Abstract
Processing conditions (potato level, frying temperature and frying time) were optimized for the development of buckwheat based chips using response surface methodology (RSM). Moisture content, oil uptake, color values, hardness and overall acceptability (OAA) were used as indices of product quality. The polynomial regression model was fitted with R2 values of 0.983, 0.982, 0.98, 0.996, 0.973, 0.984 and 0.985 for moisture content, oil uptake, L, a*, b* values, hardness, and OAA respectively indicating fitness of the models. Potato level and frying temperature showed a significant effect on all responses at linear and quadratic levels except frying temperature for OAA at the linear level and hardness at quadratic level. Frying time showed significant effect on a* value, b* value, hardness and OAA at linear level. Interaction between all processing variables had a significant effect on a* value. Interaction between potato level and frying temperature had significant negative effect on moisture content of buckwheat chips. Potato level (30%), frying temperature (169 °C) and frying time (51 s) were found to be the optimum processing conditions with maximum OAA (8.36). 0.33 aw was established as the water activity at which maximum stability of chips was shown. Buckwheat chips packed in both polypropylene (PP) and metallised polyester (MP) remained stable and acceptable for 6 months at RT whereas for 3 and 6 months in PP and MP films respectively at 37 °C.
Keywords: Buckwheat chips, RSM, Potato level, Frying time, Frying temperature, Storage stability
Introduction
Snack foods are integral part of the diet playing an essential role in consumer’s daily nutrition and calorie intake, usually produced to be durable, handy, economical and easy to eat out of a bag or package without any additional preparation. Snacks, consumed more often by people are chips, cakes, tortillas etc. The most common savoury snacks in the world are potato and corn chips which are consumed by people of all age groups (Thakur and Saxena 2000). Cereals and legumes commonly used for the manufacture of snack foods are wheat, rice, chickpea flour etc.
Buckwheat being a rich source of dietary fiber, resistant starch, vitamins, minerals, flavanoid and antioxidants is a potential food ingredient, especially for the development of snacks (Jiang et al. 2007; Das et al. 2011; Lin et al. 2009). Due to prevalence of obesity, cardiovascular and other lifestyle related diseases, people are now becoming conscious about their food choices and patterns, the market, thus, needs a variety of healthy snacks which can provide satiety value too. Consumption of buckwheat groats helps to reduce the risk of atherosclerosis, hypertension and diabetes owing to low glycemic index and reduces cholesterol level in blood (Foster-Powell and Miller 1995; Drobot et al. 2009). Buckwheat protein is supposed to reduce serum cholesterol, suppress gallstones and tumors and inhibit the angiotensin I-converting enzyme, thereby improving health (Koyama et al. 2013). Koyama et al. (2013) reported that unique medicinal properties such as antihypertensive and antihypercholesterolemic effects in humans are observed due to presence of phenolic compounds and flavones such as hyperin, quercitrin and quercetin isolated from immature buckwheat seeds (Li et al. 2010). Therefore, development of ready-to-eat chips from buckwheat, a non-gluten pseudocereal, along with potato by optimization process would be highly advantageous in obtaining a highly acceptable product for celiac and diabetic people, people on fasting and other conventional consumers.
Potato was incorporated to buckwheat flour at different proportions in this study, as potato is widely used to make snack products, highly acceptable by consumers and can be consumed even during fasting. Chips are generally prepared from potato or banana but other chips were also developed like wheat chips (Kaycier et al. 2013), water chestnut chips (Monaco et al. 2010), ackee aril flour chips (Essuman et al. 2016), etc. Buckwheat flour has not been used as the main ingredient in the preparation of fried chips until now and thus formed the basis of study.
The aim of the present study was to optimize processing conditions for development of potato incorporated buckwheat and study storage stability of chips during storage in polypropylene (PP) and metallised polyester (MP) pouches at RT and 37 °C conditions.
Materials and methods
Buckwheat groats, potato, rice bran oil (Fortune brand), spices were obtained from local market in Mysore, Karnataka, India.
Preparation of chips
Buckwheat flour with different proportions of boiled potato (cooked for 10 min at 15 psi followed by peel removal to make potato mash) was mixed (100:0, 75:25 or 50:50) thoroughly for all 17 experimental runs (Table 1) of 500 g sample each. Red chilli powder (0.6%), black pepper powder (0.6%), rock salt (2.5%) and water were added and the mixing was continued using a Kitchen Aid dough mixer (Professional 600, USA) for 5 min. Subsequently dough was covered with a plastic cover and 10 min resting time at room temperature was given for suitable hydration. Dough was sheeted manually to 1.5-2.0 mm thickness and chips were cut into circular shape of 2 cm diameter using a mold. Chips were deep fat fried in a temperature-controlled fryer (M/s. Continental, India) at different frying temperatures (160 °C, 170 °C and 180 °C) and time (40, 50 and 60 s) for the optimization of processing variables. Range for all the processing variables were selected with prior trials based on the maximum sensory acceptability by researchers. After frying process, samples were cooled to room temperature and stored in PP (75 µ) and MP (90 µ) pouches till further analysis.
Table 1.
