Abstract
Noodles are popularising among all age groups and all region throughout the World. To cater the demand of consumer noodles should be shelf-stable. Noodles prepared from chemically modified ingredients were studied for their keeping quality at two different conditions namely, ambient (27 °C, 65% RH) and accelerated (37 °C, 92% RH) for the period of 180 days (6 months). Samples were withdrawn at regular intervals and analysed for their physico-chemical and nutritional parameters. Results showed that Maximum cooking loss was observed in GP–OCT (180 days) 5.9% and was with in the acceptable range (8%). Firmness of noodles increased on storage up to 60 days later reduced. Starch digestibility increased 5–8% in all the samples upon storage. EGI of samples increased 10–15% on storage. Samples prepared with chemically modified ingredients were acceptable till the end of 180 days (2 samples) with good acceptability and low EGI without affecting its quality. Hence, it can be concluded that noodles prepared with modified ingredients using chemicals are shelf-stable up to 6 months at ambient condition.
Keywords: Low glycemic index, Noodles, Shelf-life, Modified ingredients
Introduction
Noodle products are becoming progressively popular all over the world, because of its ease of preparation and good palatability. It is consumed by most of the population irrespective of age, region and life-style (Bui and Small 2009). As noodles are mainly formulated using refined wheat four, it is not recommended for the people with diabetes. Because of its high glycemic index (GI) effect. For them low glycemic foods will be beneficial as there will be a gradual release of glucose to the blood (Thompson et al. 1984). As noodles categorised as high GI because they are sheeted and cut to strands, entrapment of starch in proteins is absent as in case of pasta, which is low GI. To cater the need of consumers with diabetes, low GI noodles are in demand. To fulfil the demand, noodles should be shelf-stable for long time. During storage period it should not lose its properties, so studying the shelf-life of the noodles is very essential. Any food product which is reaching consumers should be tested for its shelf-life before entering to the market (Gacula 1975). Shelf-life studies are conducted on all the food groups and types, including from solid, semi-solid and liquid foods. For different samples there will be different storage techniques, mainly the samples will be stored to simulate the process during transport and on shelf in market. Food can be exposed to several external conditions such as humidity, temperature, light and atmospheric oxygen (Kongkachuichai et al. 2007). Prepared noodles with respective health benefits should be shelf stable to maximum extent without deteriorating its quality characteristics until it reaches the consumers is an important task (Polydera et al. 2003). Pasta and noodle products can be stored up to 8–12 months with less deterioration in the quality characteristics (Kruger et al. 1996). As these products were dried and packed, the stability of the product has been increased to the maximum extent. For many food products in the market there will be allowed preservative levels which are regulated by the particular government of the country. These preservatives increase the shelf-life of the product especially semi-solid and liquid foods and also have minor effect on the human healthy at when consumed regularly at long run (Kruger et al. 1996). So, for the dried products such as pasta and noodles products do not require preservative, unless it is given in the form of fresh noodles (yellow alkaline noodles) (Konik et al. 1994). On basis of the literature and regular practice storage of the noodles will be performed in two conditions (ambient and accelerated) for dried noodles and refrigerated conditions for fresh noodles. Objective of the present study is to analyse the shelf-life of the noodles with modified ingredients and to determine its physico-chemical and nutritional aspects influenced by storage period.
Materials and method
Procurement and pre-processing of raw materials
All the ingredients required such as rajma beans, oats, Triticum durum wheat and Triticum dicoccum wheat were procured from local market were cleaned to remove the foreign particles and were ground in local chakki mill to suitable particle size flours. Guar pods (cluster beans) was procured from the market, cleaned and blanched according to the procedure of Ranganna (1986), cut into smaller portions and dried in a cabinet tray drier and ground in a lab mill to fine powder (100 µm). All the flours obtained were sieved and stored at air-tight packages until the end of the study. Enzymes such as pepsin, invertase, amyloglucosidase and pancreatic α-amylase were procured from Sigma Chemicals, USA. All other chemicals used for modification were of analytical grade unless and otherwise mentioned.
