Abstract
Grapefruit seed extract (GSE)–incorporated carnauba wax (CW) coating was developed to preserve Satsuma mandarins (Citrus unshiu Marc.). GSE (1.00%, w/w)–incorporated CW (GSE–CW) coating emulsions and GSE (0.50%)–oregano oil (OO, 0.50%)–incorporated CW (GSE–OO–CW) coating emulsions reduced Penicillium italicum disease incidence (%) on mandarin surfaces by 23.6 ± 3.6 and 25.0 ± 5.0%, respectively, relative to that on uncoated mandarin samples (100%). GSE (1.00%)–CW coating emulsions exhibited a higher colloidal stability than GSE (0.50%)–OO (0.50%)–CW coating emulsions. During storage at 25 °C, GSE (1.00%)–CW coating was superior to CW coating in reducing P. italicum disease incidence. CW coating significantly reduced weight loss, respiration rate, and firmness loss during storage at 4 and 25 °C (P < 0.05). The ascorbic acid concentration and peel color were not affected by GSE–CW coating (P > 0.05). These results suggest that GSE–CW coating can extend the post-harvest shelf life of mandarins by inhibiting the growth of P. italicum.
Keywords: Mandarin, Edible coating, Penicillium italicum, Carnauba wax, Grapefruit seed extract
Introduction
Citrus fruits are widely cultivated in tropical and subtropical regions and consumed as fresh fruit, salads, juice, desserts, and preserves, as well as used in cosmetic products [1]. In East Asia, including Korea, China (southern regions), and Japan, Satsuma mandarin (Citrus unshiu Marc.) is the most commercially produced citrus cultivar [1]. The annual production and per-capita annual consumption of mandarins in Korea were 672,000 tons in 2015 and 14.3 kg in 2014, respectively [2].
The genus Penicillium represents the most frequent fungal contaminant of mandarins [3]. Chemical fungicides, such as sodium-O-phenylphenol, thiabendazole, and imazalil; irradiation; and storage in controlled or ozonated atmospheres have been traditionally used for controlling post-harvest decay in citrus fruits [4]. However, the increasing public concern regarding the potential health hazards of irradiation and synthetic fungicide residues, as well as high capital investment in storage facilities with controlled conditions, have promoted the development of new post-harvest processing and preservation technologies.
Previously reported research has demonstrated the preservative effects of biopolymer-based edible coatings on citrus fruits and the formulations of coatings, including polysaccharide-based coatings and shellac-based water wax coatings applied on Valencia oranges [5] and tangerines [6]; hydroxypropyl methylcellulose-lipid edible coatings applied on ‘Clemenule’ mandarins [7] and ‘Oronule’ mandarins [8]; and chitosan-based coatings on ‘Murcott’ tangor [9] and Kinnow [10]. These coatings can cause modification of the internal atmosphere around fresh produce surfaces and can serve as carriers of antimicrobial agents and antioxidants [5]. Carnauba wax (CW), originated from the leaves of the palm Copernicia prunifera, is a natural edible coating material used for fruit coating to retard moisture loss and impart glossiness [11].
Essential oils, aliphatic aldehydes, and natural plant extracts have been studied as antimicrobial coating materials for post-harvest control in citrus fruits. A chitosan coating incorporating tea tree oil inhibited Penicillium italicum growth on oranges [12]. Castillo et al. [13] reported that the application of a commercial wax incorporating essential oil mixture (thymol and carvacrol) was effective in inhibiting the growth of yeast and molds and total aerobes on lemon surface.
Grapefruit seed extract (GSE) derived from Citrus paradisi Macf. Rutaceae is known to possess antioxidant, antibacterial, antiviral, and antifungal activities [14]. Although several reports have described biopolymer coating development for fruit preservation and reported the antifungal effects of biopolymer coatings incorporating essential oils or GSE, CW coatings incorporating GSE has not been developed and therefore the effects of the coatings on the preservation of mandarins have not been investigated. Thus, the objectives of this study were (1) to generate coating formulations with CW, GSE, and oregano oil (OO), resulting in effective antifungal activity against P. italicum and high colloidal stability, and (2) to study the effects of the CW coatings on P. italicum growth and the preservation of physicochemical properties of mandarins during storage at 4 and 25 °C.
Materials and methods
Materials
Greenhouse-grown Satsuma mandarins (C. unshiu Marc.) were harvested in Jeju Island, South Korea, in 2016. The harvested mandarins were immediately transported to the laboratory. The average size of the selected mandarins was 60 ± 5 mm in diameter and their average weight was approximately 65 ± 1 g. The selected mandarins were free from mechanical damage or fungal decay. The mandarins were kept at 4 °C until further experiments were performed. We used a commercial CW-based solution (Safepack Products, Kfar Saba, Israel) that meets the food-additive regulations of the United States Food and Drug Administration and consists of 18.1% (w/w) CW, 75.8% (w/w) water, 3.8% (w/w) morpholine salts of fatty acids, 1.5% (w/w) white shellac, and 0.8% (w/w) silicon resin. An emulsifier, Tween 80 (polyoxyethylene sorbitan monooleate), was purchased from Ilshinwells Co., Ltd. (Seoul, Korea). Pure OO and GSE were purchased from Quinabra (Quimica Natural Brasileira, São José dos Campos, Brazil) and Now Foods (Bloomingdale, IL, USA), respectively. All coating materials were of food grade.
Preparation of coating emulsions
GSE, OO, or a GSE–OO mixture (1.00%, w/w) was added to the coating solution. The ratios of GSE and OO in the GSE–OO mixture were 0.25:0.75, 0.50:0.50, and 075:0.25. Tween 80, used as an emulsifier, was added to the coating emulsions at 25% (w/w GSE or OO). These coating emulsions were prepared using a high-shear probe mixer (Model T25, IKA-Works Inc., Wilmington, NC, USA) for 1 min at 10,000 rpm and degassed under vacuum.