Experimental design and responses of buckwheat chips
| Run | Variables | Responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Potato Level (%) | Frying Temp. (°C) | Frying Time (s) | Moisture content (%) | Oil Uptake (%) | L-value | a* value | b* value | Hardness (N) | OAA | |
| 1 | 0 | 160 | 50 | 5.96 | 10.23 | 51.81 | 7.27 | 21.29 | 850.24 | 6.1 |
| 2 | 25 | 160 | 40 | 8.42 | 10.21 | 49.34 | 7.41 | 20.59 | 910.23 | 6.8 |
| 3 | 50 | 170 | 40 | 7.92 | 13.61 | 46.27 | 9.35 | 20.59 | 880.76 | 6.8 |
| 4 | 50 | 160 | 50 | 8.87 | 11.72 | 47.93 | 8.47 | 23.14 | 920.65 | 7.0 |
| 5 | 0 | 170 | 40 | 5.13 | 12.04 | 49.65 | 8.97 | 16.21 | 773.94 | 6.2 |
| 6 | 25 | 170 | 50 | 7.60 | 12.38 | 47.36 | 9.45 | 20.84 | 641.46 | 8.4 |
| 7 | 25 | 160 | 60 | 7.65 | 10.60 | 48.29 | 8.01 | 25.07 | 809.42 | 7.1 |
| 8 | 50 | 180 | 50 | 7.08 | 13.62 | 45.62 | 10.54 | 25.75 | 594.23 | 6.8 |
| 9 | 0 | 180 | 50 | 5.46 | 12.42 | 49.38 | 10.15 | 22.29 | 524.89 | 6.2 |
| 10 | 0 | 170 | 60 | 5.01 | 12.37 | 50.24 | 9.27 | 19.99 | 631.23 | 6.4 |
| 11 | 25 | 170 | 50 | 7.32 | 12.42 | 47.02 | 9.46 | 21.52 | 600.23 | 8.5 |
| 12 | 25 | 180 | 60 | 7.16 | 11.73 | 46.24 | 10.86 | 25.95 | 510.89 | 6.5 |
| 13 | 25 | 170 | 50 | 7.52 | 12.38 | 46.45 | 9.37 | 21.32 | 640.65 | 8.4 |
| 14 | 50 | 170 | 60 | 7.63 | 13.65 | 46.34 | 10.39 | 22.82 | 711.02 | 7.2 |
| 15 | 25 | 170 | 50 | 7.58 | 12.32 | 46.74 | 9.51 | 21.46 | 633.42 | 8.4 |
| 16 | 25 | 170 | 50 | 7.14 | 11.99 | 46.74 | 9.34 | 19.96 | 640.42 | 8.1 |
| 17 | 25 | 180 | 40 | 7.27 | 11.96 | 46.48 | 9.57 | 22.86 | 564.56 | 6.9 |
Physico-chemical analysis
Moisture and oil uptake of the samples were determined using standard procedures described in AOAC (1990). Tri-stimulus hunter colorimeter (M/s Miniscan XE plus, Model No. 45/O-S, Hunter Associates Laboratory, Inc., Reston, VA, USA) with D65 illuminator at an observer angle of 10° was used to determine the colour coordinates of the chips surface. Standard white and black tiles were used as a reference.
Texture analysis
Buckwheat chips samples prepared based on the experimental design were subjected to the textural analysis at room temperature via Texture Analyzer (TA.HD + , Stable Micro Systems, UK). This was equipped with Crisp fracture support rig with 5 mm diameter stainless steel ball probe operating at a test speed of 0.5 mm/s over a distance of 5 mm with a load cell of 5 kg. Hardness was expressed as the maximum force required during first compression.
Sensory evaluation
On the basis of colour, crispiness and taste, sensory acceptance of the chips was reported as overall acceptability (OAA) using a 9 point Hedonic scale according to Larmond (1977) by semi-trained panel.
Experimental design
The modelling of three processing variables: potato level (A), frying temperature (B), and frying time(C) was done via three-factor, three-level Box–Behnken experimental design (Box and Behnken 1960) with three replicates at the center point using Design Expert® 10.0.6.0 software. Predictive regression models were constructed for moisture content, oil uptake, colour, textural and sensory parameters. Second-order polynomial equation of independent variables was fitted for each response analyzed:
where Y (i = 1–7) is the predicted response for moisture, oil uptake, L, a* and b* values, hardness and OAA respectively, β0 depicts the estimated regression coefficient of the fitted response at center point of the design; β1, β2, β3 depicts the regression coefficient for linear effect terms; β11, β22, β33 are the quadratic effects and β12, β13, β23 are the interaction effects.
Effect of water activity (aw) on lipid peroxidation
150 g samples of powdered buckwheat chips was kept in desiccators of fixed aw to study effect of aw on lipid peroxidation for 40 days at room temperature. P2O5 was used to obtain 0.0 aw and saturated solutions of MgCl2, NaBr and NaNO3 were used to obtain aw of 0.33, 0.57 and 0.73 respectively. Stored samples were analyzed for moisture content, peroxide value, free fatty acid value and thiobarbituric acid initially (0th day) and after every 10 days for the period of 40 days.
Storage and evaluation
Buckwheat chips prepared by using optimized parameters of variables viz. potato concentration, frying temperature and frying time were packed in PP and MP pouches, heat sealed and stockpiled under room temperature (RT) and accelerated (37 °C) conditions for further studies.
Analysis
The buckwheat chips were developed and analysed for moisture content (%), peroxide value (meqO2/kg oil), free fatty acid (% oleic acid), thiobarbituric acid (mg malonaldehyde/kg sample) and overall acceptability during storage.
Microbial profiles was also determined using the Petri plate method as described by APHA (1992) for standard plate count (SPC) on plate count agar, coliform count on violet red bile agar, fecal coliform on Escherichia coli agar, and yeast and mold counts on potato dextrose agar. Presence of pathogens viz. coliform, Salmonella and Staphylococcus aureus was also determined by APHA (1992).
Statistical analysis
Data was analysed and response surface plot were generated using the Design Expert ®. 10.0.6.0 software (Stat Ease Inc., Minneapolis, MN). Analysis of variance (ANOVA) and regression coefficient (R2) was determined using Design Expert software to test the significance of each variable (p ≤ 0.05) and to verify the adequacy of the model respectively. The validity of the model was estimated by comparing experimental and predicted values.
