Abstract
The work attempted to study on separating the α′, α and β subunits of 7S globulin from soybean proteins by reverse micelles. The effects of six single factors (W0, time, temperature, pH, surfactant concentration and salt solution species) on three subunit partitioning were discussed. The main influence factors (time, temperature and surfactant concentration) were optimized by the response surface test (Box–Behnken design, three factor three level) in order to optimize the forward extracting three subunits of 7S globulin. It was observed that the independent factor had substantial effect on the forward extraction yield of three subunits of 7S globulin. The statistical analysis showed that the reverse micelles could significantly affect the forward extraction efficiency of protein subunits. The highest forward extraction efficiency of α′, α and β subunits achieved 78.21, 63.65 and 61.34%, respectively. The proposed method also showed that the forward extraction efficiency of subunits with larger molecular weight was higher than low molecular weight.
Keywords: Reverse micelles, Forward extraction, 7S globulin subunit, Optimization
Introduction
Soybean is probably the most ancient oilseed cultivated by mankind. This crop has been grown for centuries owing to its high quality protein content (40–45%) and oil content (17–21%). However, 7S globulin (β-conglycinin) is an important component in soybean proteins, which accounts for 36% of the total soybean proteins (Liu 1997). Most studies showed that 7S globulin had higher ability to improve the solubility and emulsion properties among soybean proteins (Fukushima 2001). The structure of β-conglycinin has been widely studied (Maruyama et al. 2001). It is a trimeric glycoprotein consisting of three subunits, α′ (Mw 83 kDa), α (Mw76 kDa) and β (Mw 53 kDa), which are linked by hydrophobic interactions and hydrogen bridging without disulphide (Liu 1997). It has been previously demonstrated that the physicochemical and functional properties of soybean proteins are affected not only by the protein composition, but also by protein subunits (James and Yang 2016). So it is very important that the subunits of β-conglycinin are studied.
Reverse micelle technology is based on microemulsion at surfactant concentration above the critical micelle concentration, which attracts much attention in food industry (Hong et al. 2015). Reverse micelles are nanometer sized aggregates by forming the surfactants in a non-polar solvents mixed with water (Leser and Luisi 1989). The core part in the reverse micelles is water microdomain, which is surrounded by organic phase (Hong et al. 2015). Some water-soluble compounds can be dissolved in the inner aqueous phase, such proteins and other biomolecules (Hong et al. 2015; Zhou et al. 2012). The large-scale separations of proteins/or other biomolecules in reverse micelles had been preferred as a attractive approach because of its various advantages, including enormous interfacial area, thermodynamically stable and optically transparent, lower cost, ease of scale up and simple control of the reaction variables et al. (Hong et al. 2015; Sereti et al. 2014).
The extraction process of molecules in reverse micelle system included forward and backward extraction steps. The protein molecules of samples could be solubilized into the water-core of reverse micelles during the forward-extraction step. The solution containing the proteins would be subsequently recovered from reverse micelles into a fresh aqueous solution during backward extraction (Leser and Luisi 1989). A number of investigations have indicated that many factors could affect the extraction yield of water soluble molecules in reverse micelles, such as nature and concentration of target proteins, pH and ionic strength et al. (Hong et al. 2015; Juang et al. 2012). Recently, soybean proteins obtained through AOT reverse micelles was reported, which resulted in a reasonable advantage in the aspect of bioconversion efficiency (Chen et al. 2014). In this respect, the extraction of subunits of 7S globulin could constitute an important first step for the investigation of the extraction yield, physicochemical and functional properties of 7S globulin and/or soybean proteins in reverse micelle system. However, little information is provided for the forward extraction yield of the corresponding subunits of soybean 7S globulin in reverse micelles.
This research focused on the reverse micelles following separation for the subunits of 7S globulin from soybean proteins. The primary purpose of this work was to investigate the influence of main factors on extraction yield of 7S globulin subunits. The six single factors were studied including water content (W0), surfactant concentration, extraction time and temperature, pH value and salt species.
Materials and methods
Materials
Bis (2-ethylhexyl) sulfosuccinate sodium (AOT) was supplied by sigma chemical Company [> 98% (purity); St. Louis, USA]. Bicinchoninic Acid (BCA) protein assay kit was purchased from USA Pierce Company (Rockford, IL., USA). Soybean separation protein was purchased from Yuwang Group (Yucheng, Shandong, China). The protein (by Kjeldhal, N × 6.25) content was above 90% (AOAC 2000). All other reagents were of analytical grade. All values were given in wt% of the total flour weight.
Isolation of 7S globulin subunits
7S globulin from soy protein isolates (SPI) was isolated according to the investigation method of Nagano et al. (1992) with slight modification. The α, α′ and β subunits from 7S globulin was separated according to the method of Zheng et al. (2009) with slight modification.