Modification of ingredients by altering pH and Esterification using octenyl–succinic anhydride and succinic anhydride
Optimized levels of the low GI ingredients were set and taken for modification on basis of preliminary studies on modified ingredients (Bharath Kumar and Prabhasankar 2017). For chemical modification by altering pH sodium bicarbonate was used. Succinic anhydride and octenylsuccinic anhydride were used to esterify the ingredients. Modification procedure was adopted by Bharath Kumar and Prabhasankar (2017). In short; optimized formulation blends were brought to alkaline pH while mixing in the Spar mixer with the addition of sodium bicarbonate, dough was checked frequently for stable pH using pH analyzer. For esterification (Han and BeMiller 2007) octenylsuccinic anhydride (3.0% of the weight of starch in the sample) and succinic anhydride (4% starch basis) were added, by maintaining the pH of the dough at 8.5. Low GI ingredients such as T. dicoccum wheat flour and rajma flour were modified by altering the pH to 9.0 (DI-9.0 and RF-9.0 respectively), oats flour is esterified with succinic anhydride (OF–SUC) and Guar powder is esterified with octenylsuccinic anhydride (GP–OCT). With these a control was formulated consisting of T. durum wheat flour.
Noodle formulation and optimization
On basis of the preliminary analysis carried out on noodles with modified ingredients five variants of noodles (including Control) were taken for the shelf-life study. These selected samples were analysed for different quality parameters at selected withdrawal period. Noodles were prepared using Lab Scale Noodle making machine (Imperia Restaurant-RM 220, Italy) adapting the procedure used by Bharath Kumar and Prabhasankar (2015). Later these dried noodles were taken for shelf-life study.
Shelf-life study
Prepared noodles were packed in HDPE bags and stored under two different conditions namely, ambient (27 °C, 65% RH) and accelerated (37 °C, 92% RH) conditions for the period of 180 days (6 months). Ambient condition represents room temperature storage in which product will retain its quality characteristics to maximum extent. Whereas, in case of accelerated condition, due to elevated temperature and humidity product behave differently. To understand the effect of these elevated conditions on the product quality parameters accelerated condition storage was planned. Samples stored at these conditions were analysed for its physico-chemical, sensory and nutritional characteristics to apprehend the influence of storage on the samples. Samples (placed at both ambient and accelerated) were withdrawn at 30 days interval to perform the above mentioned analyses. Noodles stored at both ambient and accelerated conditions were withdrawn at an interval of 1 month and analysed. Analyses were completed within 3–4 days of withdrawal, in the order accelerated samples and ambient samples.
Cooking quality
Cooking quality characteristics of noodles were analysed for each withdrawal of all the samples to determine the influence of storage on the cooking behaviour and cooking loss. Noodles were subjected to cooking quality evaluation adopting the standard method from AACC (66-50) (AACC 2000). Noodles were cut into approximately 5 cm in length. 25 g of the samples were weighed and placed in 250 ml of boiling water. Soon after that time was noted to know the cooking time of the noodles. Noodles were examined at particular intervals by placing between two glass petri plates and observed the absence of white core portion in case of dried noodles, indicating complete cooking of noodles. Total time taken to get completely cooked was recorded. Later the gruel was drained and analysed for the solid leach out analysis. Samples were analysed for its colour, texture and sensory quality characteristics soon after preparation.
Colour measurement
Noodles were analysed for its colour parameters using Lab scan-XE (Reston, USA). The colour instrument was equipped with D-65 illuminant with a 2° angle view and with a 2 mm width slit adopting the method used by Bharath Kumar and Prabhasankar (2015). The parameters analysed for the noodle samples includes L*, a* and b*. Where, L* value indicating the lightness (L = 100)/darkness (L = 0) dimensions, a* value indicating redness (+ ve) to greenness (− ve) and b* value indicating yellowness (+ ve) to blueness (− ve) of the samples (Hutchings 1994) The obtained values for the noodles were taken for the analysis of pasta colour index (PCI). For the analysis of PCI, L* (a lightness indicator) and b* (a yellowness indicator) were taken to get the standard colour index of the pasta. When the pasta incorporated with other ingredients (modified or unmodified) the PCIm was calculated using the values L*, a* and b* as explained by Ugarcic-Hardi et al. (1999) using the formulae:
For pasta colour index: For modified pasta colour index:
Firmness
Firmness of the noodles (both fresh and dried) was measured using the Texture analyser TA-XDi (Stable Micro Systems, UK) equipped with Warner–Bratzler blade for shear. Five noodles with distinct strands were selected and placed adjacent to each other on the sample plate and sheared using the blade mentioned above. Parameters used for the analysis are; Load cell: 250 kg; Pre-test speed: 1 mm/sec; Test speed: 1.67 mm/sec; Post-test speed: 10 mm/sec; Trigger force: 0.39 N. Peak force required for shearing of the noodles was recorded in Newton (N/mm). The average of five replicates was reported. The data thus obtained were analyzed statistically using Duncan’s Multiple Range Test (DMRT) (Duncan 1955).