Results and discussion
Table 1 present the Box Behnken type design for three independent variables (potato level, %; frying temperature, °C and frying time, s) and their responses (moisture content, oil uptake, L, a* and b* values, hardness and OAA). All linear, quadratic and interactive effects were calculated for each model (Table 2). Table 2 showed that the co-efficient of determination R2 was more than 80% and lack of fit (LoF) was insignificant indicating the fitness of models. Therefore, all seven responses were selected for further interpretation.
Table 2.
Regression coefficients of the fitted second-order polynomials representing the relationship between the responses and variables
| Coefficient constant | Moisture content | Oil uptake | L value | a* value | b*value | Hardness | OAA |
|---|---|---|---|---|---|---|---|
| β0 | 7.432 | 12.298 | 46.862 | 9.426 | 21.020 | 631.236 | 8.362 |
| Linear | |||||||
| A | 1.243* | 0.692* | − 1.865* | 0.386* | 1.565* | 40.795 * | 0.363* |
| B | − 0.491* | 0.871* | − 1.206* | 1.245* | 0.845* | − 161.996* | − 0.075 |
| C | − 0.161 | 0.066 | − 0.079 | 0.404* | 1.698* | − 58.366* | 0.063 |
| Interaction | |||||||
| AB | − 0.323* | − 0.073 | 0.03 | − 0.203* | 0.403 | − 0.2675 | − 0.075 |
| AC | − 0.043 | − 0.073 | − 0.13 | 0.185* | − 0.388 | − 6.7575 | 0.05 |
| BC | 0.165 | − 0.155 | 0.203 | 0.173* | − 0.348 | 11.785 | − 0.175 |
| Quadratic | |||||||
| A2 | − 0.896* | 0.746* | 1.180* | 0.107* | − 0.809* | 70.865* | − 1.006* |
| B2 | 0.307* | − 1.047* | 0.643* | − 0.426* | 2.906* | 20.402 | − 0.831* |
| C2 | − 0.114 | − 0.127 | 0.083 | − 0.038 | − 0.309 | 47.137* | − 0.706* |
| R2 | 0.983 | 0.982 | 0.980 | 0.996 | 0.973 | 0.984 | 0.985 |
| Predicted R2 | 0.839 | 0.809 | 0.823 | 0.959 | 0.832 | 0.806 | 0.876 |
| SD | 0.22 | 0.21 | 0.37 | 0.09 | 0.59 | 25.75 | 0.15 |
| Lack of fit | Not significant | Not significant | Not significant | Not significant | Not significant | Not significant | Not significant |
*Values are significant at p ≤ 0.05
A—potato level, B—frying temperature, C—frying time, R2—coefficient of determination
Effect of processing variables on physico-chemical changes in buckwheat chips
Effect on moisture content
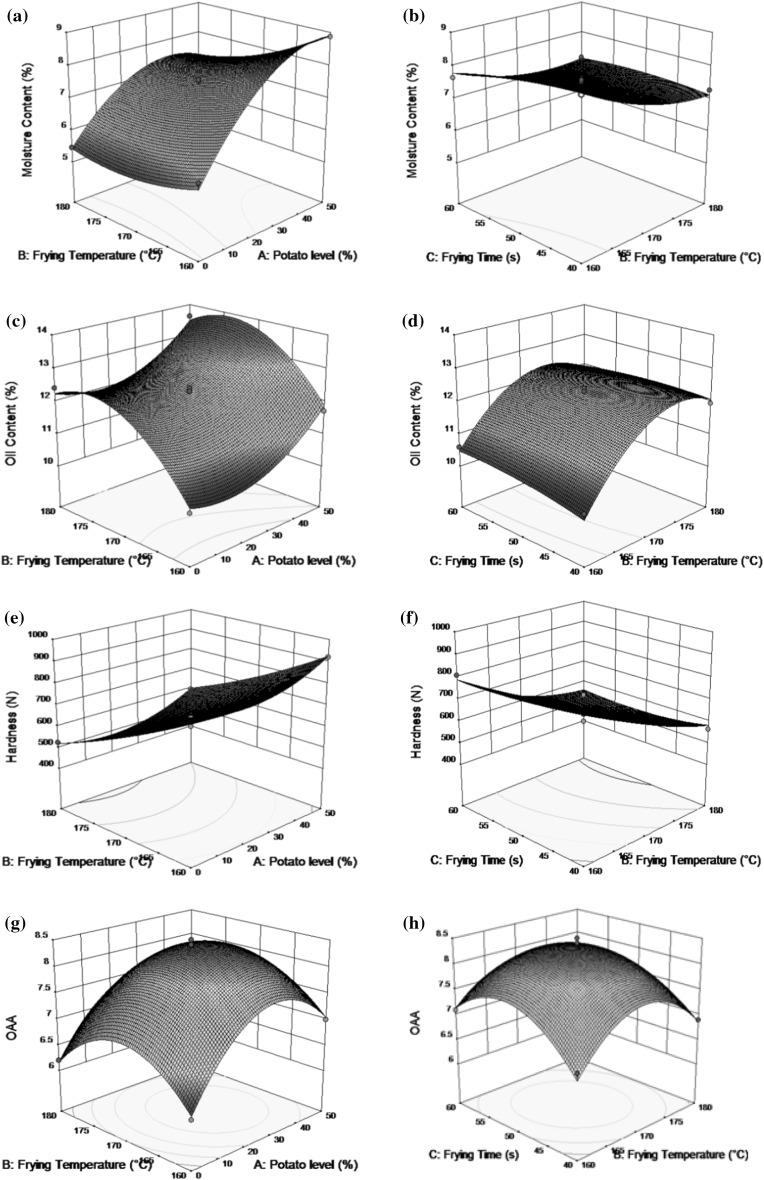
Effect of potato and frying temperature on moisture content was found significant (p ≤ 0.05) at both linear and quadratic levels. Interaction between potato level and frying temperature also showed a significant (p ≤ 0.05) negative effect on moisture content (Table 2). Moisture content was found to increase with the increase in potato level. During frying, the decrease in the moisture content was observed with increase in frying temperature and time. Response plot (Fig. 1a, b) revealed that the moisture content of chips increased slightly on increase in potato level which is attributed to the higher moisture retention and water binding property of potato (Malav et al. 2012). Increase of moisture content with increase in potato level was also observed in mashed potato incorporated egg cutlets by Singh et al. (2016). Decrease of moisture content with increasing frying temperature and time may be due to rapid removal of unbound water in fried food as boiling point of oil and water in food is reached during deep fat frying (Shyu et al. 2005). Similar trend of decrease in moisture content with increase in frying temperature and time was observed in deep fried plantain chips (Adeyanju et al. 2016), deep fried tortillas (Moreira et al. 1997) due to rapid removal of unbound moisture. Coefficient of determination (R2) for moisture content of buckwheat chips was found to be 0.839 (> 80%) indicating fitness of model.