Forward extraction of α, α′ and β subunits from 7S globulin by AOT reverse micelles
α, α′ and β subunits of 7S globulin in AOT reverse micelles according to 1:20 (w/v) ratio were extracted at different reverse micelle concentrations (0.04, 0.06, 0.08, 0.10, 0.12 g L−1), salt solution (KCl, NaCl, MgCl2, BaCl2, CaCl2, NaNO3, KNO3, Na2SO4), pH (5.0, 6.0, 7.0, 8.0, 9.0), water content (W0 = 10, 13, 18, 20, 23), extract time (15, 30, 50, 80, 120 min) and extract temperature (25, 35, 45, 55, 65 °C). After completions of extraction, the extract was centrifuged at 4000 rpm for 10 min. The supernatant was collected and analyzed for the α, α′ and β subunit contents, which were measured by BCA method (microplate procedure, microplate reader 550: Japan Bio-RAD Company) (Smith et al. 1985). The forward extraction efficiency was calculated as following equation (Umesh Hebbar et al. 2008):
| 1 |
Experimental design for optimization by response surface methodology (RSM)
The optimization of the independent parameter was studied by RSM, and the Box–Behnken design (BBD) was applied in the prediction and verification of model equation (Swamy and Muthukumarappan 2017). Based on the results of single-factor experiment, three independent variables of this work were extract time (X1), extract temperature (X2), and surfactant concentrations (X3), which were selected at three levels (low, basal and high) coded (− 1, 0, + 1). The conditions of the independent variables studied were: extract time in the range (40–90 min, X1), extract temperature in the range (40–50 °C, X2), and surfactant concentrations among (0.07–0.11 g L−1, X3). The average extraction efficiency of subunits was taken as the response, Y. The coded values for the experimental design were given in Table 1. Three replicates at the center of the design were used to allow for the estimation of precision and reproducibility of the data. The each response value in subunit extraction trial was average of triple. The experimental results of the factorial designs were expressed by a second-order polynomial equation as follows:
| 2 |
where Y represented the response to be modelled; b0 was the constant coefficient, bi, bii, bij were the coefficient of linear effect, interaction effect and coefficients of squared effect, respectively. n is the number of variables, Xi and Xj were the independent variables (Vázquez et al. 2016).
Table 1.
Experimental conditions of the experimental design 33 setting in the original and coded and uncoded form independent variables and the forward extraction efficiency of α′, α and β subunits and three repetitions at the central point
| Run | α′ | α | β | Response (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 (°C) (temperature) | X2 (min) (time) | X3 (g ml−1) (AOT concentration) | X1 (°C) (temperature) | X2 (min) (time) | X3 (g ml−1) (AOT concentration) | X1 (°C) (temperature) | X2 (min) (time) | X3 (g ml−1) (AOT concentration) | α′ | α | β | |
| 1 | 50 (+ 1) | 90 (+ 1) | 0.1 (0) | 45 (0) | 70 (− 1) | 0.09 (+ 1) | 50 (+ 1) | 40 (− 1) | 0.1 (0) | 59.22 ± 0.36 | 52.33 ± 0.31 | 27.45 ± 0.22 |
| 2 | 45 (0) | 70 (− 1) | 0.11 (+ 1) | 50 (+ 1) | 80 (0) | 0.07 (− 1) | 40 (− 1) | 50 (0) | 0.09 (− 1) | 52.04 ± 0.21 | 43.34 ± 0.24 | 49.24 ± 0.23 |
| 3 | 45 (0) | 80 (0) | 0.1 (0) | 40 (− 1) | 70 (− 1) | 0.08 (0) | 45 (0) | 50 (0) | 0.1 (0) | 76.17 ± 0.19 | 51.07 ± 0.17 | 60.82 ± 0.25 |
| 4 | 45(0) | 80 (0) | 0.1 (0) | 45 (0) | 90 (+ 1) | 0.09 (+ 1) | 45 (0) | 50 (0) | 0.1 (0) | 75.22 ± 0.22 | 28.11 ± 0.26 | 60.01 ± 0.31 |
| 5 | 45 (0) | 90 (+ 1) | 0.09 (− 1) | 45 (0) | 80 (0) | 0.08 (0) | 40 (− 1) | 40 (− 1) | 0.1 (0) | 55.74 ± 0.18 | 64.06 ± 0.26 | 28.21 ± 0.31 |
| 6 | 45 (0) | 90 (+ 1) | 0.11 (+ 1) | 45 (0) | 70 (− 1) | 0.07 (− 1) | 45 (0) | 40 (− 1) | 0.09 (− 1) | 50.11 ± 0.19 | 51.19 ± 0.18 | 27.09 ± 0.28 |
| 7 | 45 (0) | 80 (0) | 0.1 (0) | 40 (− 1) | 80 (0) | 0.07 (− 1) | 50 (+ 1) | 50 (0) | 0.11 (+ 1) | 73.17 ± 0.33 | 38.12 ± 0.22 | 46.11 ± 0.28 |
