Abstract
Chrysanthemum zawadskii (CZ) is a perennial herb belonging to the Asteraceae family. CZ is used medicinally to treat inflammatory and uterine diseases in Asia. CZ was extracted with 50% ethanol and CZ extract (CZE; at 125, 250, and 500 mg/kg body weight) was administered orally every day for 5 or 6 weeks to investigate the anti-diabetic effects in streptozotocin (STZ)-induced rats and STZ + high-fat diet (HFD)-fed mice. CZE significantly decreased fasting blood glucose levels in STZ- and STZ + HFD-induced diabetic models. In addition, glucose tolerance and insulin tolerance were improved in the STZ + HFD + CZE group by increasing insulin levels and decreasing hemoglobin A1c (HbA1c) levels in serum. Furthermore, CZE supplements decreased components of the serum lipid profile such as triglyceride, total cholesterol, and low-density lipoprotein cholesterol levels. These results suggest that CZE may be a potential candidate for controlling hyperglycemia.
Keywords: Chrysanthemum zawadskii, Hyperglycemia, Diabetes, Glucose, Insulin
Introduction
Diabetes is a common chronic metabolic disorder that is characterized by an abnormal modulation of glucose and lipid metabolism (Moller, 2001). Many other chronic diseases such as hyperlipidemia, hepatic injury, and hypertension, are closely related to the development of diabetes mellitus. Notably, hyperlipidemia is closely related with high insulin and blood glucose levels, and is a major risk factor for arteriosclerosis in diabetes mellitus (DeFronzo and Ferrannini, 1991; Talpur et al., 2003; Yudkin, 1988). The available treatment methods for diabetes include diet therapy, exercise therapy, and clinical administration of drugs. Drug therapy includes increasing the secretion of pancreatic insulin by sulfonylureas, inhibition of intestinal glucose uptake by α-glucosidase, and decreasing production of hepatic glucose by biguanides. However, using drug therapy has been known to have serious side effects such as hypoglycemia and lactic acidosis (Fratimunari et al., 1989; Moller, 2001). Therefore, there has been an active search for better agents from herbal or natural products in recent years.
Chrysanthemum zawadskii (CZ) is a perennial herb. CZ has been cultivated in Asia and northeastern Europe and is known in Korea as ‘Gujeolcho’. CZ has been used in traditional medicine to relieve inflammatory diseases, gastroenteric troubles, hypertension, bladder-related disorders, and uterine diseases such as menstrual irregularity and infertility (Han et al., 2002; Woo and Lee, 2008). CZ has been reported to have anti-oxidant (He et al., 2011; Liu et al., 2012), anti-microbial (Sassi et al., 2008), and hepatoprotective effects (Seo et al., 2010). The effective components of CZ consist of flavonoids, essential oils, and polysac-charides (Shim et al., 2012). Han et al. (2002) have shown that CZ contains flavonoids such as linarin, apigenin, acacetin, and luteolin. Among them, linarin is known as a best representative compound, which has been reported to have neuroprotective (Lou et al., 2011), hepatoprotective (Kim et al., 2014), and osteogenic differentiation effects (Li et al., 2016).
Our previous study confirmed that CZ extract (CZE) has anti-oxidant, lipase inhibitory, and α-glucosidase inhibitory activities, in addition to the confirmed correlation between these activities and linarin content. CZE showed high α-glucosidase inhibitory activity, similar to that of acarbose at the same concentration (Kim et al., 2016). Treatment with acarbose, an α-glucosidase inhibitor, has decreased fasting blood glucose and glycosylated hemoglobin levels in both diabetic animal models and human patients (Mertes, 2001; Wright et al., 1998). Our previous study confirmed that CZE has the potential to function as an α-glucosidase inhibitor (Kim et al., 2016); while this current study presents the efficiency of CZE on the control of glucose, blood insulin, and blood lipid levels in STZ and STZ + HFD-induced diabetic animal models.
Materials and methods
Preparation of standardized extract of Chrysanthemum zawadskii
Three batches (No. 20160511-20160609) of standardized CZE were manufactured and verified by Daeho Corporation Co., Ltd. (Hwaseong, Korea). Briefly, dried Chrysanthemum zawadskii (YeongPyeong Foods Co., Ltd., Sejong, Korea) were shattered and extracted by two subsequent heating at 70 °C (4 and 2 h) using 50% ethanol. The extract was then filtered and concentrated (Busung Tech, Ansung, Korea) to 10 degrees Brix at 65 °C. The concentrated extract was spray dried (Mehyun Engineering, Anyang, Korea) at an inlet temperature of 175 ± 10 °C and an outlet temperature of 80 ± 5 °C, with the addition of dextrin (40%). The extraction yield was approximately 20% (w/w) and the powder was standardized with 1.32 ± 0.22 mg/g linarin as a marker ingredient.