Fig. 1.
Effect of process variables on moisture content (a, b), oil content (c, d), hardness (e, f) and OAA (g, h) along with the second order polynomial model equation
Effect on oil uptake
Potato level and frying temperature had a significant (p ≤ 0.05) positive effect on oil uptake at linear level. At quadratic level, increase in potato level showed significant (p ≤ 0.05) positive effect and increase in frying time had a significant (p ≤ 0.05) negative effect on oil uptake. Increase in oil uptake with increase in frying time was also observed (Table 2). Maximum response for oil uptake (13.65%) was obtained with potato level (50%) at 170 °C for 60 s and minimum response for oil uptake (10.21%) was obtained with potato level 25% at 160 °C for 40 s (Table 1). During frying process, oil uptake increased with the increase in all processing variables at linear level due to transfer of heat from oil into food product and consequent replacement of moisture from food by frying oil (Krokida et al. 2000). The oil uptake followed an increasing trend with the increase in boiled potato percentage in the formulation. Similar trend was observed by Chetana et al. (2014) and Singh et al. (2016) in potato incorporated chicken cutlets and egg cutlets respectively. With increase in frying temperature and time, oil uptake increased resulting in crunchier texture. Response plot for oil uptake (Fig. 1c, d) showed increased oil uptake during deep fat frying of chips, which may be due to molecular size redistribution or modification in porosity. Similar trend of significant increase in oil uptake with increase in frying temperature and time was observed by Moreira et al. (1997) and Sobukola et al. (2007) for potato slices, tortilla chips and yam chips respectively. The coefficient of determination (R2) was obtained to be 0.809 for oil uptake indicating fitness of model.
Effect on color parameters
Potato level, frying temperature and time showed a significant (p ≤ 0.05) effect on the colour characteristics of buckwheat chips. Lightness index (L) decreased significantly (p ≤ 0.05) with change in all processing variables at linear level (Table 2). The rise in temperature of the product during frying resulted in caramelization, a non-enzymatic browning reaction (Shallenberger et al. 1959) which might have caused a decrease in the lightness value of samples. All processing variables showed significant (p ≤ 0.05) positive linear effect on a* and b* values of buckwheat chips. Also, potato level and frying temperature showed a significant (p ≤ 0.05) effect on both a* and b* values at quadratic level. Interaction between the variables attributed significant effect on a* values of final product. Increase in frying temperature and time at constant potato level resulted in increase in a* and b* value while decrease in L value. Buckwheat chips with 50% potato appeared comparatively darker and showed more redness (a*) as a result of surface non-enzymatic browning reactions which affected the final colour of the product due to formation of melanoidins due to interaction between protein and sugar molecules (Shallenberger et al. 1959). Pedreschi et al. (2005) also reported increase in a* values of potato crisps with increasing frying temperature and time. Changes in buckwheat flour to potato ratio in the preparation of buckwheat chips also affected its yellowness value significantly (p ≤ 0.05) due to maillard reaction. When potato to buckwheat ratio was changed from 0:100 to 50:50, an increase in yellowness value was observed. Torbica et al. (2012) also reported slight increase in yellowness values by decreasing the amount of buckwheat flour in rice and buckwheat flour based cookies. The coefficient of determination (R2) was obtained to be 0.823, 0.959, 0.832 respectively for L, a* and b* values respectively indicating fitness of model.
Effect on texture
Firm crispy texture which provides easy snap to produce crunchy sound is one of the most desirable characteristic of fried products. Texture of fried food depends on both raw material and processing condition. The texture of fried foods is governed by various factors which include starch content, size of the starch granules, pectic substances, cell wall and non- starch polysaccharides (Nourian and Ramaswamy 2003). Potato level, frying temperature and frying time showed a significant (p ≤ 0.05) effect on hardness of buckwheat chips at both linear and quadratic level except frying temperature at quadratic level (Table 2). Crispy and crunchy textured product with maximum acceptability was obtained when chips were fried at 170 °C for 50 s with a potato level of 25% (Table 1). Figure 1e, f illustrates increase in hardness values with increase in potato level as well as decrease in frying temperature and frying time. Increase in hardness was observed due to increase in potato level which may be due to good binding of potato with buckwheat flour as observed in egg cutlets incorporated with mashed potato by Singh et al. (2016). The decrease in hardness values with an increase in frying temperature and time may be due to loss of moisture during frying. The results were in conformity with that reported for barley chips by Prakash et al. (2015). The coefficient of determination (R2) was 0.806 for hardness values indicating goodness of fit.