| 8 | 45 (0) | 70 (− 1) | 0.09 (− 1) | 45 (0) | 80 (0) | 0.08 (0) | 50 (+ 1) | 60 (+ 1) | 0.1 (0) | 61.23 ± 0.27 | 62.56 ± 0.24 | 44.25 ± 0.19 |
| 9 | 50 (+ 1) | 80 (0) | 0.09 (− 1) | 45 (0) | 80 (0) | 0.08 (0) | 45 (0) | 60 (+ 1) | 0.11 (+ 1) | 67.03 ± 0.21 | 62.06 ± 0.18 | 43.76 ± 0.23 |
| 10 | 50 (+ 1) | 80 (0) | 0.11(+ 1) | 40 (− 1) | 80 (0) | 0.09 (+ 1) | 40 (− 1) | 60 (+ 1) | 0.1 (0) | 58.09 ± 0.25 | 40.07 ± 0.21 | 46.34 ± 0.25 |
| 11 | 40 (− 1) | 90 (+ 1) | 0.1 (0) | 45 (0) | 90 (+ 1) | 0.07 (− 1) | 45 (0) | 50 (0) | 0.1 (0) | 39.34 ± 0.26 | 36.43 ± 0.22 | 64.33 ± 0.27 |
| 12 | 40 (− 1) | 70 (− 1) | 0.1 (0) | 50 (+ 1) | 70 (− 1) | 0.08 (0) | 45 (0) | 50 (0) | 0.1 (0) | 42.99 ± 0.16 | 50.08 ± 0.23 | 58.09 ± 0.23 |
| 13 | 50 (+ 1) | 70 (− 1) | 0.1 (0) | 50 (+ 1) | 90 (+ 1) | 0.08 (0) | 40 (− 1) | 50 (0) | 0.11 (+ 1) | 61.12 ± 0.26 | 34.76 ± 0.16 | 47.01 ± 0.23 |
| 14 | 40 (− 1) | 80 (0) | 0.11(+ 1) | 45 (0) | 80 (0) | 0.08 (0) | 50 (+ 1) | 50 (0) | 0.09 (− 1) | 33.08 ± 0.18 | 59.02 ± 0.18 | 50.73 ± 0.27 |
| 15 | 45 (0) | 80 (0) | 0.1 (0) | 40 (− 1) | 90 (+ 1) | 0.08 (0) | 45 (0) | 60 (+ 1) | 0.09 (− 1) | 78.17 ± 0.21 | 30.44 ± 0.27 | 45.98 ± 0.23 |
| 16 | 45 (0) | 80 (0) | 0.1 (0) | 50 (+ 1) | 80 (0) | 0.09 (+ 1) | 45 (0) | 40 (− 1) | 0.11 (+ 1) | 76.04 ± 0.33 | 47.48 ± 0.25 | 23.87 ± 0.27 |
| 17 | 40 (− 1) | 80 (0) | 0.09 (− 1) | 45 (0) | 80 (0) | 0.08 (0) | 45 (0) | 50 (0) | 0.1 (0) | 43.41 ± 0.32 | 59.23 ± 0.22 | 57.22 ± 0.26 |
aMean ± SD of triplicate determinations from experiments
The quality of the fit of the polynomial model equation was established by the adjusted determination coefficient (R2adj). The statistical significance of the coefficients was checked by means of the Student t test (a = 0.05) and the model consistency by the Fisher F test (α = 0.05) for the model, which was significant at the 5% level (Vázquez et al. 2016).
Statistical analysis
Data for the extracts were reported as mean ± standard deviation (SD) of three replicates. The software Design Expert 8.0.7.1 (Stat Ease Inc., Minneapolis, USA) was used for regression analysis and analysis of variance (ANOVA). Differences at the 5% probability level (p < 0.05) were considered statistically significant.
Results and discussion
Influence of process variables
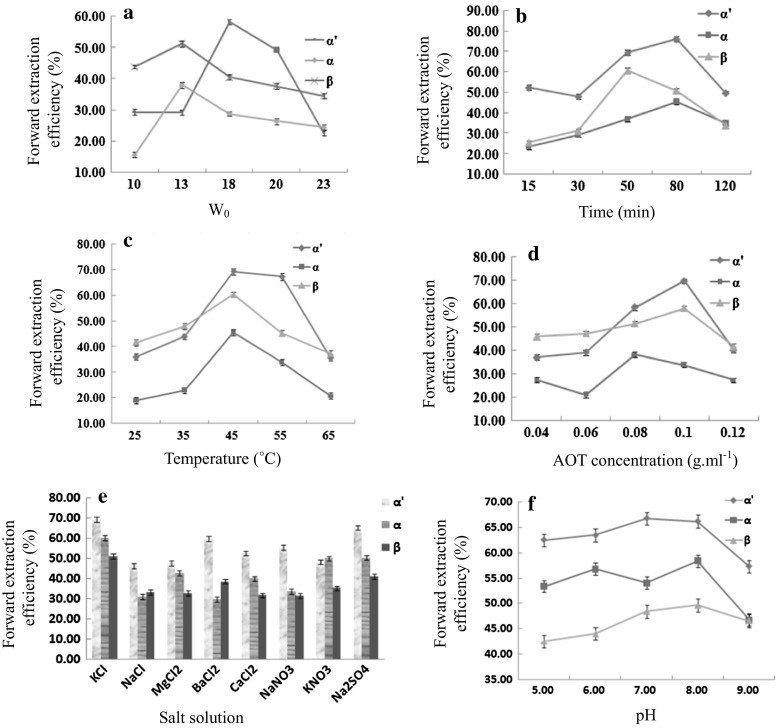
The effect of water content (W0) in reverse micellar phases on the forward extraction efficiency of subunits is shown in Fig. 1a. The forward extraction efficiency of α and β subunits from 7S globulin reached the highest at W0 13, which were 38.04% and 57.29%, respectively. However, the forward extraction efficiency of α′ subunit was the highest at W0 18, which was 58.38%. With the increase of W0, the reverse micelle size was greater, which led to the increase of the protein extraction yield. The results were agreed with the investigation of Göklen and Hatton (1987). The forward extraction efficiency of three subunits had significantly different (p < 0.05). It was noted that the molecular weight of α′ in three subunits was the largest, the extraction yield was also the highest. The result was different from some references, which reported that the molecular weight of proteins was the smaller, the forward extraction efficiency of protein was the higher (Göklen and Hatton 1987). The reason still is not still clear, which need be further studied.
Fig. 1.
Influence of the variables on forward extraction efficiency (a W0, b time, c temperature, d AOT concentration, e salt solution and f pH)
The effect of extraction time on forward extraction efficiency of subunits from 7S globulin is shown Fig. 1b. When extraction time reached 80 min, the forward extraction efficiencies of α′ and α subunits were the highest, which were 76.17% and 45.52%, respectively. While β subunit were 60.82% at 50 min. Then, the forward extraction efficiency would gradually level off. So it was suitable that the forward extraction time range of subunits from 7S globulin was from 50–80 min.