Animal models
Seven-week-old male Sprague–Dawley rats were purchased from Orient Bio Inc. (Seoul, Korea). The rats were housed in standard laboratory conditions comprising a light–dark cycle of 12:12 h, humidity of 55 ± 15%, and temperature of 23 ± 3 °C. The rats were fed a commercial diet (Cargill Agri Purina Inc., Pyeongtaek, Korea) and provided water ad libitum. The commercial diet contained 20% protein, 4.5% fat, 6% fiber, 0.5% calcium, 1% phosphorus, and 7% ash w/w. Animals were maintained in polypropylene cages, each containing a maximum of five animals. After 1 week of adaptation period, the rats were randomly divided into six groups with five rats per group. The groups were as follows: Control group (Con), Streptozotocin group (STZ), 200 mg/kg of Metformin group (Met) using 256.45 mg/kg of metformin hydrochloride as positive control, 125 mg/kg CZE group (CZE 125), 250 mg/kg of CZE group (CZE 250), and 500 mg/kg of CZE group (CZE 500). The metformin hydrochloride (Granules India Limited, Hyderabad, India), STZ and CZE groups were orally administered with equal volumes of their respective drug or extracts, while distilled water was orally administered to the control and normal groups every day for 5 weeks. Rats were administered with each sample for 2 weeks and then fasted for 12 h. All rats except for the control group were singly injected IP with 60 mg/kg of STZ (Sigma-Aldrich Co., St. Louis, MO, USA) in 0.1 M sodium citrate buffer (pH 4.5). A control group was given citrate buffer. After 72 h, blood glucose levels were measured using a monitoring device (ACCU-CHEK® Performa; Roche Diagnostics, Mannheim, Germany) in order to confirm that fasting blood glucose levels of STZ in injected rats exceeds 350 mg/dL. Afterwards, the metformin and CZE groups were orally administered for 3 weeks, while the control and STZ groups were given distilled water. Animal studies were conducted according to institutional and national guidelines; and all experimental procedures were approved by the KNOTUS Institutional Animal Care and Use Committee (KNOTUS IACUC 16-KE-255).
Seven-week-old male C57BL/6 J mice were purchased from the Central Lab. Animal Inc. (Seoul, Korea). The mice were acclimatized to standard laboratory conditions, which comprise of a light–dark cycle of 12:12 h, humidity of 40 ± 4%, and temperature of 20 ± 1 °C. After 1 week of adaptation, the mice were randomly divided into two groups. The control group was fed with a chow diet (Cargill Agri Purina Inc., Pyeongtaek, Korea) containing 20% protein, 4.5% fat, 6% fiber, 0.5% calcium, 1% phosphorus, and 7% ash w/w. The other group was fed with a high-fat diet (HFD) D12451 (Research Diets Inc., Newbrunswick, NJ, USA) containing 20% protein, 35% carbohydrate, and 45% fat for 10 weeks. The mice were provided water ad libitum. Animals were maintained in polypropylene cages, each containing a maximum of two animals. All mice except for the control group were fed with HFD for 2 weeks and then injected three times IP with 40 mg/kg of STZ in 0.1 M sodium citrate buffer after fasting for 12 h. At 2 weeks post-STZ injection, mice exceeding fasting blood glucose levels of 200 mg/dL were selected for experiments. Blood was measured using a blood glucose monitoring device, AGM-2200 GlucoDr™ (All Medicus Co., Ltd., Anyang, Korea). Mice from the HFD group were divided into five groups, with seven mice per group. The groups were as follows: STZ and high-fat diet group (STZ + HFD), 200 mg/kg of Metformin group (Met) using 256.45 mg/kg of metformin hydrochloride as positive control, 125 mg/kg of CZE group (CZE 125), 250 mg/kg of CZE group (CZE 250), and 500 mg/kg of CZE group (CZE 500). The metformin hydrochloride (Wako Pure Chemical Industries Ltd., Osaka, Japan), STZ and CZE groups were orally administered with equal volumes of their respective drug or extracts, while distilled water was orally administered to the control and normal groups every day for 6 weeks. The protocol for the current study was approved by the Animal Care and Use Committee of Chungbuk National University (Approval No. CBNUA-1046-16-01).
Oral glucose tolerance test (OGTT)
STZ + HFD-induced diabetic mice were orally administered with glucose (Sigma-Aldrich Co., St. Louis, MO, USA) at 1 g/kg body weight after fasting for 12 h. Blood samples were collected from the tail vein at 0, 15, 30, 45, 60, 90, and 120 min for measurements of plasma glucose. Collected blood samples were centrifuged at 1300×g for 10 min, after which blood glucose was measured from the serum.
Insulin tolerance test (ITT)
STZ + HFD-induced diabetic mice were intraperitoneally injected with 1 unit/kg of insulin (Eli Lilly and Company, Indianapolis, IN, USA) following 12 h of fasting. Glucose samples were obtained at 0, 15, 30, 45, 60, 90, and 120 min afterwards. Collected blood samples were centrifuged at 1300×g for 10 min, after which blood glucose was measured from the serum.
Serum biochemical parameters
STZ + HFD-induced diabetic mice were fasted for 12 h. Blood was collected and serum was obtained after centrifugation at 580×g for 15 min. The levels of blood urea nitrogen (BUN), creatinine, hemoglobin A1c (HbA1C), triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were determined using a AU480 Chemistry analyzer (Beckman Coulter Inc., Fullerton, CA, USA).