Effect on OAA
Changes in the processing variables viz potato level, frying temperature and frying time has significantly (p ≤ 0.05) affected the overall acceptability of buckwheat chips. Chips with better acceptability were obtained when fried for 50 s at 170 °C with 25% addition of potato to buckwheat flour (Table 1). Potato level showed significant (p ≤ 0.05) positive linear effect on OAA. On the other hand, all the processing variables showed a significant quadratic effect on OAA. Increase in OAA score at higher temperature (till 170 °C) may be due to shorter frying time (50 s) which gave more crispy and light coloured chips. Figure 1g, h revealed increase in OAA with frying time up to certain level (50 s) after which decrease in OAA was observed which might be due to thicker crust development and subsequent loss of moisture. The coefficient of determination (R2) was obtained to be 0.876 for overall acceptability indicating fitness of model.
Regression equations and model fitting
The lack of fit and significance of the linear, quadratic and interaction effects of the independent variables on the dependent variables was determined by ANOVA. Regression coefficient (R2) and predicted R2 for the buckwheat chips were determined by using Statisica software and results are tabulated in Table 2. All the responses were fitted with quadratic models. The significance of all the models was indicated by p values less than 0.05 (p ≤ 0.05) of all regression models. The R2 values of the dependent variables for moisture content, oil uptake, L, a*, b* values, hardness and OAA were found between 0.97–0.99. Joglekar and May (1987) reported that R2 values greater than 80% indicate the fitness of polynomial models which are used for describing the effect of variables on the responses. Results revealed that for all the response variables, the models were highly adequate because they have agreeable levels of R2 of above 80% and that there is no significant lack of fit in all the response variables. Earlier Kaycier et al. (2013) reported that in optimization of processing conditions of wheat based chips, the polynomial models were fitted as the R2 value for the responses like dry matter, ash, hardness, L, a and b values and OAA were more than 80%.
The multiple regression equations (as coded factors) obtained for the response variables are as follows:
Optimization of variables and model validation
All the variables like potato level, frying temperature and frying time were optimized by means of numerical multi-response optimization method. Numerical optimization technique is one of the paramount methods to find out the best optimal ratio of the variables in the experiment as it decreases the time and efforts required for the investigation of multifactor and multiple-response systems. Optimization for independent variables was carried out with all the responses kept in range as well as maximum overall acceptability. The optimized values for potato level, frying temperature and frying time were 30%, 169 °C and 51 s respectively with a desirability of 0.958 (Table 3).
Table 3.
Constraints and criteria for optimization along with predicted and validated values
| Name | Goal | Lower limit | Upper limit | Importance | Predicted values | Validated values |
|---|---|---|---|---|---|---|
| A: potato level (%) | In range | 0 | 50 | 3 | 30 | – |
| B: frying temperature (°C) | In range | 160 | 180 | 3 | 169 | – |
| C: frying time (s) | In range | 40 | 60 | 3 | 51 | – |
| Moisture (%) | In range | 5.01 | 8.87 | 3 | 7.65 | 7.62 ± 0.22 |
| Oil (%) | In range | 10.21 | 13.65 | 3 | 12.39 | 12.62 ± 0.21 |
| L value | In range | 45.62 | 51.81 | 3 | 46.63 | 46.95 ± 0.40 |
| a* value | In range | 7.27 | 10.86 | 3 | 9.45 | 9.64 ± 0.62 |
| b* value | Minimize | 16.21 | 25.95 | 3 | 21.33 | 21.96 ± 0.07 |
| Hardness (N) | In range | 510.89 | 920.65 | 3 | 647.56 | 652.43 ± 0.92 |
| OAA | Maximize | 6.10 | 8.50 | 3 | 8.39 | 8.36 ± 0.02 |
The predicted responses were experimentally validated using optimized values for the variables. If the predicted values are close to the experimental values during the validation tests, the model is regarded as an adequate model. No significant (p > 0.05) difference between experimental and predicted response values (Table 3) was shown from results and thereby reconfirming the appropriateness of the models.
Proximate composition of optimized buckwheat chips
The optimized buckwheat chips had 7.62% moisture content, 12.62% fat, 11.52% protein, 2.91% dietary fiber, 1.74% ash content, 66.5% carbohydrate and energy value of 425.66 kcal/100 g. Potato level significantly (p ≤ 0.05) affected the OAA of chips.
Shelf stability of optimized buckwheat chips
Storage stability of optimized buckwheat chips in different packaging materials (PP and MP) at room temperature (15–34 °C) and 37 ± 2 °C conditions was assessed on the basis of changes observed in moisture content (MC), peroxide value (PV), free fatty acid value (FFA), thiobarbituric acid value (TBA) and overall acceptability (OAA).
The moisture content in all the samples increased significantly (p ≤ 0.05) from a minimum value of 7.65–9.29 and 9.16% at RT and 37 °C temperature conditions respectively in samples packed in PP films during 9 months of storage. However, the increase in moisture content was observed less in MP packed samples and it increased from 7.65 to 8.06 and 8.07% at RT and 37 °C temperature conditions respectively during 9 months, due to its better barrier property for moisture as compared to PP films (Table 4). The same trend of comparatively more moisture content increase in PP than MP films was reported during storage of instant wheat porridge mix by Khan et al. (2014). There was a significant (p ≤ 0.05) increase in moisture content of chips packed in PP films during 9 months at both RT and 37 °C. There was also a significant (p ≤ 0.05) difference between moisture content of chips packed in PP and MP films at both RT and 37 °C during storage.
Table 4.