Colloidal stability
The colloidal stabilities of the GSE (1.00%)–CW (GSE–CW) and GSE (0.50%)–OO (0.50%)–CW (GSE–OO–CW) coating emulsions were studied according to the method of Lee et al. [15]. Backscattering profiles of coating emulsions in the entire length of the sample cell (40 mm) were analyzed for 7 days at 23 ± 2 °C, using an emulsion stability analyzer (Turbiscan AGS, Formulaction, Toulouse, France). Turbiscan Stability Index (TSI) was also calculated to evaluate colloid stability of coating emulsions, using the following formula [15]:
where h is the sample height in the measurement cell, scani is the final backscattering (%), and scani−1 is the initial backscattering (%).
Fungal culture and preparation of inoculum
Penicillium italicum (KACC 40826), a strain isolated from Mandarin, was obtained from the Rural Development Administration-Genebank Information Center (Jeonju, Korea). The stock culture was maintained on potato dextrose agar (PDA, Difco, sparks, MD, USA) slants at 4 °C. Fresh cultures were grown on PDA plates at 25 °C before use. Spore suspensions were prepared by collecting spores from 5-day-old cultures and filtered through a single layer of cheesecloth to remove fungal mycelium. The filtrate was centrifuged at 4000×g for 15 min (GyroSpin, Gyrozen, Seoul, Korea), washed twice, and suspended in 0.1% peptone water. Spores were enumerated with a hemocytometer (Paul Marienfeld GmbH & Co. KG, Lauda-Konigshofen, Germany) and adjusted to 9.0 log spores/mL. Spore suspensions were diluted to the desired concentration (~ 6.0 log spores/mL) for inoculation.
Fungal inoculation and coating
Uniformly sized mandarins without physical injuries and signs of fungal infection were selected. On the day of the experiment, selected mandarins were lightly washed with 70% ethanol for 1 min, rinsed with running tap water for 1 min and sterile distilled water for 3 min, and air-dried for 1 h on a clean bench (HB-402; Hanbaek Co., Ltd., Bucheon, Korea). Mandarins were inoculated with P. italicum as described by Won et al. [16]. Needle punctures were performed on the sample surfaces, and 20 µL of spore suspension (~ 6.0 log spores/mL) was spotted into the puncture (approximately a 1-mm depth). For coating, mandarin samples were immersed in the prepared coating emulsion for 30 s and allowed to dry for 1 h at 23 ± 2 °C and 26 ± 3% relative humidity (RH). After drying, the integrity of the coating on each mandarin was visually inspected. If uncoated areas were found, additional coating emulsion was applied across the entire surface. Mandarin coating was made before (C + I) or after (I + C) inoculating with the spores. The C + I treatment simulated a situation in which coating was made on mandarins before contamination (post-process contamination), and the I + C treatment simulated coating mandarins after contamination [17]. The thickness of the coating was approximately 0.1 mm, determined based on weight measurements before and after coating. Each sample was packaged in a sterile stomacher bag (710 mL, Nasco Whirl–Pak®, Fort Atkinson, WI, USA) and stored at 25 °C and 75–80% RH for 7 days. Fungal disease incidence rates were expressed as a percentage of mandarins showing fungal decay symptoms (visible mycelial growth) over the initial number of the mandarins.
Storage study
Among the various coatings (Table 1), GSE (1.00%)–CW coating was selected for the storage study. The antifungal effects of coating against P. italicum as well as the physicochemical properties, including weight loss, CO2 concentration, firmness, and ascorbic acid concentration, were analyzed during storage at 4 and 25 °C for 7 or 28 days. Each mandarin sample was placed in a sterile bag (710 mL; Nasco Whirl–Pak®). The RH in mandarin sample-containing bags during storage at 4 and 25 °C was 80–85%, as determined using a data logger (Testo 174H, Testo AG, Lenzkirchen, Germany).
Table 1.
Effects of carnauba wax (CW) coating incorporating grapefruit seed extract (GSE) or oregano oil (OO) on the inhibition of P. italicum on mandarin peels
| Coating | Treatment | Disease incidence (%) |
|---|---|---|
| No coating | C + I1 | 100 ± 1.1a3 |
| I + C2 | 100 ± 0.0a | |
| CW coating (CW only) | C + I | 75.0 ± 5.0b |
| I + C | 80.0 ± 0.0b | |
| GSE (1.00%)–CW coating | C + I | 23.6 ± 3.6g |
| I + C | 30.0 ± 1.0fg | |
| OO (1.00%)–CW coating | C + I | 37.5 ± 7.5def |
| I + C | 31.7 ± 0.3efg | |
| GSE (0.50%)–OO (0.50%)–CW coating | C + I | 25.0 ± 5.0g |
| I + C | 36.9 ± 2.0def | |
| GSE (0.75%)–OO (0.25%)–CW coating | C + I | 55.0 ± 5.0c |
| I + C | 41.7 ± 8.4de | |
| GSE (0.25%)–OO (0.75%)–CW coating | C + I | 52.5 ± 7.5c |
| I + C | 45.0 ± 15cd |
1Inoculation after coating on fruits
2Inoculation before coating on fruits
3Different letters within a column are significantly different (P < 0.05)
Weight loss
The weight of individual mandarins of each sample type was recorded on each sampling day, and the same samples were weighed each time on the storage day at 4 and 25 °C. The weight loss was calculated as percentage loss relative to the initial mass. The precision balance used for the weight measurements had an accuracy of 0.01 g.
Respiration rate
The respiration rate of the fruits was measured according to the method of Won et al. [16]. Four mandarin fruits were loaded into a gas container (1.5 L volume). After equilibrating in the container for 1 h at 23 ± 2 °C, CO2 and O2 levels (%) in the headspace were analyzed at each sampling time, using a portable gas analyzer (Check Point 2, PBI Dansensor, Ringsted, Denmark). An injection needle connected to a built-in pump was used to withdraw gas (10 cm3) from the container headspace, which was subsequently analyzed.