Sensory analysis
Prepared noodles were evaluated for its quality characteristics and for its acceptance. Panelists who regularly participate in evaluating the noodles were selected (male and female: 10–15). Quantitative descriptive analysis (QDA) method to analyze the products was used for the study (Bharath Kumar and Prabhasankar 2015).
In vitro starch digestibility (IVSD)
In vitro digestibility of starch was analysed using the modified method used by Bharath Kumar and Prabhasankar (2015). In brief; Freeze dried and ground sample (50 mg) was dispersed in 4 ml of sodium acetate buffer (pH 4.6, 0.4 M) containing Amyloglucosidase was incubated in water bath for 45 min at 60 °C. Later enzyme was inactivated by placing the tubes in boiling water bath for 15 min. The tubes were cooled to room temperature and then centrifuged at 5000 rpm for 10 min. Supernatant was measured for its glucose content using a glucose oxidase–peroxidase (GOD–POD) kit (Autospan, Span Diagnostics limited, India). Absorption was measured at 505 nm and the glucose concentration was converted into starch content using a conversion factor 0.9. Each sample was analysed in triplicates.
Estimated glycemic index (EGI)
In vitro glycemic index was analysed using the method of Englyst et al. (1992). 50 mg of sample was cooked in 5 ml of distilled water for 3–5 min. Ten ml of hydrochloric acid and potassium chloride (HCl–KCl) buffer (pH 1.5) was added to the cooked sample with 0.2 ml of pepsin solution (1 g pepsin in 10 ml HCl–KCl buffer). This was incubated at 40 °C for 1 h at shaking water bath. After incubation the mixture was made up to 25 ml with Tris-Maleate buffer (pH 6.5) with the enzyme solution α-amylase (5 µl–2.6 U). The reaction mixture was incubated at 37 °C in a shaking water bath maintaining the constant shaking for the continuous reaction. During incubation an aliquot of 1 ml in duplicates were withdrawn from the mixture at 30, 60, 90, 120, 150 and 180 min time intervals into different tubes. These tubes were immediately kept in boiling water bath (100 °C) for 5 min to inactivate the enzyme activity and stored in refrigerator until the end of the incubation time (180 min). At 180 min the tubes were removed from the shaking water bath and the enzyme was inactivated. Later all the tubes from the refrigerator were removed and equilibrated to 60 °C in the water bath set at the same temperature. To the tubes 3 ml of sodium-acetate buffer (0.4 M, pH 4.7) was added with 60 µl of amyloglucosidase enzyme solution. These tubes were incubated at 60 °C for 45 min at shaking water bath. Later volume of the reaction mixture was adjusted to 10 ml with distilled water. An aliquot of 0.5 ml was incubated with GOD–POD reagent to estimate the glucose content in each withdrawal.
The values obtained for the starch hydrolysis were plotted against different time intervals. The hydrolysis index (HI) was calculated considering the area under the hydrolysis curve expressed as a percentage ratio of area under the curve for the test food and standard food (white bread).
Estimated glycemic index (EGI) was calculated using the values obtained for HI and substituting in the following equation stated by Goni et al. (1997):
Starch profile analysis
Analysis of in vitro rapidly digestible starch (RDS), slowly digestible starch (SDS), resistant starch (RS) and total starch (TS) were analysed and also free glucose (FG) and total glucose (TS) were determined using the method of Englyst et al. (1992). Conversion factor used was 0.9 to convert glucose to starch. Each sample was analysed in triplicates.
RDS, SDS, RS and TS were calculated using the following equations;
where, G20 is the value of glucose hydrolysed during the first 20 min of in vitro digestion, G120 is the value of glucose hydrolysed after 120 min of in vitro digestion.