Figure 1c shows the influence of temperature on the forward extraction efficiency of subunits of 7S globulin. With the increase of temperature from 25 to 45 °C, the forward extraction efficiency of three subunits from 7S globulins gradually increased. The forward extraction yield of α′, α and β reached the highest at 45 °C, which were 69.42, 45.52 and 60.31%, respectively. Then, as the increase of temperature, the forward extraction efficiency clearly decreased. The reason was that the interaction between protein and micellar molecules in the reverse micelles would be enhanced at appropriate temperature. The water molecule movement would be accelerated, which was beneficial to the solubilization of protein in the reverse micelles (Noritomi et al. 2006). In addition, at very high temperature, the reverse micelle system could be destroyed, which resulted in the decrease of forward extraction efficiency of 7S globulin subunits (Hilhorst et al. 1992).
The effect of the surfactant AOT concentration on the forward extraction efficiency of subunits of 7S globulin is shown Fig. 1d. With AOT concentration from 0.04 to 0.08 g mL−1 or 0.1 g mL−1, the forward extraction efficiency of subunits increased. When the AOT concentration was above 0.1 g mL−1, the extraction yield of subunits would begin to decline. The extraction efficiency of α subunit reached the highest at AOT concentration 0.08 g mL−1, while the extraction efficiency of α′ and β subunits were highest at AOT concentration 0.1 g mL−1, which were 69.55% and 57.82%, respectively. The results were in agreement with the investigation of Chen et al. (2014). The reason was that the right increase of surfactant content in reverse micelles could lead to the increase of surface tension, which had higher interfacial activity. So the solubilization of protein would be improved (Krishna et al. 2002). But when AOT concentration was excessively high, the reverse micellar system had lager viscosity and lower surface tension, which led to the decrease of protein solubilization (Krishna et al. 2002).
Figure 1e shows the effect of salt species on the forward extraction efficiency of subunits from 7S globulin. The results showed that the forward extraction efficiency of subunits with different salt solutions in reverse micelles was also different. When KCl and Na2SO4 were added in reverse micelles, the forward extraction efficiency of subunits was significantly higher than other salt solution (p < 0.05). Especially, in reverse micelles with adding KCl solution, the extraction yield of α′, α and β subunits reached the highest, which were 69.42%, 60.06% and 51.05% respectively. The results were supported by the investigation of Chen et al. (2014). The reasons were that different salt ions had different electrostatic effect, which was closely related to the ionic strength of the aqueous phase, and affected the protein solubilization due to micelle size changes or screening of electrostatic interactions between proteins and micelle wall (Kinugasa et al. 2003).
The effect of aqueous pH on subunit solubilization of 7S globulin into the reverse micellar phase is shown in Fig. 1f. As the pH of aqueous phase increased from 5 to 7.0 or 8.0, the forward extraction efficiency of subunits increased. Maximum efficiency of α′ (66.78%) subunit was found at pH 7.0, while α and β subunits (58.39% and 49.64%, respectively) was found at pH 8.0. Then, with the increase of pH, the extraction yield of α′, α and β subunits decreased. pI values of α′, α and β subunits was 5.2, 4.9 and 5.7, respectively. It was interesting that the extraction of three subunits at pH was higher than the pI, the results were agreed with the investigation of Chen et al. (2014). Although the extraction protein mechanism using anion surfactants had not yet been elucidated, it was well known that these surfactants were compounds with negative charges. It was indicated the hydrophobic interaction or steric hindrance interaction of micelles and proteins would affect the extraction yield of 7S globulin subunits (Ronnie et al. 1989).
Optimization parameter analysis by response surface method (RSM)
The optimization of the extraction temperature, time and AOT concentration in terms of forward extraction efficiency of α′, α and β subunits from 7S globulin was carried out using an experimental plan based on a 33 full factorial central composite design. The corresponding response values of α′, α and β subunits from the experimental design are presented in Table 1. As be seen in Table 1, the forward extraction efficiency of α′, α and β subunits varied from 33.08 to 78.17%, 28.11 to 64.06% and 23.87 to 64.33%, respectively. The highest values of α′, α and β subunits were observed in the assays employing the temperature 45, 45 and 45 °C, time 80, 80 and 50 min, AOT concentration 0.1, 0.08 and 0.1 g mL−1, respectively. The forward extraction efficiency of α′ was significantly higher than other two subunits (p < 0.05).
The influence of critical factors and model efficiency was checked by ANOVA according to Fisher’s statistical analysis (Table 2). It was known that criterion for significant contribution of each variable was p < 0.05. The p values more than 0.05 indicated that model terms were not significant. From Table 2, the model F-value of 81.67, 30.68 and 57.53 from α′, α and β subunits indicated that the model was significant (Ma et al. 2010). In this case, X21, X22, and X23 for α′, α and β subunits were significant model terms. X1 and X3 for α′ subunit were significant model terms, X3 for α and β subunits was significant model term. The “Lack of Fit F-value” of α′, α and β subunits 1.17, 2.36 and 0.16, implied that the “Lack of Fit” was not significant, which suggested that the model adequately fitted the data.
Table 2.