Firmness
Firmness was evaluated as the maximum force required to penetrate the whole mandarin fruit [16]. A texture analyzer (TX-XT express 2007, Stable Micro Systems Ltd., Surrey, UK) with a cylindrical probe of 5-mm diameter was used, under the following conditions: a pre-test speed of 3.0 mm/s, a test speed of 1.0 mm/s, a post-test speed of 3.0 mm/s, and a penetration distance of 3 mm.
Ascorbic acid concentration
The ascorbic acid concentration (mg/g) in mandarin flesh was measured using a high-performance liquid chromatography system (LC-10ATvp, liquid chromatograph, Shimadzu Co., Kyoto, Japan) [16]. The mobile phase was 2% (v/v) acetic acid in acetonitrile (95:5 v/v). A Symmetry® C18 column (5 µm, 4.6 mm × 250 mm I.D., Waters Co., MA, USA) was placed in a column oven (Shinkwang Scientific Co., Taipei Hsien, Taiwan) set at 23 ± 2 °C. Mandarin juice was obtained from peeled mandarin flesh and blended using a food processor (Philips HR-1372, Koninklijke Philips Electronics N.V., Amsterdam, Netherlands). Mandarin juice samples were prepared by filtering the blended juice through four layers of cheesecloth. The samples (20 µL) were prepared by filtering the supernatant of mandarin juice obtained after centrifugation at 12,500×g for 10 min (23 °C). A syringe filter (Dismic®-25CP, cellulose acetate, pore size: 0.45 µm, Advance MFS, Inc., CA, USA) was used for filtration. The effluent was monitored using an ultraviolet–visible detector at 254 nm.
Color
The mandarin peel color was measured using a colorimeter (Chroma Meter CR-400; Minolta Camera Co., Osaka, Japan) and evaluated in terms of L* (lightness), a*, and b* values or/and hue (h°), based on CIELAB coordinates. The mandarin peel color was recorded in terms of the L* and h° values. The colorimeter was calibrated using a white standard tile (Illuminate D 65) and a 10° standard observer. The h° was calculated from L*, a*, and b* values, as described below.
Experimental design and statistical analyses
All experiments were performed in duplicate and were analyzed three times per sample, with each replicate. Ten samples per treatment were used for the P. italicum-inhibition study. Three measurement samples were used to determine weight loss, CO2 concentration, firmness, and ascorbic acid content in each replicate. Color coordinates were recorded five times for each sample. Analysis of variance was performed to evaluate differences among means; in cases of statistical significance, means were evaluated using Duncan’s multiple range test for significant differences (α = 0.05). PASW statistics software (version 18.0.0; IBM SPSS Inc., New York, NY, USA) was used for all statistical analyses.
Results and discussion
Inhibitory activity of coatings against P. italicum
The inhibitory effects of the coating emulsions against P. italicum inoculated on coated mandarins are presented in Table 1. All coatings exhibited higher inhibitory activity against P. italicum compared to that in untreated mandarins (P < 0.05). Even the CW coating without external antimicrobials exhibited antifungal activity in both C + I and I + C treatments. The antifungal effect of CW might be caused by lytic enzymes in CW, such as chitinase and β-1,3-glucanases, capable of inhibiting the early growth of Fusarium oxysporum, Colletotrichum lindemuthianum and Colletotrichum musae [18]. Abd-Alla et al. [19] reported that the decay incidences of tomato fruits dipped in CW for 2 h were 35.6% for Botrytis cinerea, 32.9% for Rhizopus stolonifera, and 30.0% for Alternaria alternate, compared to uncoated fruits (100%).
GSE- and OO–incorporated CW coatings significantly inhibited P. italicum growth compared to the CW coating (P < 0.05). GSE or OO contained in the coating appeared to effectively inhibit the growth of P. italicum by diffusing on the coating surface. Previous reports have described the antifungal activities of GSE and OO. For example, Xu et al. [20] reported that intact grape berries treated by immersion in 0.5% GSE solution for 1 min showed a 9.3% incidence of Botrytis cinerea infection after 7 day of storage (25 °C), while this incidence with untreated grape berries was 75%. The antimicrobial activities of GSE could be attributed to its high concentration of flavonoids and its derivatives, including naringin, hesperidin, limonoid, quercetin, and kaempferol [21]. Cho et al. [22] confirmed the bactericidal activity of GSE using transmission electron microscopy, which damaged microbial cell membranes. Additionally, Xu et al. [20] demonstrated that the germination rate of Botrytis cinerea spores exposed to 0.5% GSE for 1 min was reduced by 14%. Previous reports have shown that the antimicrobial effects of OO on various fruits and vegetables were higher when OO was used in a mix with other antimicrobials than when OO was used alone [23, 24]. The GSE and OO were mixed at different ratios and examined for a potential improved antifungal effect by mixing. The ratio of GSE to OO, 1:1, which was used in the formulation of the GSE (0.50%)–OO (0.50%)–CW coating, resulted in a higher inhibition than that was observed with the OO (1.00%)–CW coating when C + I treatment was applied (Table 1), agreeing with the previous reports. The results suggest that the antifungal effects depended on the ratio of the amounts of antimicrobials. There might be an optimum ratio of GSE and OO amounts in exhibiting the highest antifungal effect.
The inhibitory effects against innate P. italicum spores before packaging and against P. italicum infected after packaging were tested by the C + I and I + C treatments, respectively. Both C + I and I + C treatments effectively inhibited P. italicum. With the C + I treatment, P. italicum growth may have been inhibited after the antimicrobial compounds (GSE or OO) diffused from the coating emulsion to the smooth coating surface, with the coating creating an environment that made it difficult for hyphae of P. italicum to settle. With I + C treatment, the growth of P. italicum could be inhibited by the action of the incorporated antimicrobial compounds, GSE and OO, and the formation of oxygen barrier between the fruits and their immediate surroundings [25]. Additionally, the growth inhibition might have resulted from a failure of P. italicum to secure sufficient space for hyphal extension and proliferation. However, no significant differences between C + I and I + C-treated samples were found in terms of the disease-incidence rate with each coating (P > 0.05), except for the GSE (0.50%)–OO (0.50%) and GSE (0.75%)–OO (0.25%)–CW coatings. Therefore, the prepared antimicrobial coatings were effective not only in preventing post-harvest contamination, but also in inhibiting the growth of pre-existing P. italicum spores on the mandarin surface.