Microstructural characterization
Microstructural characterization was performed for samples stored till 180 days (6 months) to understand the interaction of the matrix and changes during storage. Only the samples which are acceptable and had a promising result in the in vitro analyses were taken for the analysis as per the procedure given by Bharath Kumar and Prabhasankar (2017).
Statistical analysis
The mean scores of individual attributes of all the tests were calculated according to DMRT (Duncan 1955) to the data to find the significance difference between mean values of the samples using Statistica 99, V 5.5, Stat Soft, USA.
Results and discussion
Results of the shelf-life study are discussed in this section, including the effect of modification on the noodle quality characteristics. Noodles were analysed during different time intervals. Noodles were also analysed for the changes in cooking quality, physico-chemical, sensory and nutritional properties.
Analysis of initial sample (0 day)
Initial samples were analysed before proceeding to the storage process in two different conditions. Analysis includes chemical composition, physico-chemical, sensory, microstructural and nutritional analysis.
Chemical composition analysis (Table 1) revealed that with modification there was a reduction in moisture content of some samples. This may be due to the modification and processing steps, which alters the internal structural arrangement and also loss of moisture in the samples. Proximate analysis resulted (Table 1) higher moisture in DI (9.0) with 8.2%, protein in RF (9.0) with 1.4%, fat in DI (9.0), OF (SUC) with 1.19%, 1.14% respectively and ash in DI (9.0) with 1.59% compared to control and other samples. Cooking quality analysis results (Table 1) were almost similar for the modified initial samples. For the initial sample cooking time was highest for RF (9.0) sample, as the rajma requires more time to cook completely compared to other ingredients (Parveen and Chakravarty 2014). Least cooking time was observed in OF (SUC) sample, because of its rapid cooking properties. Cooked weight increased with different modification process and all the samples had higher cooked weight compared to Control noodles. Cooking loss was within the standard acceptable range (8%) for all the noodle. Colour and firmness values did not show any significant difference compared to Control sample.
Table 1.
Chemical compositions of noodles and Quality characteristics of the noodles prepared with low glycemic index ingredients (0 day)
| Moisture (%) | Protein (%) | Fat (%) | Ash (%) | Cooking time (min) | Cooked weight (g) | Cooking loss (%) | Colour | Firmness (N/mm) | IVSD (%) | RDS (%) | SDS (%) | RS (%) | EGI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||||||||||
| CON | 6.73a ± 0.09 |
12.08a ± 0.05 |
1.01a ± 0.09 |
0.74a ± 0.09 |
9.02b ± 0.15 |
58.9b ± 1.1 |
4.4b ± 0.2 |
68.84c ± 0.21 |
0.97a ± 0.11 |
10.22a ± 0.98 |
1.47a ± 0.10 |
78.3c ± 1.1 |
77.3c ± 1.7 |
11.4a ± 1.2 |
1.8a ± 1.6 |
75.9c ± 1.2 |
| DI (9.0) | 8.21b ± 0.12 |
13.89b ± 0.09 |
1.19a ± 0.07 |
0.89a ± 0.04 |
9.15b ± 0.12 |