Analysis of variance (ANOVA) for Box–Behnken design
| Source of variation | Sum of square | Degree of freedom | Mean square | F-value | p value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α′ | α | β | α′ | α | β | α′ | α | β | α′ | α | β | α′ | α | β | |
| Model | 3157.09 | 2098.37 | 2539.5 | 9 | 9 | 9 | 350.79 | 233.15 | 282.17 | 81.67 | 30.68 | 57.53 | < 0.0001 | < 0.0001 | < 0.0001 |
| X1 | 938.31 | 31.84 | 0.64 | 1 | 1 | 1 | 938.31 | 31.84 | 0.64 | 218.47 | 4.19 | 0.13 | < 0.0001 | 0.0799 | 0.7289 |
| X2 | 21.03 | 701.81 | 679.15 | 1 | 1 | 1 | 21.03 | 701.81 | 679.15 | 4.9 | 92.34 | 138.46 | 0.0626 | < 0.0001 | < 0.0001 |
| X3 | 145.27 | 0.15 | 18.88 | 1 | 1 | 1 | 145.27 | 0.15 | 18.88 | 33.82 | 0.02 | 3.85 | 0.0007 | 0.8928 | 0.0906 |
| X1 X2 | 0.77 | 7.05 | 0.44 | 1 | 1 | 1 | 0.77 | 7.05 | 0.44 | 0.18 | 0.93 | 0.09 | 0.6855 | 0.3676 | 0.7727 |
| X1 X3 | 0.48 | 1.2 | 1.43 | 1 | 1 | 1 | 0.48 | 1.2 | 1.43 | 0.11 | 0.16 | 0.29 | 0.7472 | 0.7031 | 0.6062 |
| X2 X3 | 3.17 | 22.37 | 0.25 | 1 | 1 | 1 | 3.17 | 22.37 | 0.25 | 0.74 | 2.94 | 0.051 | 0.4188 | 0.1299 | 0.8278 |
| X21 | 913.82 | 402.77 | 114.6 | 1 | 1 | 1 | 913.82 | 402.77 | 114.6 | 212.77 | 53 | 23.36 | < 0.0001 | 0.0002 | 0.0019 |
| X22 | 451.43 | 422.57 | 1412.3 | 1 | 1 | 1 | 451.43 | 422.57 | 1412.3 | 105.11 | 55.6 | 287.94 | < 0.0001 | 0.0001 | < 0.0001 |
| X23 | 474.84 | 368.33 | 183.66 | 1 | 1 | 1 | 474.84 | 368.33 | 183.66 | 110.56 | 48.46 | 37.44 | < 0.0001 | 0.0002 | 0.0005 |
| Residual | 30.06 | 53.2 | 34.33 | 7 | 7 | 7 | 4.29 | 7.60 | 4.90 | ||||||
| Lack of fit | 17.01 | 33.97 | 3.58 | 3 | 3 | 3 | 5.67 | 11.32 | 1.19 | 1.74 | 2.36 | 0.16 | 0.2972 | 0.2131 | 0.9211 |
| Pure error | 13.05 | 19.23 | 30.75 | 4 | 4 | 4 | 3.26 | 4.81 | 7.69 | ||||||
| Total error | 3187.15 | 2151.57 | 2573.84 | 16 | 16 | 16 | |||||||||
From Table 3, for the model fitted, the coefficients of determination (R2) of α′, α and β subunits could check the goodness of a model, were 0.9906, 0.9753 and 0.9867, respectively. It implied that at least 95% of experimental data suited the model. The adjusted-R2 of α′, α and β subunits were 0.9784, 0.8502 and 0.9695, respectively, which indicated high correlation of the actual and predicted values. Adequacy precision determined the signal of noise ration. The ratio was greater than 4, which were desirable (Swamy and Muthukumarappan 2017). The present ration of 25.66, 9.34 and 21.15 suggested adequate signal. This model could be applied to navigate the design space. Swamy et al. (2014) reported that the coefficient of variation (CV) represented the deviation of the actual points from the predicated ones and a low coefficient of variation, which showed the least variation in the mean value. The CV values of α′, α and β subunits were 3.52, 8.33 and 4.82, respectively, which indicated high precision and reliability of the experiments.
Table 3.
The credibility analysis of the regression equations
| Index mark | Average forward extraction efficiency | ||
|---|---|---|---|
| α′ | α | β | |
| SD | 2.07 | 4.01 | 2.21 |
| Mean | 58.95 | 48.22 | 45.91 |
| C.V.(%) | 3.52 | 8.33 | 4.82 |
| Adequacy precision | 25.66 | 9.34 | 21.15 |
| Press | 292.56 | 1807.57 | 105.34 |
| R-squared | 0.9906 | 0.9344 | 0.9867 |
| Adjust R-squared | 0.9784 | 0.8502 | 0.9695 |
| Predicted R-squared | 0.9082 | − 0.0489 | 0.9591 |
CV coefficient of variation
The final average forward extraction efficiencies of α′, α and β subunits (Y) were given by following equation:
| 3 |
| 4 |
| 5 |
Influence of the independent factor in the extraction process
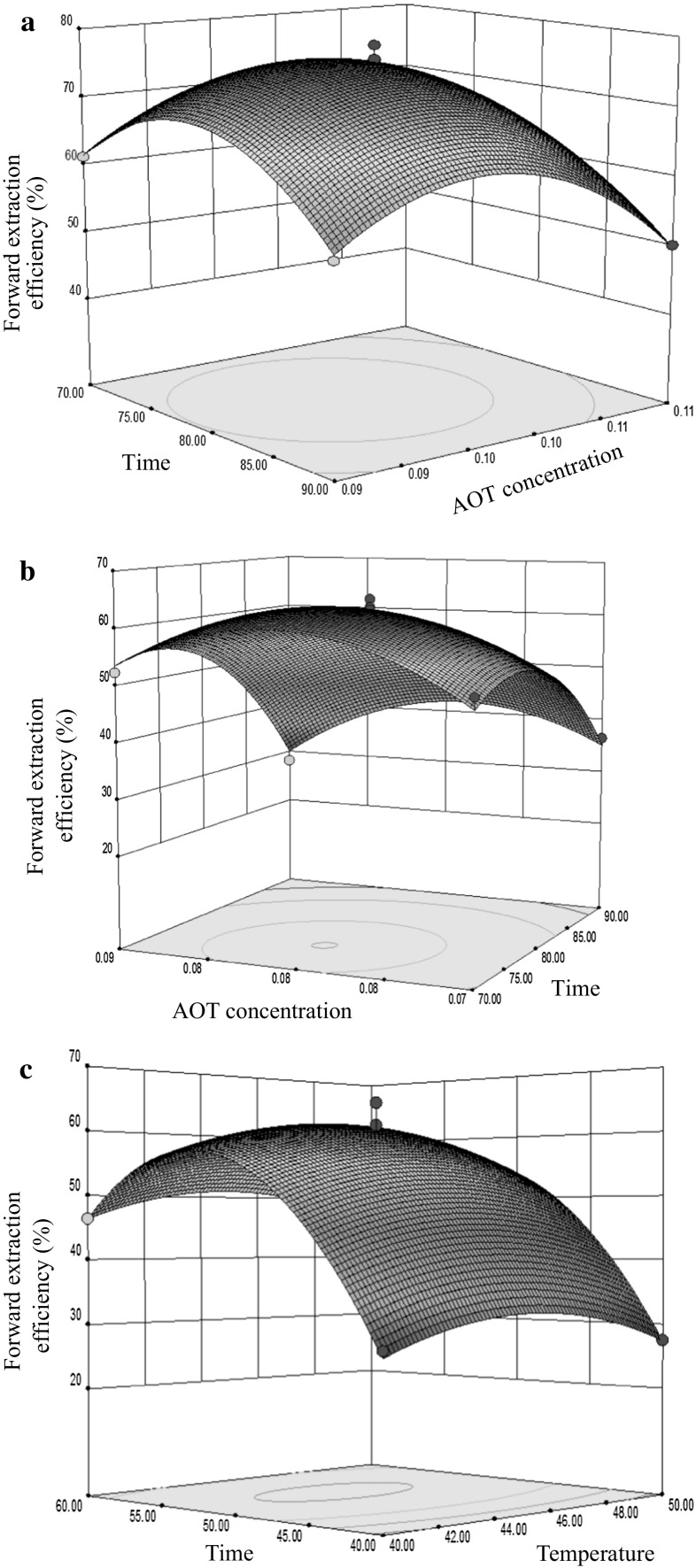