Colloidal stability
Colloidal stability was evaluated in emulsions formed with GSE (1.00%)–CW and GSE (0.50%)–OO (0.50%)–CW coatings, which appeared to represent the most effective coatings against P. italicum growth. Figure 1 presents a representative graph of the height-dependent backscattering of the GSE–CW coating emulsion (A) or the GSE–OO–CW coating emulsion (B). The GSE–CW coating emulsion was more stable than GSE–OO–CW, as judged by the lack of change in backscattering, and did not form creaming, which was studied at the top layer (38–40 mm height from the bottom of the sample cuvette). These results can be associated with the solubility of GSE in CW solution. GSE contains many aliphatic compounds [26]. CW contained 82% wax esters, mostly present in the forms of aliphatic co-hydroxy, aliphatic cinnamic, and aliphatic diesters [27]. GSE could be evenly distributed in the CW solution due to abundance of aliphatic compounds in both GSE and CW. In contrast, backscattering of the GSE–OO–CW coating resulted in creaming, as demonstrated by a peak with the top layer [Fig. 1(B)]. A decrease in colloidal stability was also demonstrated with increased TSI [28]. The rate of TSI increase in the GSE–OO–CW coating emulsion was higher than the rate of GSE emulsion, especially after day 3 in storage [Fig. 1(C)]. An unstable emulsion solution is not suitable to form a coating barrier on food surfaces due to considerable variation in the thickness of the coating formed from the emulsion, as well as blistering and cracking generated by the instability of coating-forming solution [29]. Food applications of coating emulsions with stable disperse systems provide a high barrier against gas, volatile compounds, and moisture on the food surface; thus, GSE–CW coating could improve the quality attributes of coated fruits, which can enhance mandarin market value and consumer’s preferences.
Fig. 1.
Backscattering (BS) profiles of the carnauba wax (CW) coating incorporating grapefruit seed extract (GSE, 1.00%) (A) and GSE 0.50% and oregano oil (OO) 0.50% (B), and the variation in BS (Turbiscan Stability Index, TSI) of CW coating emulsions incorporating GSE (1.00%) and both GSE (0.50%) and OO (0.50%) in the 0–40 mm zone at 25 °C for 7 days (C)
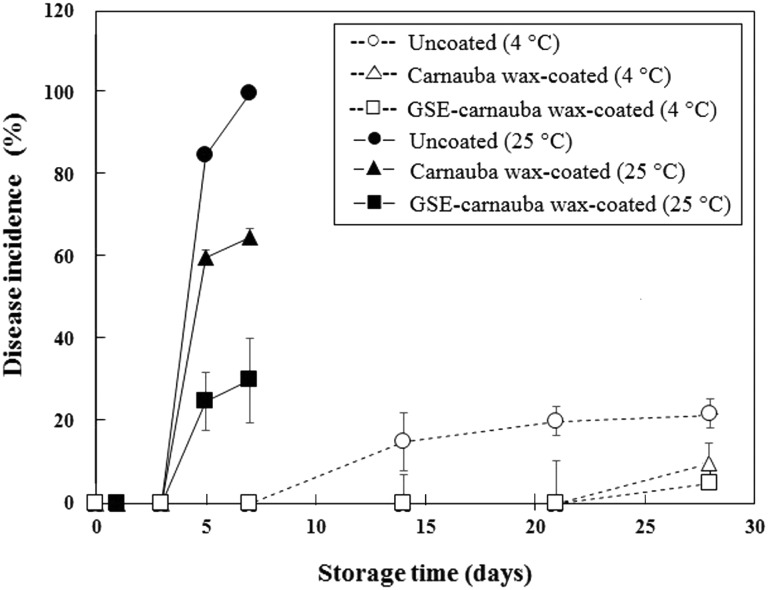
Effects of coating on P. italicum growth during storage
To investigate the effects of coating on P. italicum growth during storage, C + I treatment with GSE (1.00%)–CW coating was chosen due to its relatively high antifungal activity with a high colloidal stability. The inhibitory activities of CW coatings with and without GSE on P. italicum inoculated on mandarin peel are shown in Fig. 2. During storage at 4 °C, no incidence of disease symptoms in coated mandarin samples appeared until day 21 (Fig. 2). The GSE–CW-coated mandarins showed significantly higher antifungal activity than CW-coated and uncoated mandarins after day 5 at 25 °C (P < 0.05). The difference in the microbial counts of CW-coated and GSE–CW-coated samples was more pronounced at 25 °C than at 4 °C. This may have been due to a faster diffusion of the GSE antimicrobials at a higher temperature. Previously, Lee et al. [30], reported change of antimicrobial activity by temperature within coating emulsion due to difference in diffusion coefficient, in which the diffusion coefficient of antimicrobial substance (hypothiocyanite ion) contained in the defatted soybean meal film increased as temperature increased from 5 to 22 °C.
Fig. 2.