Histopathology
The pancreatic tissues of STZ-induced diabetic rats, and liver tissues of STZ + HFD-induced mice were collected for histopathological examination. The tissues were fixed in 10% neutral buffered formalin (BBC Biochemical, Mount Vernon, WA, USA), embedded in paraffin, and serial sections were cut. Paraffin was removed with alcohol and xylene. The sections were stained with hematoxylin and eosin (H&E, BBC Biochemical, Mount Vernon, WA, USA). Atrophy scores of the islet tissue stained with H&E was graded according to the method used by Liu XQ: 0, no islet atrophy; 1, very mild islet atrophy; 2, mild islet atrophy; 3, moderate islet atrophy; 4, marked islet atrophy (Liu et al., 2009). In addition, apoptosis levels of other sections were analyzed using a commercial kit for the TUNEL assay, ApopTag® Peroxidase In Situ Apoptosis Detection Kit S7100 (Merck Millipore Corporation, Darmstadt, Germany). After dehydration with alcohol, images were obtained under a light microscope (Olympus, Tokyo, Japan). The histopathological analyses were performed blindly.
Statistical analysis
The results are presented as mean ± standard error of the mean (SEM). The differences between groups were evaluated using a one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test, or a two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test, using the GraphPad Prism 5 software (San Diego, CA, USA).
Results and Discussion
Body weight and clinical observations
The body weight of all STZ-induced rats was markedly decreased compared to control rats (p < 0.001) after STZ injection. This result is similar to a previously reported study (Swanston-Flatt et al., 1989). The body weight of CZE groups did not differ from the STZ + HFD group, while the body weight of STZ + HFD-induced mice was elevated compared to the control group, but the difference was not statistically significant (Table 1). CZE had no influence on body weight, food, and water intake in the diabetic animals (data not shown).
Table 1.
Body weight of STZ-induced type 1 diabetic rats and STZ + HFD-induced type 2 diabetic mice treated with CZE
| Model | Control | STZa | STZ + HFDb | Metc | CZE 125d | CZE 250e | CZE 500f |
|---|---|---|---|---|---|---|---|
| STZ-induced rats | 461.70 ± 14.07### | 288.85 ± 5.39 | 286.47 ± 16.93 | 272.84 ± 4.98 | 287.52 ± 13.32 | 279.98 ± 8.65 | |
| STZ + HFD-induced mice | 27.48 ± 0.60 | 28.61 ± 0.45 | 29.73 ± 0.66 | 28.46 ± 0.94 | 28.51 ± 0.46 | 27.93 ± 0.59 |
Values are presented as mean ± SEM (n = 5–7 per group) (n = 5 per group of STZ-induced rats, n = 7 per group of STZ + HFD-induced mice). ###p < 0.001 compared to STZ group
aSTZ: streptozotocin
bSTZ + HFD: STZ and high-fat diet
cMet: STZ + HFD + Metformin 200 mg/kg
dCZE 125: STZ + HFD + CZE 125 mg/kg
eCZE 250: STZ + HFD + CZE 250 mg/kg
fCZE 500: STZ + HFD + CZE 500 mg/kg
Effect of CZE on fasting blood glucose of diabetic rats and mice
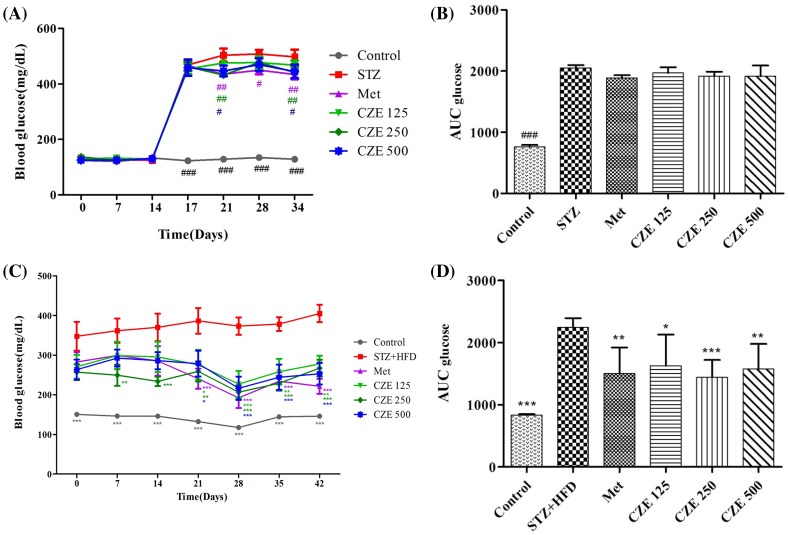
Diabetes is a common metabolic disorder worldwide characterized by chronic dyslipidemia and hyperglycemia due to insulin resistant peripheral tissue and damaged insulin secretion in the pancreas (Saltiel, 2001; Sharma et al., 2011). Diabetes is also related to severe changes in insulin, glucose, lipid, and lipoprotein (Arvind et al., 2002; Zheng et al., 2011) levels. In this study, STZ-induced rats and STZ + HFD-induced mice were used to determine the effects of CZE on diabetes. STZ causes appreciable reduction in insulin secretion through