61.2bc ± 0.9 |
3.9a ± 0.3 |
58.03ab ± 0.09 |
3.24b ± 0.54 |
19.44d ± 0.47 |
1.17a ± 0.14 |
39.8a ± 1.9 |
39.2a ± 1.2 |
34.7c ± 1.4 |
3.7b ± 0.8 |
41.5a ± 1.9 |
| RF (9.0) | 6.54a ± 0.04 |
14.01c ± 0.08 |
1.01a ± 0.08 |
1.59bc ± 0.09 |
10.32c ± 0.15 |
59.4b ± 1.5 |
3.5a ± 0.2 |
54.22a ± 2.11 |
5.72c ± 0.91 |
12.52b ± 1.11 |
2.85bc ± 0.77 |
44.4b ± 1.4 |
44.4b ± 1.4 |
21.9b ± 0.9 |
6.8d ± 1.8 |
47.5b ± 0.9 |
| OF (SUC) | 6.87a ± 0.03 |
13.89b ± 0.14 |
1.14a ± 0.04 |
1.21b ± 0.05 |
6.98a ± 0.17 |
49.2a ± 0.9 |
4.1b ± 0.5 |
61.14b ± 1.12 |
3.84b ± 0.81 |
15.42c ± 1.81 |
2.11b ± 0.47 |
41.9b ± 1.7 |
42.5ab ± 1.7 |
42.0d ± 1.7 |
5.2c ± 1.5 |
45.2b ± 1.5 |
| GP (OCT) | 6.92a ± 0.05 |
13.22b ± 0.07 |
1.11a ± 0.02 |
1.89c ± 0.07 |
9.55b ± 0.12 |
61.8bc ± 1.2 |
3.8a ± 0.0 |
57.45ab ± 1.81 |
3.12b ± 0.91 |
17.43cd ± 1.20 |
2.54bc ± 0.14 |
41.2b ± 1.9 |
41.2a ± 1.9 |
40.2d ± 1.9 |
4.9bc ± 1.7 |
41.8a ± 1.7 |
Mean values in the same column with different alphabets differ significantly (p ≤ 0.05)
CON control noodles with 100% T. durum, DI(9.0) T. dicoccum flour modified with pH 9.0, RF(9.0) Rajma flour (20%) with T. durum modified with pH 9.0, OF (SUC) Oats flour (30%) with T. durum modified with succinic anhydride, GP (OCT) Guar powder (10%) with T. durum modified with octenyl–succinic anhydride, IVSD in vitro starch digestibility, RDS rapidly digestible starch, SDS slowly digestible starch, RS resistant starch, EGI estimated glycemic index
In vitro analyses of the noodles (Table 1) indicated that with modification, IVSD reduced significantly with the lowest in case of DI (9.0) with the percentage digestibility of 46, which was half the value as compared to control noodles (74%). Starch fraction analysis indicated the reduction in RDS and increase in SDS, RS with modification. Lowest RDS was observed in DI (9.0) compared to Control. Simultaneously SDS increased significantly with modification. Highest SDS value of 42% was reported in OF (SUC) and in RF (9.0) with lowest (22%). This may be due to high amount of soluble dietary fiber in oats and high amount of insoluble dietary fiber in rajma flour. Resistant starch content of the sample also differ significantly with modification with highest of 6.8% observed in RF (9.0), compared to Control (1.8%). Estimated glycemic index content of the modified samples reduced to maximum extent with modification and all the samples fall below the value of 55, considered to be low GI foods.
Sensory analysis of the modified noodles (Fig. 1-0 day) indicated that with modification there was a significant change in firmness and chewiness of the noodles. The results revealed that modification of ingredients improved some of the quality attributes of noodles and are acceptable even after the modification. Strands were distinct and firmness was improved for modified RF sample, even after modification. All the samples were acceptable after modification, with overall quality of all the samples was above 8.0 on 15 cm QDA scale.
Fig. 1.
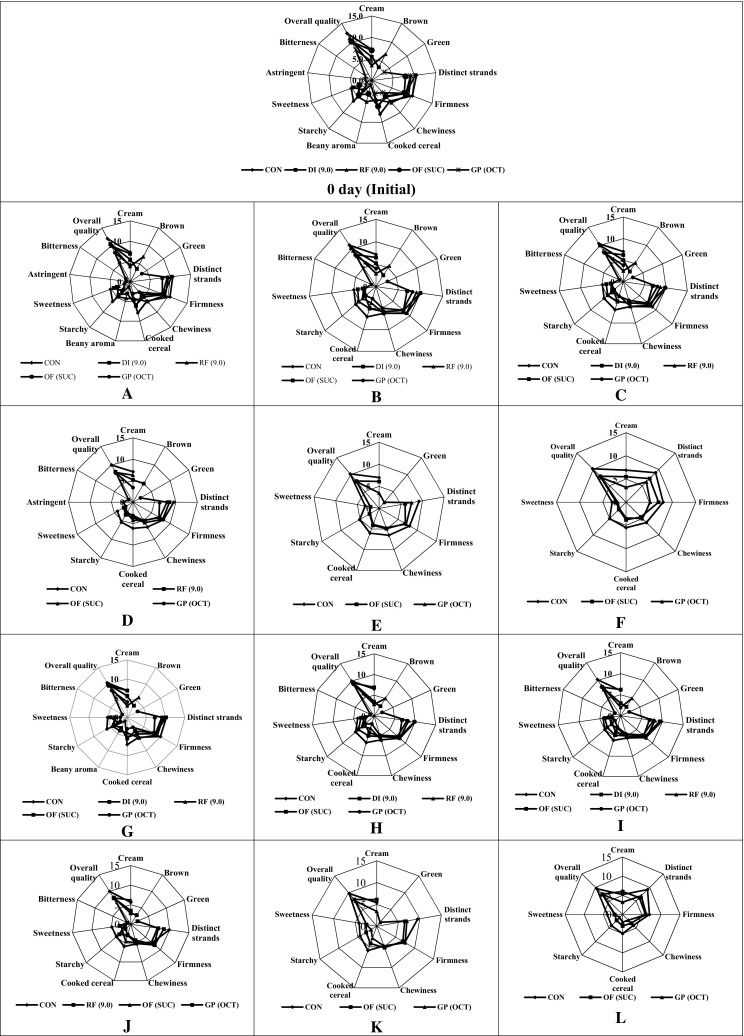