Effects of carnauba wax (CW) coating incorporating grapefruit seed extract (GSE, 1.00%) on the inhibition of P. italicum on mandarin peels during storage at 4 and 25 °C. Uncoated (4 °C); open circle, carnauba wax-coated (4 °C); open triangle, GSE-carnauba wax-coated (4 °C); open square, uncoated (25 °C); filled circle, carnauba wax-coated (25 °C); filled triangle, GSE-carnauba wax-coated (25 °C); filled square
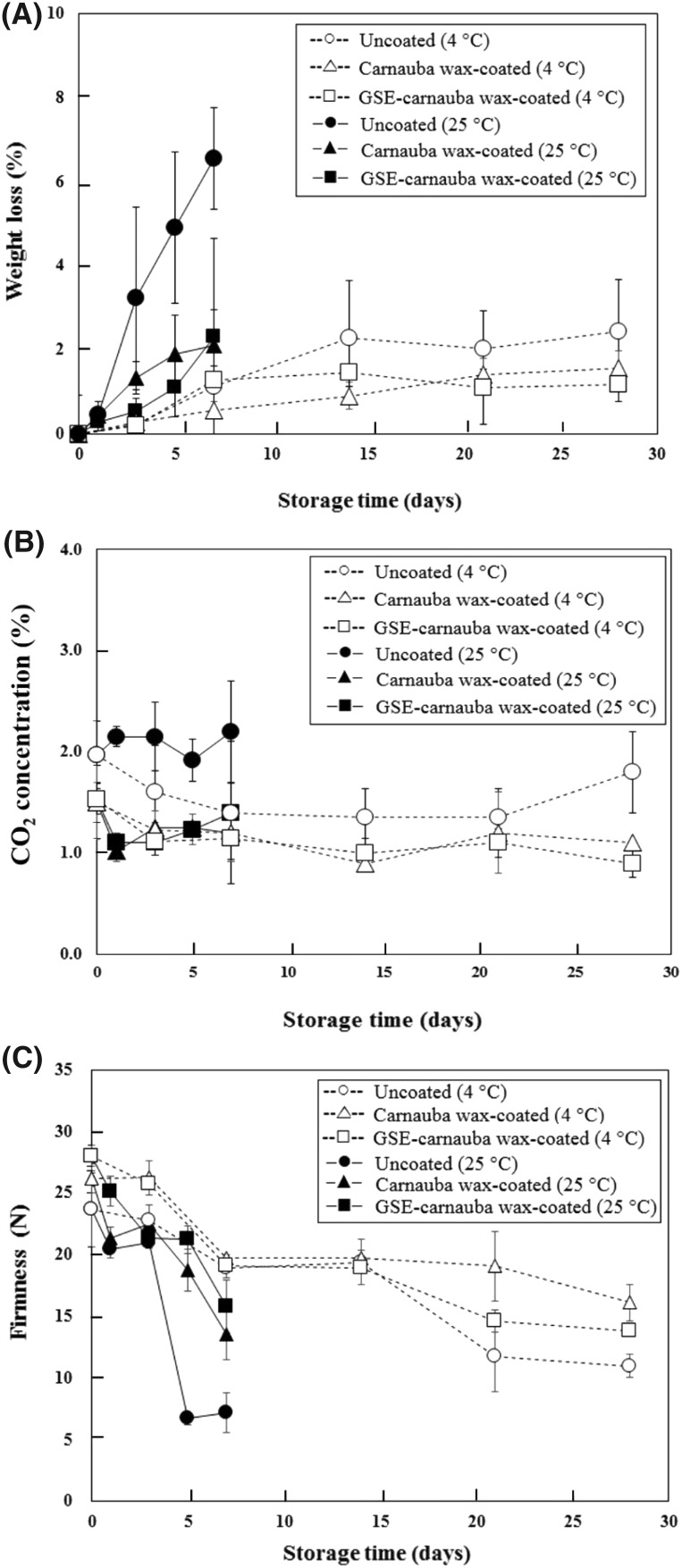
Effects of coating on weight loss during storage
The effects of coating on mandarin weight loss are shown in Fig. 3(A). The weight loss of all mandarin samples at 4 °C was maintained without significant changes between groups (P > 0.05). No morphological changes such as wilting and pitting generated by chilling injury were visually observed with any mandarins. At 25 °C, a dramatic increase in the weight loss of uncoated mandarins appeared after day 3. These results could be explained by the increased metabolic activity of mandarins, tissue senescence, and moisture loss during storage at 25 °C. However, the weight loss rates of coated mandarins were significantly lower than those of uncoated mandarins after day 3 of 25 °C storage (P < 0.05). The reduced weight loss could be due to a reduction in moisture loss [17]. No significant difference between CW and GSE–CW coating was observed on the same day of sampling during storage at 25 °C (P > 0.05). Thus, the results suggest that CW coatings with or without GSE acted as a physical barrier to minimize weight loss of mandarins and the incorporation of GSE into coatings had no effect on weight loss.
Fig. 3.
Effects of carnauba wax (CW) coating incorporating grapefruit seed extract (GSE, 1.00%) on the weight loss (A), CO2 concentration (B), and firmness (C) of mandarin during storage at 4 and 25 °C. Uncoated (4 °C); open circle, carnauba wax-coated (4 °C); open triangle, GSE-carnauba wax-coated (4 °C); open square, uncoated (25 °C); filled circle, carnauba wax-coated (25 °C); filled triangle, GSE-carnauba wax-coated (25 °C); filled square
Effects of coating on respiration during storage
Changes in the CO2 concentration inside sample containers, representing the respiration rate of mandarins are demonstrated in Fig. 3(B). The CO2 concentrations of uncoated mandarins at 4 °C showed higher levels than those of coated mandarins on day 28 [Fig. 3(B)] (P < 0.05), indicating that the coatings decreased the respiration rate of mandarins stored at 4 °C. The coated mandarins maintained a similar CO2 concentration throughout the entire storage period. At 25 °C, the coated mandarins had lower CO2 concentrations than uncoated mandarins (P < 0.05). Such a reduction in the respiratory rate may be attributable to blocking oxygen influx by the wax barrier formed by coating [31]. Limited gas exchange due to the coating treatment could extend the shelf life by delaying the maturation of mandarins during storage. Nonetheless, GSE–CW-coated mandarins showed no significant difference in CO2 concentrations compared to CW-coated mandarins at both temperatures, indicating that the GSE incorporation into the wax coating did not influence the respiration of the wax-coated fruit.
Effects of coating on firmness during storage
The firmness of all kinds of mandarin samples decreased during storage [Fig. 3(C)]. These changes in fruit firmness are reported to be correlated with reduced cellular turgor pressure resulting from structural degradation in fruit cell walls and water loss during fruit maturation [32]. At 4 °C, no significant difference in firmness was found among the coated and uncoated groups after storage for 2 weeks (P > 0.05), but higher firmness was exhibited with coated mandarins fruits after day 21. Retardation of firmness loss demonstrated by the coatings might be induced by potential carbon dioxide accumulation and oxygen depletion inside coating by increase respiration of the fruit, which can lower the activity of enzymes that responsible for structural degradation in fruit cell walls, including polygalacturonase, pectin methylesterase, pectate lyase, β-galactosidase, and cellulose [32].