the selective damage of β-cells within Langerhans islets (Kim and Kim, 2006; Lemhadri et al., 2006). It has been reported that when fed with a HFD and injected with a low dose of STZ, mice show many characteristics of insulin resistance such as hyperglycemia, hyperinsulinemia, as well as kidney and liver damage (Reed et al., 2000; Sharma et al., 2011; Zhang et al., 2003). In the present study, CZE supplementation ameliorated insulin resistance and glycemic control in STZ-induced type 1 diabetic rats and STZ + HFD-induced type 2 diabetic mice. The effects of CZE on FBG in normal and STZ-induced diabetic rats are shown in Fig. 1A, B. The FBG levels of rats injected with STZ were increased from the third day of injection. The NC group showed a normal FBG levels, which is different from those of STZ, Met, and CZE groups. The FBG levels of diabetic rats were significantly inhibited by 250 mg/kg (p < 0.05) and 500 mg/kg (p < 0.05) of CZE. The effect of CZE on FBG levels in STZ + HFD-induced diabetic mice during feeding is shown in Fig. 1C, D. FBG levels of the 250 CZE group have prominently (p < 0.01) decreased from day 7, while those of the Met group have markedly (p < 0.001) decreased from day 21. On the 42nd day, FBG levels of all CZE groups were notably decreased. These results suggest that CZE may act positively in modulating FBG levels of diabetic rats and mice.
Fig. 1.
Effect of CZE on fasting glucose levels in STZ-induced type 1 diabetic rats and STZ + HFD-induced type 2 diabetic mice. The fasting blood glucose in STZ-induce rats (A, B) and STZ + HFD-induced mice (C, D). (A) STZ, streptozotocin; Met, STZ + Metformin 200 mg/kg; CZE 125, STZ + CZE 125 mg/kg; CZE 250, STZ + CZE 250 mg/kg; CZE 500, STZ + CZE 500 mg/kg. Area under the curve for glucose (AUC glucose) (B). (C) STZ + HFD, streptozotocin and high-fat diet; Met, STZ + HFD + Metformin 200 mg/kg; CZE 125, STZ + HFD + CZE 125 mg/kg; CZE 250, STZ + HFD + CZE 250 mg/kg; CZE 500, STZ + HFD + CZE 500 mg/kg. Area under the curve for glucose (AUC glucose) (D). Values are presented as mean ± SEM (n = 5–7 per group). #p < 0.05, ##p < 0.01, ###p < 0.001 compared to STZ group, *p < 0.05, **p < 0.01, ***p < 0.001 compared to STZ + HFD group
Effect of CZE on oral glucose tolerance test and insulin tolerance test of diabetic mice
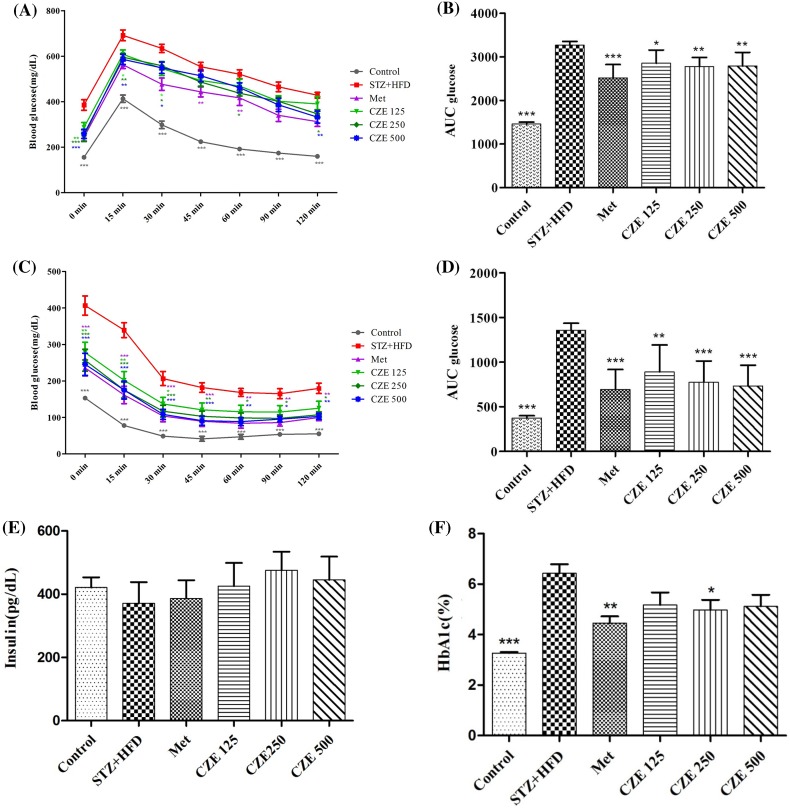
Diabetes causes impairment in the response of specific tissues to insulin, leading to glucose tolerance (Reaven, 1988). Postprandial hyperglycemia can trigger protein glycosylation and chronic complications (Dunn et al., 1979; Steiner et al., 1984). Hanefeld and Temelkova-Kurktschiev (1997) reported that the risk of cardiovascular diseases is related to postprandial blood glucose, rather than fasting blood glucose levels. In the current study, we have shown that treating diabetic mice with CZE improves postprandial glucose levels. The OGTT results of all groups showed the highest blood glucose levels at 15 min after glucose administration, followed by steady decreases. The glucose levels of CZE 125 group were decreased at 15 min (p < 0.05) and 30 min (p < 0.05) after glucose administration, with marked difference compared to the STZ + HFD group. The mice treated with 250 mg/kg of CZE had decreased blood glucose levels at 15 min (p < 0.01), 30 min (p < 0.05), 60 min (p < 0.05), and 120 min (p < 0.01). In the CZE 500 group, the glucose levels were decreased at 15 min (p < 0.01), 30 min (p < 0.05), and 120 min (p < 0.01, Fig. 2A, B).