Sensory profiles of noodles prepared with modified ingredients. CON control noodles with 100% T. durum, DI(9.0) T. dicoccum flour modified with pH 9.0, RF(9.0) Rajma flour (20%) with T. durum modified with pH 9.0, OF (SUC) Oats flour (30%) with T. durum modified with succinic anhydride, GP (OCT) Guar powder (10%) with T. durum modified with octenyl–succinic anhydride. Ambient condition: A (30 days), B (60 days), C (90 days), D (120 days), E (150 days) and F (180 days); Accelerated condition: G (30 days), H (60 days), I (90 days), J (120 days), K (150 days) and L (180 days)
Storage study of noodles with chemically modified ingredients
Influence of storage on cooking quality
Samples stored at ambient and accelerated conditions during the storage period were analysed for the cooking quality characteristics and the results were compared with the 0 day samples. Results of the analysis indicated that during storage at ambient condition cooking time reduced significantly in all the samples, simultaneous reduction in the cooked weight of the noodles was also reported. Reduction in cooking time may be due to slight increase in the moisture content of the noodles stored at ambient condition (Pangloli et al. 2000). Cooking loss indicated that on storage the loss increased significantly in all the samples till the end of the study. Maximum increase was observed in 5.9% (GP–OCT, 180 days). Samples stored at accelerated condition resulted in increased cooking time in all the samples, this may be due to the reduction in moisture content of the sample during storage. This reduction in moisture content of the noodles increases the time to hydrate and cook the noodles compared to the initial sample (0 day). Cooking loss increased in all the modified samples except in case of Control noodles, but within the standard limits (8%). Increase in cooking loss may be due to the disruption of internal matrix during storage eventually reducing water uptake and increasing the leach out during cooking. Samples such as Control noodles, OF (SUC) and GP (OCT) were stable till the end of 120 days at accelerated condition.
Influence of storage on colour parameters
Colour values of the sample stored at ambient temperature did not show any significant difference on storage up to 60 days (Table 2). Samples stored beyond 60 days showed decline in L* value indicating the deterioration of the surface colour of the noodles. This may be due to the moisture uptake by the samples during storage. Oxidation of the samples also contributes to the loss of surface colour during storage. Other colour values such as a* and b*, indicators of noodle quality also followed the same trend as L* value. Samples stored at accelerated condition indicated significant changes in the colour values during storage compared to 0 day samples. These obtained colour values were higher than the 0 days sample values. Results of the study indicated that storage condition and period of storage has a direct effect on altering the surface colour of the noodles and also other food products (Chakraborty et al. 2003). This is due to changes in the surface charateristics of the products during storage.
Table 2.