The impact of the independent factor on the extraction procedure was analyzed by three dimensional response surface plots, which showed the impact of two factors on the process while holding the third variable constant. Figure 2a–c show the response surfaces for the effect of independent variable on forward extraction efficiency of three subunits. The curve nature of the response surface methodology (RSM) figure shows the presence of strong interaction between variables. The surface plots in Fig. 2a–c showed that under lower extraction time, extraction temperature and AOT concentration, the forward extraction efficiency of three subunits was lower. Figure 2a, b present the interaction of extraction time and AOT concentration during extracting α′ and α subunits. Figure 2a, b initially showed increasing the partitioning of α′ and α subunits with the increment of extraction time and AOT concentration, but the drop of partitioning coefficient was observed at longer extraction time and higher AOT concentration. It suggested a significant difference in the partition coefficient at low and high level of the extraction time and AOT concentration. The reasons of the extraction yield change could be due to as following factors. There were the hydrophobic interactions or interfacial resistance between α′ and α subunits and the reverse micelle system forming components, which were probably responsible for the selective partitioning of them to the reverse micelle phase (Hagen et al. 1990). The water-head group localized in the interior of reverse micelles resulted in the interaction of surfactant (AOT) and protein molecule, which could promote the partition of proteins (Hagen et al. 1990). The α′ and α subunits had very high surface hydrophobicity, which could promote the partition of the proteins to the reverse micelle phase (Andrews and Haywood 1994). The addition of surfactant also caused an electrical potential in reverse micelles that were able to drive proteins into reverse micelles depending on their charge. Such as, proteins with positive charge were partitioned more to the reverse micelles with negative charge surfactant (Ding et al. 2016). In this case, the pH of the operating system during the forward extracting was maintained at pH 7 or 8, which was higher than the pI of the α′, α and β subunits. It was presumed that the hydrophobic effects or interfacial resistance were the driving forces during extracting α′, α and β subunits using AOT reverse micelle phase (Paradkar and Dordick 1994). The protein partitioning was also aided by the temperature beside of the forces of surfactant, as most proteins strongly favored the higher temperature in reverse micelle phase (Dekker et al. 1991).
Fig. 2.
Three dimensional graphic surface optimisation—effect of AOT concentration and time on α′ (a), α (b), β (c) subunit forward extraction efficiency
Plot Fig. 2c illustrates that extraction time and temperature have a strong way in the process of extracting β subunit. The graph initially showed increasing the partitioning of β subunit with the increment of extraction time and temperature, but the drop of partitioning coefficient was observed at longer extraction time and higher temperature. It suggested a significant difference in the partition coefficient at low and high level of the extraction time and temperature. The reasons were that the molecule motion with the increase of temperature in reversed micelles enhanced, the partitioning coefficient of proteins would increase at certain extraction time (Dekker et al. 1991). So the forward extraction efficiency of β subunit increased.
Optimal solutions for α′, α and β subunits
For the optimization of extraction factors from α′, α and β subunits, the BBD method as optimal design for the desired response of the system was employed. The extraction parameters of α′, α and β subunits via reverse micelles were optimized based on the usage of KCl 0.05 mol L−1, while extraction time, temperature and AOT concentration was in the range of experimentation. The system pH and W0 were maintained with buffer at pH 7 or 8 and W0 13 or 18. The optimized input variables were attained. The forward extraction efficiency of α′ subunit reached 78.21% under 0.1 g mL−1 AOT concentration, 47 min extraction time, 47 °C extraction temperature; α subunit reached 63.65% under 0.08 g mL−1 AOT concentration, 75 min extraction time, 45 °C extraction temperature; β subunit reached 61.34% under 0.1 g mL−1 AOT concentration, 53 min extraction time, 45 °C extraction temperature.