Fig. 2.
Effect of CZE on glucose and insulin tolerance in STZ + HFD-induced type 2 diabetic mice. The oral glucose tolerance test (A) and area under the curve for glucose (AUC glucose) (B). The Insulin tolerance test in STZ + HFD-induce diabetic mice (C), area under the curve for glucose (AUC glucose) (D). Insulin levels (E) and HbA1c levels in serum (F). STZ + HFD, streptozotocin and high-fat diet; Met, STZ + HFD + Metformin 200 mg/kg; CZE 125, STZ + HFD + CZE 125 mg/kg; CZE 250, STZ + HFD + CZE 250 mg/kg; CZE 500, STZ + HFD + CZE 500 mg/kg. Values are presented as mean ± SEM (n = 7 per group). *p < 0.05, **p < 0.01, ***p < 0.001 compared to STZ + HFD group
CZE showed appreciable reduction effects on ITT levels compared to the STZ + HFD group (Fig. 2C, D). All CZE groups had significantly decreased blood glucose levels at 15 and 30 min after insulin administration, followed by continuous differences in the CZE 250 and 500 groups. The CZE 250 and CZE 500 groups had reduced blood glucose levels between 60 and 120 min (p < 0.05). Insulin resistance was reported to increase glucose production and decrease glucose utilization in the liver, leading to hyperglycemia (Gerich, 2003). Based on this result, CZE may decrease the risk of insulin resistance and prevent diseases such as hyperglycemia.
Effects of CZE on serum insulin and HbA1c levels
The serum insulin levels of STZ + HFD-induced diabetic mice were decreased compared to the control group, while the levels for CZE supplement groups increased compared to the STZ + HFD group (Fig. 2E). However, the aforementioned changes were not significant. HbA1c levels in the serum of diabetic mice were observed to increase compared to control (p < 0.001); while those of treated diabetic mice (at 250 mg/kg of CZE) were decreased compared with the STZ + HFD group (p < 0.05, Fig. 2F). Although there were no significant differences, diabetic mice treated with 125 and 500 mg/kg of CZE had lower serum HbA1c levels than the STZ + HFD group. High levels of serum insulin can induce insulin resistance to insulin-mediated glucose uptake (Harris et al., 2001). Thus, the results of the present study indicate that CZE supplement can improve insulin sensitivity.
Effects of CZE on serum biochemical parameters
STZ + HFD-induced type 2 diabetes mice had increased serum levels of TG, TC, and LDL compared to the control group (Table 2). Treatment of diabetic mice with CZE decreased both TG and LDL levels, while increasing HDL levels; however, these differences were not significant when compared to the STZ + HFD group. BUN and creatinine levels did not significantly change. In this study, CZE did not lead to renal toxicity. Glycemic control level is an important determinant of serum LDL and triglycerides (26). Thus, the lowering effects of CZE on serum lipids, including TG and LDL, and its increasing effects on HDL levels in STZ-induced and STZ + HFD-induced diabetic animals can be associated with a glycemic control resulting from increased insulin.
Table 2.
