: bioactive components and their effect on human health. Nutrition. 2014;30:134–144. doi: 10.1016/j.nut.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Ouwehand AC, Tuomola EM, Tolkko S, Salminen S. Assessment of adhesion properties of novel probiotic strains to human intestinal mucus. Int. J. Food Microbiol. 2001;64:119–126. doi: 10.1016/S0168-1605(00)00440-2. [DOI] [PubMed] [Google Scholar]
- Park SY, Ko YT, Jeong HK, Yang JO, Chung HS, Kim YB, Ji GE. Effect of various lactic acid bacteria on the serum cholesterol levels in rats and resistance to acid, bile and antibiotics. Korean J. Appl. Microbiol. Biotechnol. 1996;24:304–310. [Google Scholar]
- Ramos CL, Thorsen L, Schwan RF, Jespersen L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013;36:22–29. doi: 10.1016/j.fm.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Randey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics-a review. J. Food Sci. Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe RD. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000;130:396S–402S. doi: 10.1093/jn/130.2.396S. [DOI] [PubMed] [Google Scholar]
- Ryu EH, Yoon HH, Jung JH. Characteristics of lactic acid fermentation of black raspberry juice using the Lactobacillus plantarum GBL17 Strain. Korean J. Food Cook Sci. 2015;31:773–780. doi: 10.9724/kfcs.2015.31.6.773. [DOI] [Google Scholar]
- Saarela M, Mogensen G, Fondn R, Matto J, Mattila-sandholm T. Probiotic bacteria: safety, functional and technological properties. J. Biotechnol. 2000;84:197–215. doi: 10.1016/S0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Chang HC. Antifungal activity of Lactobacillus plantarum isolated from kimchi. Korean J. Microbiol. Biotechnol. 2008;36:276–284. [Google Scholar]