The effect of coating in reducing the firmness loss was demonstrated earlier at 25 °C than 4 °C [Fig. 3(C)]. This could be due to rapid decrease in the firmness of uncoated fruits at 25 °C. Activity of the structure-degrading enzymes and moisture loss in the samples might increase at higher rates at 25 °C than 4 °C. Dramatic increase in weight loss at 25 °C compared to 4 °C was demonstrated in this study [Fig. 3(A)]. Nonetheless, the firmness values of the mandarin samples were not differed by the incorporation of GSE in the coating formulation during storage at both 4 and 25 °C, suggesting that GSE incorporation does not affect the fruit firmness.
Effects of coating on peel color during storage
Table 2 shows the effect of coating on the L* and h° values of mandarin peels during storage at 4 and 25 °C. The L* and h° were measured in the range of 59.6–65.2 and 3446.2–3610.7, respectively. Regardless of the storage temperature and period, no significant differences in L* and h° values were found between the uncoated and coated samples (P > 0.05). Most citrus peels contain high levels of pigments, such as chlorophylls and carotenoids [33]. Such pigments in most citrus fruits undergo various changes during ripening and senescence. Among them, chlorophyll pigments in citrus peels gradually disappear during maturation, while carotenoid pigments gradually accumulate. Accumulation of sugars, such as sucrose, also occurs during ripening, which can promote color break of citrus peel and thus the change of peel color [34]. However, no significant change in color of mandarins was observed in the present study. This finding may be due to the fact that fully ripened mandarins were used as samples, so that maturation of mandarin peel had already progressed.
Table 2.
Effects of carnauba wax (CW) coating incorporating grapefruits seed extract (GSE, 1.00%) on color of mandarin peels during storage 4 and 25 °C
| Storage temperature (°C) | Storage time (days) | L* | Hue angle | ||||
|---|---|---|---|---|---|---|---|
| Uncoated | CW-coated | CW–GSE-coated | Uncoated | CW-coated | CW–GSE-coated | ||
| 4 | 0 | 63.4 ± 1.1ab | 64.3 ± 1.3ab | 59.6 ± 11.9b | 3535.7 ± 43.4a | 3505.3 ± 70.5a | 3590.0 ± 75.9a |
| 3 | 62.3 ± 1.2ab | 62.5 ± 2.1ab | 64.9 ± 1.8a | 3461.5 ± 62.9a | 3520.2 ± 40.0a | 3579.3 ± 37.4a | |
| 7 | 62.3 ± 1.6ab | 62.4 ± 0.7ab | 63.2 ± 0.9ab | 3610.7 ± 47.8a | 3548.6 ± 18.6a | 3495.8 ± 23.4a | |
| 14 | 63.4 ± 1.3ab | 64.4 ± 1.3ab | 63.3 ± 0.8ab | 3527.2 ± 33.9a | 3545.6 ± 34.1a | 3552.2 ± 55.5a | |
| 21 | 64.4 ± 0.6ab | 65.2 ± 1.5a | 65.1 ± 0.7a | 3540.9 ± 21.0a | 3441.2 ± 134.6a | 3570.3 ± 143.5a | |
| 25 | 0 | 63.4 ± 1.1ab | 64.3 ± 1.3ab | 59.6 ± 11.9b | 3535.7 ± 43.4a | 3505.3 ± 70.5a | 3590.0 ± 75.9a |
| 1 | 63.7 ± 1.7b | 63.2 ± 0.8b | 64.6 ± 0.3b | 3494.9 ± 87.8a | 3448.1 ± 39.7a | 3572.3 ± 28.9a | |
| 3 | 64.7 ± 1.7ab | 62.3 ± 1.2ab | 63.3 ± 1.4ab | 3504.0 ± 84.7a | 3432.8 ± 31.1a | 3591.7 ± 56.0a | |
| 5 | 65.0 ± 7.1ab | 62.8 ± 1.4ab | 63.6 ± 1.1ab | 3446.2 ± 72.5a | 3422.0 ± 56.9a | 3452.1 ± 54.9a | |
| 7 | 64.2 ± 1.8ab | 63.3 ± 2.4ab | 64.0 ± 0.9ab | 3542.8 ± 168.6a | 3519.3 ± 34.3a | 3581.9 ± 37.5a | |
a, b Different letters indicate a significant difference (P < 0.05) within the same parameters
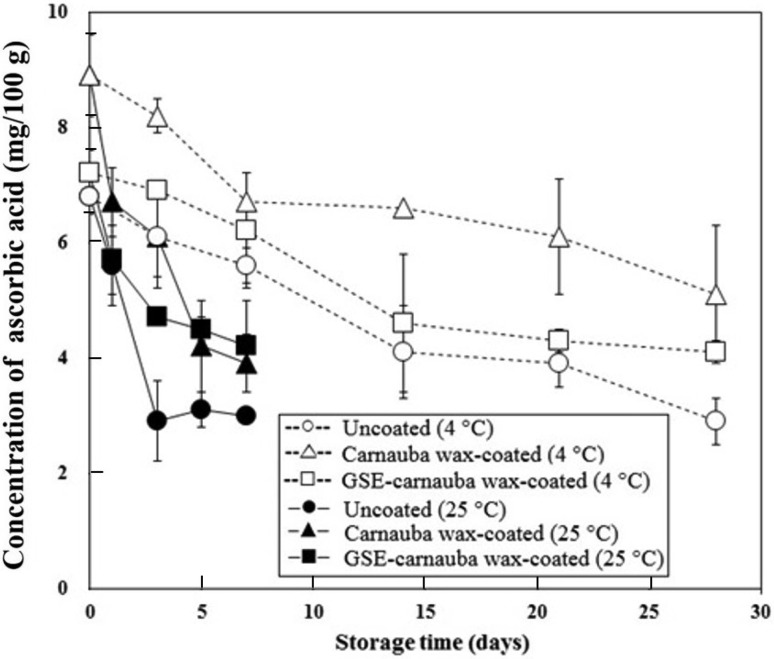
Figure 4 shows the effects of mandarin coatings on the ascorbic acid content. During storage at 4 °C, ascorbic acid contents were not significantly different among samples until day 21 (P > 0.05), but coated mandarins had higher ascorbic acid contents than uncoated mandarins on day 28. At a storage temperature of 25 °C, the ascorbic acid contents of all mandarin samples dramatically decreased by day 7 (P < 0.05). Compared to that in the uncoated mandarins, the effect of coating on ascorbic acid contents at 25 °C became apparent beginning at day 3, indicating that CW coating effectively preserved the ascorbic acid, regardless of the GSE content. This result suggests that CW coating blocked the inflow of oxygen into the flesh during storage, thereby inhibiting ascorbic acid oxidation.