Concentration of serum biochemical parameters in STZ + HFD-induced type 2 diabetic mice treated with CZE
| Ingredient (mg/dL) | Control | STZ + HFDa | Metb | CZE 125c | CZE 250d | CZE 500e |
|---|---|---|---|---|---|---|
| BUNf | 31.51 ± 0.88 | 30.96 ± 2.09 | 32.20 ± 1.24 | 33.49 ± 2.46 | 34.20 ± 1.74 | 32.26 ± 1.62 |
| Creatinine | 0.43 ± 0.01 | 0.41 ± 0.01 | 0.42 ± 0.01 | 0.40 ± 0.01 | 0.41 ± 0.01 | 0.41 ± 0.01 |
| TGg | 21.83 ± 1.51 | 40.07 ± 8.24 | 33.19 ± 7.74 | 35.09 ± 2.25 | 25.36 ± 3.28 | 35.04 ± 6.45 |
| TCh | 110.43 ± 1.93*** | 164.66 ± 7.55 | 163.31 ± 8.06 | 172.84 ± 6.61 | 156.31 ± 3.49 | 163.00 ± 3.87 |
| HDL-Ci | 72.43 ± 0.86 | 71.43 ± 2.50 | 73.89 ± 2.21 | 75.87 ± 2.05 | 73.63 ± 2.07 | 75.23 ± 1.45 |
| LDL-Cj | 4.68 ± 0.18*** | 12.26 ± 1.06 | 10.13 ± 0.92 | 13.76 ± 1.21 | 11.10 ± 0.74 | 11.43 ± 1.08 |
Values are presented as mean ± SEM (n = 7 per group). ***p < 0.001 compared to STZ + HFD group
aSTZ + HFD: streptozotocin and high-fat diet
bMet: STZ + HFD + Metformin 200 mg/kg
cCZE 125: STZ + HFD + CZE 125 mg/kg
dCZE 250: STZ + HFD + CZE 250 mg/kg
eCZE 500: STZ + HFD + CZE 500 mg/kg
fBUN blood urea nitrogen
gTG triglyceride
hTC total cholesterol
iHDL-C high-density lipoprotein cholesterol
jLDL-C low-density lipoprotein cholesterol
Effects of CZE on histopathology
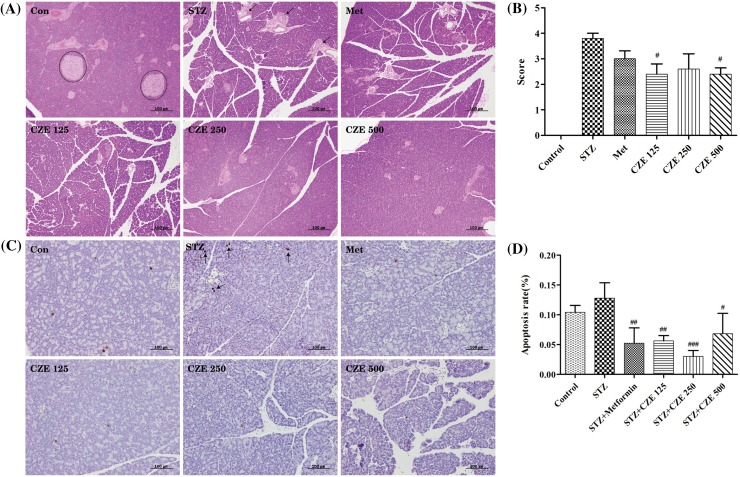
Histopathological observation and atrophy scores of pancreatic islet tissues stained with H&E are shown in Fig. 3. STZ-induced diabetic mice had increased atrophy scores, while CZE treatment was able to decrease the score. Treatment of diabetic rats with CZE at 125 and 500 mg/mL notably reduced (p < 0.05) atrophy; while CZE at 250 mg/mL had the same trend, albeit insignificantly. The TUNEL assay showed that apoptosis of pancreatic islets was significantly decreased upon treatment with 125 mg/kg (p < 0.01), 250 mg/kg (p < 0.001) and 500 mg/kg (p < 0.05) of CZE. These results suggested that CZE may inhibit the atrophy and apoptosis of pancreatic islets in diabetic animals.
Fig. 3.
Histopathological characteristics of pancreatic islets in STZ-induced type 1 diabetic rats treated with CZE. Pancreatic sections were stained with hematoxylin and eosin (A) and scored for atrophy (B). Representative images from the TUNEL assay (C) and apoptotic index (D). Con, Control; STZ, streptozotocin; Met, STZ + Metformin 200 mg/kg; CZE 125, STZ + CZE 125 mg/kg; CZE 250, STZ + CZE 250 mg/kg; CZE 500, STZ + CZE 500 mg/kg. H&E stain and TUNEL assay, magnification: ×200. Values are presented as mean ± SEM (n = 5 per group). #p < 0.05, ##p < 0.01, ###p < 0.001 compared to STZ group
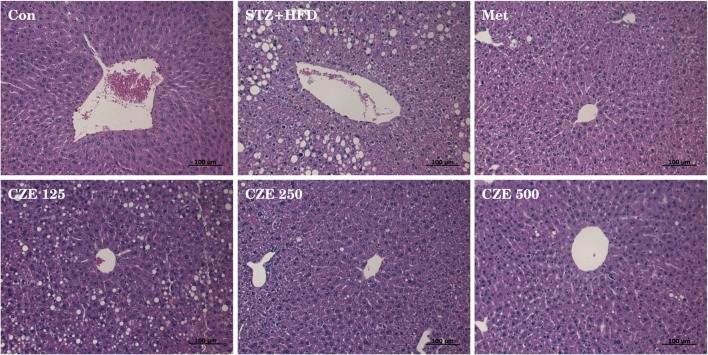
To determine whether CZE affects hepatic lipid accumulation, liver tissues of mice were stained with H&E to investigate histopathologic changes in the liver (Fig. 4). The lipid droplets were prominently increased in STZ + HFD-induced diabetic mice compared with the control group. The cord arrangement around the central vein of diabetic mice was partially collapsed. The lipid droplets in the liver of diabetic mice supplied with CZE at 125 mg/mL decreased in size, but the number of lipid droplets was observed to increase. On the other hand, lipid droplets of mice treated CZE at 250 and 500 mg/mL were significantly decreased, and arrangement of hepatocytes remained constant. Based on these results, CZE appears to prevent fatty livers caused by STZ + HFD-induced type 2 diabetes by inhibiting lipid accumulation in the liver.