Changes in physic-chemical, sensory and microbiological analysis of buckwheat chips
| Parameters | Packaging material | 0 M | 3 M RT |
6 M RT |
9 M RT |
3 M 37 °C |
6 M 37 °C |
9 M 37 °C |
|---|---|---|---|---|---|---|---|---|
| Moisture content* | Poly propylene | 7.65ab | 7.96cd | 8.62e | 9.29g | 8.05d | 8.83f | 9.16g |
| Metallised polyester | 7.69ab | 7.76abx | 8.06dx | 7.79abc | 7.93bcdx | 8.07dx | ||
| Peroxide value* | Poly propylene | 3.21a | 6.62b | 11.98d | 22.46g | 6.86b | 13.21e | 25.24h |
| Metallised polyester | 6.42b | 10.61cx | 20.39fx | 6.79b | 11.79dx | 22.76gx | ||
| Free fatty acid* | Poly propylene | 0.56a | 1.36b | 2.12cd | 2.96e | 1.42b | 2.36d | 3.34f |
| Metallised polyester | 1.25b | 1.94cx | 2.75ex | 1.30b | 2.15cdx | 2.92ex | ||
| Thiobarbituric acid* | Poly propylene | 0.08a | 0.11bc | 0.15e | 0.25hi | 0.14de | 0.19f | 0.27i |
| Metallised polyester | 0.10ab | 0.13cdex | 0.22gx | 0.12bcdx | 0.15ex | 0.24ghx | ||
| Overall acceptability** | Poly propylene | 8.23h | 8.12fgh | 7.65de | 6.12b | 8.02fgh | 6.86c | 5.16a |
| Metallised polyester | 8.16gh | 7.87efg | 6.74cx | 8.05fgh | 7.49dx | 5.87bx | ||
| Total plate count* (colonies/g) | Poly propylene | 75a | 90b | 124d | 151f | 106c | 156f | 180g |
| Metallised polyester | 82a | 104cx | 132ex | 90bx | 125dx | 158fx | ||
| Yeast and mold* (cfu/g) | Poly propylene | Nil | Nil | Nil | 5c | Nil | 4 | 6d |
| Metallised polyester | Nil | Nil | 3ax | Nil | Nil | 4bx |
*Values are mean of 3 determinations of mean ± SD (n = 3)
**Values are mean of 15 determinations of mean ± SD (n = 15)
a–hValues within same row with different superscript differ significantly (p ≤ 0.05)
xValues are significantly different from their corresponding PP (polypropylene) packed samples (p ≤ 0.05)
PV, an index to assess the lipid oxidation level which form hydroperoxides as primary product (Kashyap et al. 2012) was found significantly (p ≤ 0.05) higher in samples packed in PP films as compared to those packed in MP films (Table 4). The peroxide value of chips increased from 3.21 to 25.24 and 22.76 meqO2/kg fat packed in PP and MP pouches during 9 months of storage at 37 °C. The increase in PV at RT was from 3.21 to 22.46 and 20.49 meqO2/kg fat in PP and MP films respectively. The changes in peroxidation were found to be significantly (p ≤ 0.05) less in MP as compared to sample stored in PP films during 6 months. Ikpeme et al. (2007) reported that peroxide value of less than 25 meqO2/kg fat is the safe limit for storage of chips and it is evident from our study that PV of the samples stored at RT and samples stored in MP films at 37 °C remained within the safe limit during entire storage.
FFA value increased significantly (p ≤ 0.05) from 0.56 to 2.94 and 2.75% oleic acid in PP and MP films at RT, from 0.56 to 3.34 and 2.92% oleic acid in PP and MP films at 37 °C during 9 months of storage. FFA in chips increased significantly (p ≤ 0.05) during storage irrespective of the packaging materials. However, no significant changes between samples stored in PP and MP films at RT was observed. Lipase activity was destroyed during thermal processing (frying) employed for chips and therefore, the decomposition of hydroperoxide may have resulted in the formation of free fatty acids in chips as previously reported in processed cereal product by Thakur and Arya (1990).
TBA test is a method for measurement of secondary metabolites. With increase in PV, an increase in TBA values was observed due to formation of secondary metabolites after lipid peroxidation. The TBA values during 9 months of storage increased significantly (p ≤ 0.05) from 0.08 to 0.27 and 0.24 mg malonaldehyde/kg sample in PP and MP packed samples respectively at 37 °C. The same has been increased significantly (p ≤ 0.05) from 0.08 to 0.25 and 0.22 mg malonaldehyde/kg sample in PP and MP packed samples respectively stored at RT. The same trend of comparatively higher TBA values in PP as compared to MP films was reported during storage of instant wheat porridge mix by Khan et al. (2014).
The increase in lipid oxidation during storage has undoubtedly affected the sensory attributes of chips stored at both the temperature conditions. The OAA score of 8.23 obtained initially decreased significantly at each interval of the study conducted at both temperature conditions and was decreased to 6.12 & 6.74 and 5.16 & 5.87 in samples packed in PP and MP films stored during 9 months at RT and 37 °C temperature conditions respectively. There was development of unacceptable rancid off-flavor and the decrease in crispiness due to which sensory evaluation was not carried out further. However, between the samples packed in PP and MP films, the OAA score did not vary significantly except for samples stored at RT during 9 months as well as 37 °C during 6 and 9 months. The accelerated temperature conditions (37 °C) decreased the OAA values significantly during storage. Since OAA score of 7 was considered as the lowest limit for the acceptability of the product which reveals more than 75% acceptability from the researchers, the product was found to be acceptable for 6 months at RT irrespective of packaging material and 3 months in PP films, 6 months in MP films stored at 37 °C.
There was practically no significant change in the microbiological status of chips during the entire storage period. The total plate count of chips prepared at 0th day ranged from 75 to 100 colonies/g and in 9 months stored samples, the same was found to be from 125 to 165 colonies/g irrespective of storage conditions and packaging materials. Fresh and stored chips showed below 10 cfu/g of yeast and mold count, no coliform and fecal coliform during 9 months. The pathogens viz E. coli, S. aureus and Salmonella were also absent in all stored samples.