Influence of storage at different conditions on colour and firmness of noodles
| Storage days | Parameters | Ambient condition | Accelerated condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | DI (9.0) | RF (9.0) | OF (SUC) | GP (OCT) | CON | DI (9.0) | RF (9.0) | OF (SUC) | GP (OCT) | ||
| 0 | Colour (L*) | 68.84c ± 1.23 |
58.03b ± 2.45 |
54.22c ± 2.14 |
61.14c ± 2.14 |
57.45c ± 3.01 |
68.84b ± 1.98 |
58.03a ± 2.14 |
54.22b ± 2.41 |
61.14c ± 1.28 |
57.45b ± 2.17 |
| Firmness (N) | 1.47p ± 0.05 |
1.17p ± 0.14 |
2.85p ± 0.14 |
2.11p ± 0.17 |
2.54pq ± 0.18 |
1.47p ± 0.44 |
1.17p ± 0.00 |
2.85p ± 0.08 |
2.11p ± 0.15 |
2.54p ± 0.09 |
|
| 30 | Colour (L*) | 65.48b ± 1.15 |
52.66a ± 2.22 |
43.54a ± 1.99 |
56.66b ± 2.22 |
41.59a ± 2.48 |
61.70a ± 1.11 |
60.62a ± 2.11 |
45.54a ± 2.22 |
54.83b ± 1.92 |
36.66a ± 1.97 |
| Firmness (N) | 4.09t ± 0.11 |
4.52r ± 0.15 |
3.81q ± 0.24 |
3.58r ± 0.11 |
4.22r ± 0.00 |
3.30q ± 0.14 |
4.47q ± 0.02 |
4.59r ± 0.97 |
3.50q ± 0.36 |
4.51q ± 0.88 |
|
| 60 | Colour (L*) | 65.42b ± 2.54 |
52.60a ± 2.87 |
43.51a ± 2.87 |
56.60b ± 1.95 |
41.55a ± 1.97 |
61.00a ± 2.11 |
60.51a ± 2.98 |
45.50a ± 1.21 |
54.71b ± 1.24 |
36.51a ± 3.05 |
| Firmness (N) | 3.31s ± 0.09 |
3.37q ± 0.25 |
3.62q ± 0.11 |
2.64q ± 0.00 |
3.38q ± 0.28 |
7.40r ± 0.44 |
7.51r ± 0.13 |
6.13s ± 0.18 |
5.45r ± 0.37 |
5.90r ± 0.17 |
|
| 90 | Colour (L*) | 63.44ab ± 1.98 |
53.93a ± 1.57 |
46.81ab ± 1.55 |
53.21a ± 1.98 |
41.48a ± 2.48 |
60.96a ± 1.95 |
– | 45.61a ± 2.44 |
55.11b ± 1.47 |
37.40a ± 2.28 |
| Firmness (N) | 3.41s ± 0.24 |
3.09q ± 0.01 |
3.73q ± 0.14 |
2.24pq ± 0.17 |
3.04q ± 0.14 |
3.22q ± 0.19 |
– | 3.66q ± 0.19 |
2.84pq ± 0.19 |
3.06p ± 0.17 |
|
| 120 | Colour (L*) | 62.01a ± 2.55 |
– | 47.89b ± 1.01 |
56.25b ± 2.55 |
42.33a ± 2.22 |
67.38b ± 1.14 |
– | – | 42.45a ± 1.59 |
53.52b ± 2.19 |
| Firmness (N) | 2.89r ± 0.11 |
– | 3.32q ± 0.14 |
2.26pq ± 0.17 |
3.04q ± 0.14 |
4.05q ± 0.78 |
– | – | 5.53r ± 0.07 |
2.87p ± 0.12 |
|
| 150 | Colour (L*) | 61.39a ± 2.90 |
– | – | 55.22b ± 2.14 |
41.24a ± 2.98 |
– | – | – | – | – |
| Firmness (N) | 2.54q ± 0.01 |
– | – | 2.01p ± 0.19 |
2.11p ± 0.05 |
– | – | – | – | – | |
| 180 | Colour (L*) | 61.21a ± 1.14 |
– | – | 53.22a ± 3.89 |
49.24b ± 5.41 |
– | – | – | – | – |
| Firmness (N) | 2.11q ± 0.00 |
– | – | 1.99p ± 0.05 |
2.98pq ± 0.08 |
– | – | – | – | – | |
Mean values in the same column with different alphabets differ significantly (p ≤ 0.05) (a,b,c…for colour and p,q,r,…for firmness); Sample abbreviations as per Table 1
Influence of storage on firmness
Firmness of the noodles stored at ambient condition increased significantly up to 120 days, maximum increase was observed in Control and RF (9.0) samples with 2.9N and 3.8N respectively (Table 2). After 120 days of storage, further increase in the storage period decreased the firmness of the samples. This may be due to the increase in the moisture content of the noodles during storage. The increase and decrease in the firmness may be due to the moisture uptake during storage and water uptake during cooking of noodles (Rho et al. 1988). Increase in the firmness of the noodles can also be attributed to the surface characteristics, which losses to retain moisture after cooking when the stored samples were cooked after certain period of time. Samples stored at accelerated condition showed higher firmness during 60 days of storage. Samples such as Control noodles, DI (9.0), RF (9.0) and OF (SUC) showed firmness of about 7.4N, 7.5N, 6.1N and 5.4N respectively at the end of 60 days, which were around 3.3N, 4.4N, 4.5N and 3.5N respectively at 30 days of storage. This may be due to the loss of moisture content in the noodles during storage at accelerated condition. Further storage reduced the firmness of the samples to 4.0N and 2.5N (Control and OF-SUC). This may be due to disruption of starch-protein matrix and fiber matrix during storage. So, from the result it is evident that firmness of the noodles is directly affected by the storage condition and storage period.