Fig. 4.
Histopathological characteristics of the liver in STZ and HFD-induced type 2 diabetic mice treated with CZE. Liver sections were stained with hematoxylin and eosin. Con, Control; STZ + HFD, Streptozotocin and high-fat diet; Met, STZ + HFD + Metformin 200 mg/kg; CZE 125, STZ + HFD + CZE 125 mg/kg; CZE 250, STZ + HFD + CZE 250 mg/kg; CZE 500, STZ + HFD + CZE 500 mg/kg. H&E stain, magnification: × 200
In conclusion, the current study provides evidence that CZE ameliorates by reducing fasting blood glucose, increasing insulin levels in serum, as well as improving insulin resistance and lipid metabolism. In our previous study, we had confirmed that CZE correlates with linarin content, total polyphenol content, total flavonoid content, ABTS and DPPH radical scavenging ability, α-glucosidase and lipase inhibitory activity. Thus, we standardized CZE with linarin. Linarin as a CZE marker ingredient may influence the anti-diabetic effect in current study. These results suggest that supplementation with CZE may be beneficial for controlling hyperglycemia, hyperlipidemia and may also prevent diabetic complications.
Acknowledgements
This research was supported by the Ministry of Trade, Industry and Energy (R0004042) of Korea.
References
- Arvind K, Pradeepa R, Deepa R, Mohan V. Diabetes and coronary artery disease. Indian J. Med. Res. 2002;116:163–176. [PubMed] [Google Scholar]
- DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- Dunn P, Cole R, Soeldner J, Gleason R, Kwa E, Firoozabadi H, Younger D, Graham C. Temporal relationship of glycosylated haemoglobin concentrations to glucose control in diabetics. Diabetologia. 1979;17:213–220. doi: 10.1007/BF01235857. [DOI] [PubMed] [Google Scholar]
- Fratimunari A, Altamiranob E, Rodriguezbarcenas N, Arizaandraca R, Lopezledesma R. Hypoglycemic Effect Of Opuntia-Streptacantha Lemaire-Research With Crude Extracts. Arch. Invest. Med. (Mex.) 1989;20:321–325. [Google Scholar]
- Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch. Intern. Med. 2003;163:1306–1316. doi: 10.1001/archinte.163.11.1306. [DOI] [PubMed] [Google Scholar]
- Han S, Sung KH, Yim D, Lee S, Lee CK, Ha NJ, Kim K. The effect of linarin on LPS-induced cytokine production and nitric oxide inhibition in murine macrophages cell line RAW264.7. Arch. Pharm. Res. 2002;25:170–177. doi: 10.1007/BF02976559. [DOI] [PubMed] [Google Scholar]
- Hanefeld M, Temelkova-Kurktschiev T. The postprandial state and the risk of atherosclerosis. Diabet. Med. 1997;14:6–11. doi: 10.1002/(SICI)1096-9136(199708)14:3+<S6::AID-DIA438>3.3.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Harris RB, Mitchell TD, Yan X, Simpson JS, Redmann SM. Metabolic responses to leptin in obese db/db mice are strain dependent. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:115–132. doi: 10.1152/ajpregu.2001.281.1.R115. [DOI] [PubMed] [Google Scholar]
- He J, Chen F, Chen S, Lv G, Deng Y, Fang W, Liu Z, Guan Z, He C. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. Journal of plant physiology. 2011;168:687–693. doi: 10.1016/j.jplph.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Kim M-J, Kim HK. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006;79:2288–2292. doi: 10.1016/j.lfs.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Kim S-J, Cho H-I, Kim S-J, Park J-H, Kim J-S, Kim YH, Lee SK, Kwak J-H, Lee S-M. Protective effect of linarin against d-galactosamine and lipopolysaccharide-induced fulminant hepatic failure. Eur. J. Pharmacol. 2014;738:66–73. doi: 10.1016/j.ejphar.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kim SE, Lee HS, Hong SY, Kim SE, Kim YJ, Lee JH, Park SJ, Kim JH, Park YJ, Kim HK. Comparison of Linarin Content and Biological Activity in Ethanol Extraction of Chrysanthemum zawadskii. J. Korean Soc. Food Sci. Nutr. 2016;45:1414–1421. doi: 10.3746/jkfn.2016.45.10.1414. [DOI] [Google Scholar]