Authentication of optimized solutions and predictive model
For validation of the model, the experiment was carried out at the optimum conditions within the experimental range obtained from the above study, and the extracted subunit was determined. The experimental data were compared with the predicted data in order to determine the validity of the model. Under the predicted conditions, the fitness of the model equations was examined and to verify the validity of the optimized solutions, experiments were carried out.
The results showed that the efficiency of the forward extraction of α′, α and β subunits by the experimental operation was 78.21 ± 1.21%, 63.65 ± 1.26% and 61.34 ± 1.21%, respectively. These values were compared with the anticipated numbers, the validation results were within 95% of forecast values. So, it appeared that the quadratic models were well-suited and the optimal values were valid inside the range of factor levels selected. Comparing the results of three subunits obtained through AOT reverse micelles, it was seen that the forward extraction efficiency of three subunits significantly different (p < 0.05), the value α′ subunit was the highest. It indicated that the reverse micelles had greater influence on the extraction efficiency of α′ subunit. The reason probably was that the strength of interaction between α′ subunit and surfactant was higher than other two subunits, or the interfacial resistance strength in the process of extracting α′ subunit could decrease (Ding et al. 2016).
Conclusion
This study focused on the optimization of the α′, α and β subunit forward extraction of 7S globulin from soybean proteins in reverse micelles by BBD design, and evaluated the effect of the reverse micelles on the three subunits extraction yield. The experimental data indicated that the independent factor had a marked effect on the forward extraction procedure and RSM plots assessed the interactive effect input factors on the extraction. The polynomial repression model was offered to reasonably depict the experimental results. Be based on the proposed model, the optimal condition for α′ subunit forward extraction yield was at 0.1 g mL−1 AOT concentration, 47 min extraction time, 47 °C extraction temperature; α subunit was 0.08 g mL−1 AOT concentration, 75 min extraction time, 45 °C extraction temperature; β subunit was 0.1 g mL−1 AOT concentration, 53 min extraction time, 45 °C extraction temperature. At these conditions, the predicted α′, α and β subunit forward extraction yield were 78.17, 64.06 and 64.33%, respectively, which had a good correlation with experimental values (78.21, 63.65 and 61.34%, respectively). Under the optimized conditions, the forward extraction efficiency of α′ subunit was the highest comparing with α and β subunits. It indicated that the reverse micelles were beneficial to extracting α′ subunit, which straightly affected the forward extraction efficiency of soybean proteins. Different forward extraction yield of subunits in reverse micelles would change the content of different subunits of 7S globulin, which resulted in the changes of structure or functional properties of soybean proteins. During the extraction subunits of reverse micelles, the hydrophobic interaction or interfacial resistance might play important roles in transporting α′, α and β subunits.
Acknowledgements
Financial support of this work by National Natural Science Foundation of China (Grant No. 21406133) and National Key Technology Support Program of China (2015BAD29B04).
References
- Andrews BA, Haywood K. Effect of pH, ion type and ionic strength on partitioning of proteins in reverse micelle systems. J Chromatogr A. 1994;66:55–60. doi: 10.1016/0021-9673(94)80091-X. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 17. Arlington: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Chen J, Chen FL, Wang XC, Zhao XY, Ao Q. Forward and backward transport processes in the AOT/hexane reversed micellar extraction of soybean protein. J Food Sci Technol. 2014;51(10):2851–2856. doi: 10.1007/s13197-012-0801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker M, Van’t Riet K, Van Der Pol Jan WA, Baltussen JJ, Riet Hilhorst RHBH. Effect of temperature on the reversed micellar extraction of enzymes. Chem Eng J. 1991;46(3):B69–B74. doi: 10.1016/0300-9467(91)87007-W. [DOI] [Google Scholar]
- Ding X, Cai J, Guo X. Extraction of ovalbumin with gemini surfactant reverse micelles—effect of gemini surfactant structure. Sep Purif Technol. 2016;158:367–373. doi: 10.1016/j.seppur.2015.12.042. [DOI] [Google Scholar]
- Fukushima D. Recent progress in research and technology on soybeans. Food Sci Technol Res. 2001;7(1):8–16. doi: 10.3136/fstr.7.8. [DOI] [Google Scholar]
- Göklen KE, Hatton TA. Liquid-liquid extraction of low molecular-weight proteins by selective solubilization in reversed micelles. Sep Purif Technol. 1987;22:831–841. [Google Scholar]
- Hagen AJ, Hatton TA, Wang D. Protein refolding in reversed micelles: interactions of the protein with micelle components. Biotechnol Bioeng. 1990;35(10):966–975. doi: 10.1002/bit.260351003. [DOI] [PubMed] [Google Scholar]