Effect of water activity on storage
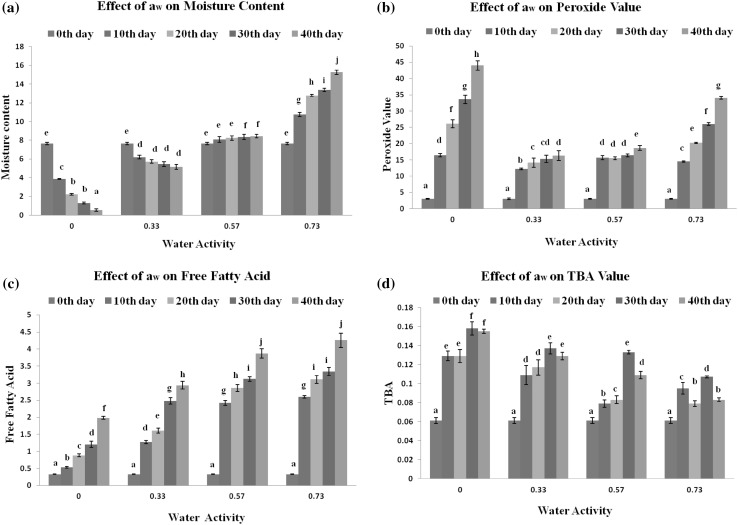
Changes in moisture content, PV, FFA and TBA values of optimized buckwheat chips under aw of different levels (0–0.73) are shown in Fig. 2a–d. The product equilibrated to 0.54, 5.15, 8.44 and 15.26% moisture levels at 0.0, 0.33, 0.57 and 0.73 aw respectively after 40 days of storage. aw up to 0.73 showed no visible microbial spoilage for 40 days. The gradual increase in PV and FFA values were observed at all aw during storage but least values were observed at 0.33 aw suggesting that monolayer acts as a barrier towards the oxidation of lipid present in food resulting in the maximum stability of the product. Both below and above 0.33 aw, the rate of increase of PV and FFA values was more. Labuza (1978) reported similar results as water forms hydrogen bonds with hydroperoxides and arresting decomposition of peroxides into free radicals at 0.33 aw. The similar results depicting maximum stability of product at 0.33 aw were also obtained previously for groundnut burfi (Khan et al. 2008) and flaxoat nutty bar (Padmashree et al. 2013).
Fig. 2.
Effect of aw on a moisture content, b peroxide value, c free fatty acid and d TBA values of optimized buckwheat chips. Asterisk values with different superscript at each day differ significantly (p ≤ 0.05)
Conclusion
RSM was found to be efficient in optimizing the best optimal values of processing variables to obtain buckwheat chips with maximum acceptability. The present study shows that buckwheat flour can be substituted with 30% boiled potato and deep fried at 169 °C for 51 s to obtain chips with maximum overall acceptability. The effect of aw on lipid peroxidation established the maximum stability of the chips at 0.33 aw. Buckwheat chips remained acceptable for 6 months in both the packaging materials at RT and also 6 months in MP pouches at 37 °C storage conditions.
Acknowledgements
The author, Charu Goel acknowledge Department of Science and Technology, Ministry of Science and Technology, India, for awarding doctoral fellowship under the INSPIRE fellowship programme. The authors sincerely thank Director, DFRL for providing all the necessary facilities for carrying out the study.
References
- Adeyanju JA, Olajide JO, Adedeji AA. Optimisation of deep fat frying of plantain chips (Ipekere) using response surface methodology. J Food Process Technol. 2016;7(5):584–590. [Google Scholar]
- AOAC . Official methods of analysis. 15. Washington: Association of Official Analytical Chemists, AOAC International Publisher; 1990. [Google Scholar]
- APHA (1992) Compendium of methods for the microbiological examination of food, 2nd edn. In: Speck ML (ed) American Public Health Association, Washington, DC
- Box GEP, Behnken DW. Some new three level designs for the study of quantitative variables. Technometrics. 1960;7:455–475. doi: 10.1080/00401706.1960.10489912. [DOI] [Google Scholar]
- Chetana P, Yogesh K, Anita Bharti SK, Tanwar VK. Effect of incorporation of potato on the quality of chicken cutlets. J Agric Vet Sci. 2014;7:12–15. [Google Scholar]
- Das A, Raychaudhuri U, Chakraborty R. Cereal based functional food of Indian subcontinent: a review. J Food Sci Technol. 2011;49(6):665–672. doi: 10.1007/s13197-011-0474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobot V, Mikhonik L, Grischenko A. Products of functional purposes: prospects for using of products of processing cereals in bread making. Mir produktov. 2009;9:6–8. [Google Scholar]
- Essuman EK, Osei JA, Gyimah V. Proximate Composition and sensory qualities of chips produced from ackee aril flour. Am J Food Sci Technol. 2016;4(2):38–42. [Google Scholar]
- Foster-Powell K, Miller JB. International tables of glycemic index. Am J Clin Nutr. 1995;62(4):871–890. doi: 10.1093/ajcn/62.4.871S. [DOI] [PubMed] [Google Scholar]
- Ikpeme CAE, Eneji CA, Essiet U. Storage stability and sensory evaluation of taro chips fried in palm oil, palmolein oil, groundnut oil, soybean oil and their blends. Pakistan J Nutr. 2007;6:570–575. doi: 10.3923/pjn.2007.570.575. [DOI] [Google Scholar]
- Jiang P, Burczynski F, Campbell C, Pierce G, Austria JA, Briggs CJ. Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F. tataricum and F. homotropicum and their protective effects against lipid peroxidation. Food Res Int. 2007;40(3):356–364. doi: 10.1016/j.foodres.2006.10.009. [DOI] [Google Scholar]
- Joglekar AM, May AT. Product excellence through design of experiments. Cereal Food World. 1987;32:857–868. [Google Scholar]
- Kashyap A, Thind SS, Kaur A. Development of chicken meat patties incorporating natural antioxidants. Int J Food Sci Technol. 2012;2:27–40. [Google Scholar]