Fig. 4.
Effects of carnauba wax (CW) coating incorporating grapefruit seed extract (GSE, 1.00%) on the ascorbic acid concentration of mandarin flesh during storage 4 and 25 °C. Uncoated (4 °C); open circle, carnauba wax-coated (4 °C); open triangle, GSE-carnauba wax-coated (4 °C); open square, uncoated (25 °C); filled circle, carnauba wax-coated (25 °C); filled triangle, GSE-carnauba wax-coated (25 °C); filled square
GSE addition to CW coatings could raise the colloidal stability and the GSE–CW coating was more effective in decreasing fungal disease caused by P. italicum on the mandarin surface compared to CW coatings without antifungal agents. Most coating formulations containing GSE and/or OO demonstrated no significant difference in P. italicum inhibition between the I + C and C + I treatments. GSE–CW coating effectively inhibited P. italicum growth during storage at 4 and 25 °C where the inhibitory effect was more obviously demonstrated at 25 °C than 4 °C. The results obtained in this study demonstrate that GSE–CW coating is an effective postharvest technology for protecting mandarins from P. italicum without altering the physicochemical properties of mandarins.
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (314059-03) and by a research grant from Seoul Women's University (2018).
References
- 1.Dugo G, Di Giacomo A. Citrus: the genus citrus. London: Taylor and Francis Group; 2002. [Google Scholar]
- 2.Statistics Korea (KOSTAT). Available from: http://kostat.go.kr/office/hnro/rohn_ki/5/index.board?bmode=read&aSeq=356996. Accessed Nov. 01, 2017.
- 3.Kinay P, Yildiz F, Sen F, Yildiz M, Karacali I. Integration of pre-and postharvest treatments to minimize Penicillium decay of Satsuma mandarins. Postharvest Biol. Tec. 2005;37(1):31–36. doi: 10.1016/j.postharvbio.2005.02.008. [DOI] [Google Scholar]
- 4.Palou L. Control of citrus postharvest diseases by physical means. Tree For. Sci. Biotech. 2009;3:127–142. [Google Scholar]
- 5.Baldwin EA, Nisperos-Carriedo M, Shaw PE, Burns JK. Effect of coatings and prolonged storage conditions on fresh orange flavor volatiles, degrees Brix, and ascorbic acid levels. J. Agr. Food Chem. 1995;43(5):1321–1331. doi: 10.1021/jf00053a037. [DOI] [Google Scholar]
- 6.Hagenmaier RD, Shaw PE. Changes in volatile components of stored tangerines and other specialty citrus fruits with different coatings. J. Food Sci. 2002;67(5):1742–1745. doi: 10.1111/j.1365-2621.2002.tb08716.x. [DOI] [Google Scholar]
- 7.Valencia-Chamorro SA, Palou L, Del Rio MA, Pérez-Gago MB. Performance of hydroxypropyl methylcellulose (HPMC)-lipid edible coatings with antifungal food additives during cold storage of ‘Clemenules’ mandarins. LWT-Food Sci. Technol. 2011;44(10):2342–2348. doi: 10.1016/j.lwt.2011.02.014. [DOI] [Google Scholar]
- 8.Contreras-Oliva A, Rojas-Argudo C, Pérez-Gago MB. Effect of solid content and composition of hydroxypropyl methylcellulose–lipid edible coatings on physico-chemical and nutritional quality of ‘Oronules’ mandarins. J. Sci. Food Agr. 2012;92(4):794–802. doi: 10.1002/jsfa.4649. [DOI] [PubMed] [Google Scholar]
- 9.Chien PJ, Sheu F, Lin HR. Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chem. 2007;100(3):1160–1164. doi: 10.1016/j.foodchem.2005.10.068. [DOI] [Google Scholar]
- 10.Abbas H, Abassi NA, Yasin T, Maqbool M, Ahmad T. Influence of irradiated chitosan coating on postharvest quality of kinnow (Citrus reticulata Blanco.) Asian J. Chem. 2008;20(8):6217. [Google Scholar]
- 11.Jo WS, Song HY, Song NB, Lee JH, Min SC, Song KB. Quality and microbial safety of ‘Fuji’ apples coated with carnauba-shellac wax containing lemongrass oil. LWT-Food Sci. Technol. 2014;55:490–497. doi: 10.1016/j.lwt.2013.10.034. [DOI] [Google Scholar]
- 12.Cháfer M, Sánchez-González L, González-Martínez C, Chiralt A. Fungal decay and shelf life of oranges coated with chitosan and bergamot, thyme, and tea tree essential oils. J. Food Sci. 2012;77(8):E182–E187. doi: 10.1111/j.1750-3841.2012.02827.x. [DOI] [PubMed] [Google Scholar]
- 13.Castillo S, Pérez-Alfonso CO, Martínez-Romero D, Guillén F, Serrano M, Valero D. The essential oils thymol and carvacrol applied in the packing lines avoid lemon spoilage and maintain quality during storage. Food Control. 2014;35:132–136. doi: 10.1016/j.foodcont.2013.06.052. [DOI] [Google Scholar]
- 14.Heggers John P., Cottingham John, Gusman Jean, Reagor Lana, McCoy Lana, Carino Edith, Cox Robert, Zhao Jian-Gang. The Effectiveness of Processed Grapefruit-Seed Extract as An Antibacterial Agent: II. Mechanism of Action and In Vitro Toxicity. The Journal of Alternative and Complementary Medicine. 2002;8(3):333–340. doi: 10.1089/10755530260128023. [DOI] [PubMed] [Google Scholar]