- Hilhorst R, Fijneman P, Heering D, Wolbert RBG, Dekker M, Riet KV, Bijsterbosch BH. Protein extraction using reversed micelles. Pure Appl Chem. 1992;64:1765–1770. doi: 10.1351/pac199264111765. [DOI] [Google Scholar]
- Hong SC, Park KM, Son YH, Jung HS, Kim K, Choi SJ, Chang PS. AOT/isooctane reverse micelles with a microaqueous core act as protective shells for enhancing the thermal stability of Chromobacterium viscosum lipase. Food Chem. 2015;179:263–269. doi: 10.1016/j.foodchem.2015.01.120. [DOI] [PubMed] [Google Scholar]
- James AT, Yang A. Interactions of protein content and globulin subunit composition of soybean proteins in relation to tofu gel properties. Food Chem. 2016;194:284–289. doi: 10.1016/j.foodchem.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Juang RS, Chen HL, Tsao SC. Recovery and separation of surfactin from pretreated Bacillus subtilis broth by reverse micellar extraction. Biochem Eng J. 2012;61:78–83. doi: 10.1016/j.bej.2011.12.008. [DOI] [Google Scholar]
- Kinugasa T, Kondo A, Mouri E, Ichikawa S, Nakagawa S, Nishii Y, Watanabe K, Takeuchi H. Effects of ion species in aqueous phase on protein extraction into reversed micellar solution. Sep Sci Technol. 2003;31:251–259. [Google Scholar]
- Krishna SH, Srinivas ND, Raghavarao KSMS, Karanth NG. Reverse micellar extraction for downstream processing of proteins/enzymes. Adv Biochem Eng/Biotechnol. 2002;75:119–183. doi: 10.1007/3-540-44604-4_5. [DOI] [PubMed] [Google Scholar]
- Leser ME, Luisi PL. The use of reverse micelles for the simultaneous extraction of oil and proteins from vegetable meal. Biotechnol Bioeng. 1989;34:1140–1146. doi: 10.1002/bit.260340904. [DOI] [PubMed] [Google Scholar]
- Liu K. Soybeans: chemistry, technology, and utilization. New York: Chapman & Hall; 1997. p. 532. [Google Scholar]
- Ma TZ, Wang Q, Wu HW. Optimization of extraction conditions for improving solubility of peanut protein concentrates by response surface methodology. LWT Food Sci Technol. 2010;43(9):1450–1455. doi: 10.1016/j.lwt.2010.03.015. [DOI] [Google Scholar]
- Maruyama N, Adachi M, Takahashi K, Yagasaki K, Kohno M, Takenaka Y, Okuda E, Nakagawa S, Mikami B, Utsumi S. Crystal structures of recombinant and native soybean beta-conglycinin beta homotrimers. Eur J Biochem. 2001;268(12):3595–3604. doi: 10.1046/j.1432-1327.2001.02268.x. [DOI] [PubMed] [Google Scholar]
- Nagano T, Hirotsuka M, Mori H, Kohyama K, Nishinarit K. Dynamic viscoelastic study on the gelation of 7S globulin from soybeans. J Agric Chem. 1992;40:941–944. doi: 10.1021/jf00018a004. [DOI] [Google Scholar]
- Noritomi H, Kojima N, Kato S, Nagahama K. How can temperature affect reverse micellar extraction using sucrose fatty acid ester. Colloid Polym Sci. 2006;284:683–687. doi: 10.1007/s00396-005-1452-9. [DOI] [Google Scholar]
- Paradkar VM, Dordick JS. Mechanism of extraction of Chymotrypsin into isooctane at very low concentrations of aerosol OT in the absence of reversed micelles. Biotechnol Bioeng. 1994;43:529–540. doi: 10.1002/bit.260430614. [DOI] [PubMed] [Google Scholar]
- Ronnie BG, Wolbert RH, Gijsbert V, Nachtegaal H, Dekker M, Van’t Riet K, Bijsterbosch BH. Protein transfer from an aqueous phase into reversed micelles the effect of protein size and charge distribution. Eur J Biochem. 1989;184:627–633. doi: 10.1111/j.1432-1033.1989.tb15059.x. [DOI] [PubMed] [Google Scholar]
- Sereti V, Zoumpanioti M, Papadimitriou V, Pispas S, Xenakis A. Biocolloids based on amphiphilic block copolymers as a medium for enzyme encapsulation. J Phys Chem B. 2014;118(32):9808–9816. doi: 10.1021/jp504449y. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartener FH, Rovenzano MDP, Fujimoto EK, Goeke NM, Olson BJ, Klenk KC. Measurement of protein using bichinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Swamy GJ, Muthukumarappan K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017;220:108–114. doi: 10.1016/j.foodchem.2016.09.197. [DOI] [PubMed] [Google Scholar]
- Swamy GJ, Sangamithra A, Chandrasekar V. Response surface modeling and process optimization of aqueous extraction of natural pigments from Beta vulgaris using Box–Behnken design of experiments. Dyes Pig. 2014;111:64–74. doi: 10.1016/j.dyepig.2014.05.028. [DOI] [Google Scholar]
- Umesh Hebbar H, Sumana B, Raghavarao KSMS. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresour Technol. 2008;99:4896–4902. doi: 10.1016/j.biortech.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Vázquez JA, Blanco M, Fraguas J, Pastrana L, Pérez-Martín R. Optimisation of the extraction and purification of chondroitin sulphate from head by-products of Prionace glauca by environmental friendly processes. Food Chem. 2016;198:28–35. doi: 10.1016/j.foodchem.2015.10.087. [DOI] [PubMed] [Google Scholar]
- Zheng HG, Yang XQ, Ahmad I, Min W, Zhu JH, Yuan DY. Soybean β-conglycinin constituent subunits: isolation, solubility and amino acid composition. Food Res Int. 2009;42:998–1003. doi: 10.1016/j.foodres.2009.04.018. [DOI] [Google Scholar]
- Zhou B, Wan JF, Wang JA, Cao XJ. Effect of chaotropes in reverse micellar extraction of kallikrein. Process Biochem. 2012;47:229–233. doi: 10.1016/j.procbio.2011.10.033. [DOI] [Google Scholar]