- Kaycier A, Yuksel F, Karaman S. Response surface methodology study for optimization of effects of fiber level, frying temperature and frying time on some physicochemical, textural and sensory properties of wheat chips enriched with apple fiber. Food Bioprocess Tech. 2013;7(1):133–147. doi: 10.1007/s11947-013-1096-6. [DOI] [Google Scholar]
- Khan MA, Semwal AD, Sharma GK, Yadav DN, Srihari KA. Studies on the development and shelf stability of groundnut (Arachis hypogea) burfi. J Food Qual. 2008;31(6):612–626. doi: 10.1111/j.1745-4557.2008.00224.x. [DOI] [Google Scholar]
- Khan MA, Semwal AD, Sharma GK, Bawa AS. Studies on the optimization and stability of instant wheat porridge (Dalia) mix. J Food Sci Technol. 2014;51:1154–1160. doi: 10.1007/s13197-012-0630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M, Nakamura C, Nakamura K. Changes in phenols contents from buckwheat sprouts during growth stage. J Food Sci Technol. 2013;50:86–93. doi: 10.1007/s13197-011-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokida MK, Oreopoulou V, Maroulis ZB. Water loss and oil uptake as a function of frying time. J Food Engg. 2000;44:39–46. doi: 10.1016/S0260-8774(99)00163-6. [DOI] [Google Scholar]
- Labuza TP. Oxidative changes in food at low and intermediate moisture foods. In: Duckworth RB, editor. Water relation of foods. London: Academic Press; 1978. pp. 455–474. [Google Scholar]
- Larmond E. Laboratory methods for sensory evaluation of foods. Canada: Canada Department of Agricultural Publication, Ottawa; 1977. p. 1637. [Google Scholar]
- Li D, Li XL, Ding XL. Composition and antioxidative Properties of the flavonoid- rich fractions from tartary buckwheat grains. Food Sci Biotechnol. 2010;19(3):711–716. doi: 10.1007/s10068-010-0100-4. [DOI] [Google Scholar]
- Lin LY, Liu HM, Yu YW. Quality and antioxidant property of buckwheat enhanced wheat bread. Food Chem. 2009;112(4):987–991. doi: 10.1016/j.foodchem.2008.07.022. [DOI] [Google Scholar]
- Malav OP, Sharma BD, Talukder S, Kumar RR, Mendiratta SK. Quality characteristics and textural attributes of restructured chicken meat blocks extended with potato. Indian J Poult Sci. 2012;47(1):93–97. [Google Scholar]
- Monaco RD, Miele NA, Cavella S, Masi P. New chestnut-based chips optimization: effects of ingredients. LWT-Food Sci Technol. 2010;43:126–132. doi: 10.1016/j.lwt.2009.07.005. [DOI] [Google Scholar]
- Moreira RG, Sun X, Chen Y. Factors affecting oil uptake in tortilla chips in deep-fat frying. J Food Engg. 1997;31(4):485–498. doi: 10.1016/S0260-8774(96)00088-X. [DOI] [Google Scholar]
- Nourian F, Ramaswamy HS. Kinetics of quality changes during cooking and frying of potatoes: part 1. Texture. J Food Process Engg. 2003;26(4):377–394. doi: 10.1111/j.1745-4530.2003.tb00608.x. [DOI] [Google Scholar]
- Padmashree A, Sharma GK, Govindaraj T. Development and evaluation of shelf stability of flaxoat nutty bar in different packaging materials. Food Nutr Sci. 2013;4:538–546. [Google Scholar]
- Pedreschi F, Moyano P, Kaack K, Granby K. Color changes and acrylamide formation in fried potato slices. Food Res Int. 2005;38:1–9. doi: 10.1016/j.foodres.2004.07.002. [DOI] [Google Scholar]
- Prakash J, Naik HR, Hussain SZ, Singh B. Effect of processing conditions on the quality characteristics of barley chips. J Food Sci Technol. 2015;52(1):294–302. doi: 10.1007/s13197-013-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallenberger RS, Smith O, Treadway RH. Role of sugars in the browning reaction in potato chips. J Agric Food Chem. 1959;7:274–277. doi: 10.1021/jf60098a010. [DOI] [Google Scholar]
- Shyu S, Hau L, Hwang L. Effects of processing conditions on the quality of vacuum-fried carrot chips. J Sci Food Agric. 2005;85:1903–1908. doi: 10.1002/jsfa.2195. [DOI] [Google Scholar]
- Singh V, Mehta N, Chatli MK, Kumar P, Malav OP. Development of egg cutlets from whole egg liquid incorporated with mashed potato as binder and its economics of production. J Anim Res. 2016;6(4):619–628. doi: 10.5958/2277-940X.2016.00072.3. [DOI] [Google Scholar]
- Sobukola OP, Dairo OU, Afe TT, Coker OJ. Water sorption isotherms and crispness of fried yam chips in the temperature range from 293K to 313K. Int J Food Prop. 2007;10(3):561–575. doi: 10.1080/10942910601035538. [DOI] [Google Scholar]
- Thakur BR, Arya SS. Packaging requirement and stability of fried snacks (Trisnacks) J Food Sci Technol. 1990;27:76–81. [Google Scholar]
- Thakur S, Saxena DC. Formulation of extruded snack food (gum based cereal-pulse blend): optimization of ingredients levels using response surface methodology. LWT Food Sci Technol. 2000;33:354–361. doi: 10.1006/fstl.2000.0668. [DOI] [Google Scholar]
- Torbica A, Hadnadev M, Dapcevic HT. Rice and buckwheat flour characterization and its relation to cookie quality. Food Res Int. 2012;48:277–283. doi: 10.1016/j.foodres.2012.05.001. [DOI] [Google Scholar]