- Lemhadri A, Hajji L, Michel J, Eddouks M. Cholesterol and triglycerides lowering activities of caraway fruits in normal and streptozotocin diabetic rats. J. Ethnopharmacol. 2006;106:321–326. doi: 10.1016/j.jep.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Li J, Hao L, Wu J, Zhang J, Su J. Linarin promotes osteogenic differentiation by activating the BMP-2/RUNX2 pathway via protein kinase A signaling. Int. J. Mol. Med. 2016;37:901–910. doi: 10.3892/ijmm.2016.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liu H, Yuan Z, Wei D, Ye Y. Evaluation of antioxidant activity of chrysanthemum extracts and tea beverages by gold nanoparticles-based assay. Colloids Surf. B Biointerfaces. 2012;92:348–352. doi: 10.1016/j.colsurfb.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu L, Guo X-J. Effect of Bu-Zhong-Yi-Qi-Tang on deficiency of N-glycan/nitric oxide and islet damage induced by streptozotocin in diabetic rats. World J. Gastroenterol. 2009;15:1730–1737. doi: 10.3748/wjg.15.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Fan P, Perez RG, Lou H. Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-β-induced neuronal cell death. Bioorg. Med. Chem. 2011;19:4021–4027. doi: 10.1016/j.bmc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Mertes G. Safety and efficacy of acarbose in the treatment of type 2 diabetes: data from a 5-year surveillance study. Diabetes Res. Clin. Pract. 2001;52:193–204. doi: 10.1016/S0168-8227(01)00221-2. [DOI] [PubMed] [Google Scholar]
- Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–827. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metab. Clin. Exp. 2000;49:1390–1394. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–529. doi: 10.1016/S0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- Sassi AB, Harzallah-Skhiri F, Bourgougnon N, Aouni M. Antimicrobial activities of four Tunisian Chrysanthemum species. Indian J. Med. Res. 2008;127:183–192. [PubMed] [Google Scholar]
- Seo JY, Lim SS, Park J, Lim JS, Kim HJ, Kang HJ, Park Y, Han J, Kim JS. Protection by Chrysanthemum zawadskii extract from liver damage of mice caused by carbon tetrachloride is maybe mediated by modulation of QR activity. Nutr. Res. Pract. 2010;4:93–98. doi: 10.4162/nrp.2010.4.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Bharti S, Ojha S, Bhatia J, Kumar N, Ray R, Kumari S, Arya DS. Up-regulation of PPARγ, heat shock protein-27 and-72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br. J. Nutr. 2011;106:1713–1723. doi: 10.1017/S000711451100225X. [DOI] [PubMed] [Google Scholar]
- Shim SY, Kang HS, Sun HJ, Lee YJ, Park JR, Chun SS, Song YH, Byun DS. Isolation and identification of flavonoids from Gujeolcho (Chrysanthemum zawadskii var. latilobum) as inhibitor of histamine release. Food Sci. Biotechnol. 2012;21:613–617. doi: 10.1007/s10068-012-0079-0. [DOI] [Google Scholar]
- Steiner G, Haynes FJ, Yoshino G, Vranic M. Hyperinsulinemia and in vivo very-low-density lipoprotein-triglyceride kinetics. Am. J. Physiol. 1984;246:187–192. doi: 10.1152/ajpendo.1984.246.2.E187. [DOI] [PubMed] [Google Scholar]
- Swanston-Flatt SK, Day C, Flatt PR, Gould BJ, Bailey C. Glycaemic effects of traditional European plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetes Res. 1989;10:69–73. [PubMed] [Google Scholar]
- Talpur N, Echard BW, Yasmin T, Bagchi D, Preuss HG. Effects of niacin-bound chromium, Maitake mushroom fraction SX and (-)-hydroxycitric acid on the metabolic syndrome in aged diabetic Zucker fatty rats. Mol. Cell. Biochem. 2003;252:369–377. doi: 10.1023/A:1025564930088. [DOI] [PubMed] [Google Scholar]
- Woo JH, Lee CH. Effect of harvest date on antioxidant of Dendranthema zawadskii var. latilobum (Maxim.) Kitam and D. zawadskii var. yezoense (Maek.) Y.M. Lee & H.J. Choi. Korean Journal of Plant. Resources. 2008;21:128–133. [Google Scholar]
- Wright BE, Vasselli JR, Katovich MJ. Positive effects of acarbose in the diabetic rat are not altered by feeding schedule. Physiol. Behav. 1998;63:867–874. doi: 10.1016/S0031-9384(98)00013-4. [DOI] [PubMed] [Google Scholar]
- Yudkin J. Sucrose, coronary heart disease, diabetes, and obesity: do hormones provide a link? Am. Heart J. 1988;115:493–498. doi: 10.1016/0002-8703(88)90508-X. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Ye C, Li G, Ding W, Zhou W, Zhu H, Chen G, Luo T, Guang M, Liu Y, Zhang D, Zheng S, Yang J, Gu Y, Xie X, Luo M. The rat model of type 2 diabetic mellitus and its glycometabolism characters. Exp. Anim. 2003;52:401–407. doi: 10.1538/expanim.52.401. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang L, Wang W, Wu Y, Zhang Q, Feng W. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. J. Ethnopharmacol. 2011;137:662–668. doi: 10.1016/j.jep.2011.06.018. [DOI] [PubMed] [Google Scholar]