- 15.Lee YJ, Ryu HS, Chun SS. Quality characteristics of salad dressing prepared with mulberry fruit powder. Korean J. Food Cook. Sci. 2010;26(5):537–544. [Google Scholar]
- 16.Won MY, Lee SJ, Min SC. Mandarin preservation by microwave-powered cold plasma treatment. Innov. Food Sci. Emerg. Tech. 2017;39:25–32. doi: 10.1016/j.ifset.2016.10.021. [DOI] [Google Scholar]
- 17.Kim IH, Oh YA, Lee H, Song KB, Min SC. Grape berry coatings of lemongrass oil incorporating nanoemulsion. LWT-Food Sci. Technol. 2014;58(1):1–10. doi: 10.1016/j.lwt.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Cruz MAL, Gomes VM, Fernandes KV, Machado OL, Xavier-Filho J. Identification and partial characterization of a chitinase and a β-1, 3-glucanase from Copernicia cerifera wax. Plant Physiol. Biochem. 2002;40(1):11–16. doi: 10.1016/S0981-9428(01)01340-7. [DOI] [Google Scholar]
- 19.Abd-Alla MA, El-Mougy NS, El-Gamal NG. Formulation of essential oils and yeast for controlling postharvest decay of tomato fruits. Plant Pathol. Bull. 2009;18(1):23–33. [Google Scholar]
- 20.Xu WT, Huang KL, Guo F, Qu W, Yang JJ, Liang ZH, Luo YB. Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea. Postharvest Biol. Tec. 2007;46(1):86–94. doi: 10.1016/j.postharvbio.2007.03.019. [DOI] [Google Scholar]
- 21.Saalu LC, Ajayi GO, Adeneye AA, Imosemi I, Osinubi AA. Ethanolic seed extract of grapefruit (Citrus paradisi Macfad) Int. J. Cancer Res. 2009;5:44–52. doi: 10.3923/ijcr.2009.44.52. [DOI] [Google Scholar]
- 22.Cho S, Lee SY, Kim JW, Ko GH, Seo IW. Development and application of natural antimicrobial agent isolated from grapefruit seed extract-antimicrobial activity of grapefruit seed extract. J. Hyg. Safety. 1995;10:33–39. [Google Scholar]
- 23.Seydim AC, Sarikus G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006;39(5):639–644. doi: 10.1016/j.foodres.2006.01.013. [DOI] [Google Scholar]
- 24.Rojas-Graü MA, Raybaudi-Massilia RM, Soliva-Fortuny RC, Avena-Bustillos RJ, McHugh TH, Martín-Belloso O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Tec. 2007;45(2):254–264. doi: 10.1016/j.postharvbio.2007.01.017. [DOI] [Google Scholar]
- 25.Alonso J, Alique R. Influence of edible coating on shelf life and quality of “Picota” sweet cherries. Eur. Food Res. Technol. 2004;218(6):535–539. doi: 10.1007/s00217-004-0908-3. [DOI] [Google Scholar]
- 26.Zhang X, Li L, Xu Z, Liang Z, Su J, Huang J, Li B. Investigation of the interaction of naringin palmitate with bovine serum albumin: spectroscopic analysis and molecular docking. PLoS One. 2013;8(3):e59106. doi: 10.1371/journal.pone.0059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donhowe G, Fennema O. Water vapor and oxygen permeability of wax films. J. Am. Oil Chem. Soc. 1993;70(9):867–873. doi: 10.1007/BF02545345. [DOI] [Google Scholar]
- 28.Sun C, Wu T, Liu R, Liang B, Tian Z, Zhang E, Zhang M. Effects of superfine grinding and microparticulation on the surface hydrophobicity of whey protein concentrate and its relation to emulsions stability. Food Hydrocolloid. 2015;51:512–518. doi: 10.1016/j.foodhyd.2015.05.027. [DOI] [Google Scholar]
- 29.Reinoso Elsy, Mittal Gauri S., Lim Loong-Tak. Influence of Whey Protein Composite Coatings on Plum (Prunus Domestica L.) Fruit Quality. Food and Bioprocess Technology. 2007;1(4):314–325. doi: 10.1007/s11947-007-0014-1. [DOI] [Google Scholar]
- 30.Lee H, Min SC. Development of antimicrobial defatted soybean meal-based edible films incorporating the lactoperoxidase system by heat pressing. J. Food Eng. 2014;120:183–190. doi: 10.1016/j.jfoodeng.2013.07.035. [DOI] [Google Scholar]
- 31.Erbil HY, Muftugil N. Lengthening the postharvest life of peaches by coating with hydrophobic emulsions. J. Food Process. Pres. 1986;10(4):269–279. doi: 10.1111/j.1745-4549.1986.tb00025.x. [DOI] [Google Scholar]
- 32.Brummell DA, Harpster MH. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001;47(1–2):311–339. doi: 10.1023/A:1010656104304. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigo MJ, Alquézar B, Alós E, Lado J, Zacarías L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013;163:46–62. doi: 10.1016/j.scienta.2013.08.014. [DOI] [Google Scholar]
- 34.Shemer TA, Harpaz-Saad S, Belausov E, Lovat N, Krokhin O, Spicer V, Standing KG, Goldschmidt EE, Eyal Y. Citrus chlorophyllase dynamics at ethylene-induced fruit color-break: a study of chlorophyllase expression, posttranslational processing kinetics, and in situ intracellular localization. Plant Physiol. 2008;148(1):108–118. doi: 10.1104/pp.108.124933. [DOI] [PMC free article] [PubMed] [Google Scholar]