Influence of storage on in vitro starch digestibility
Results indicated that on storage IVSD increased significantly in all the samples (Table 3). Starch digestibility increased from 74.3, 39.8, 44.4 and 41.2% to 78.9, 44.2, 49.5 and 45.4% for Control, DI (9.0), RF (9.0) and GP (OCT) samples respectively at the end of 30 days. In case of OF (SUC) there was slight reduction in IVSD from 41.9 to 41%. The increase in the digestibility may be due to the moisture uptake during storage, leading to the disruption of the matrix in the sample by increasing the digestibility. This is also supported by the increased solid leach out during cooking of the stored noodles. This increasing trend of digestibility was reported in the samples stored further up to 180 days with digestibility percentage of 81.2 and 52.2 for Control and OF (SUC) samples respectively. Samples stored at accelerated condition reported the starch digestibility in the increasing manner. The digestibility increased to 79.5, 41.2, 46.8, 48.1 and 41.4% for Control, DI (9.0), RF (9.0), OF (SUC) and GP (OCT) at the end of 60 days of storage. The results indicate that during storage at accelerated condition the samples were increased with the starch digestibility.
Table 3.
Influence of storage at different conditions on IVSD and EGI of noodles
| Storage days | Parameters | Ambient condition | Accelerated condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | DI (9.0) | RF (9.0) | OF (SUC) | GP (OCT) | CON | DI (9.0) | RF (9.0) | OF (SUC) | GP (OCT) | ||
| 0 | IVSD (%) | 74.3a ± 1.25 |
39.8a ± 2.74 |
44.4a ± 2.78 |
41.9a ± 2.48 |
41.2a ± 1.87 |
74.3a ± 2.22 |
39.8a ± 1.44 |
44.4a ± 1.27 |
41.9a ± 2.14 |
41.2a ± 2.78 |
| EGI | 75.9p ± 2.11 |
41.5p ± 2.18 |
47.5p ± 2.17 |
45.2p ± 2.14 |
41.8p ± 2.74 |
75.9p ± 2.78 |
41.5p ± 2.87 |
47.5p ± 2.22 |
45.2q ± 2.22 |
41.8p ± 1.14 |
|
| 30 | IVSD (%) | 78.9b ± 1.98 |
44.4b ± 1.11 |
49.5b ± 2.97 |
41.2a ± 1.47 |
42.1a ± 2.54 |
79.5b ± 1.25 |
39.9a ± 2.78 |
45.6a ± 2.17 |
45.5b ± 2.78 |
41.4a ± 3.01 |
| EGI | 76.1p ± 1.98 |
40.2p ± 2.97 |
47.5p ± 3.68 |
45.2p ± 2.98 |
46.2q ± 1.87 |
76.8p ± 2.78 |
42.2p ± 2.14 |
49.5p ± 2.47 |
42.1p ± 2.78 |
39.2p ± 2.17 |
|
| 60 | IVSD (%) | 79.1b ± 3.59 |
45.2b ± 2.14 |
50.2b ± 2.17 |
42.2a ± 2.47 |
44.8b ± 2.48 |
79.5b ± 1.89 |
41.2a ± 3.10 |
46.8a ± 2.78 |
48.1bc ± 2.78 |
41.4a ± 1.98 |
| EGI | 78.1pq ± 1.79 |
41.2p ± 2.97 |
48.5p ± 2.78 |
45.9p ± 1.99 |
47.5q ± 2.87 |
79.2q ± 2.87 |
46.3q ± 1.10 |
49.8p ± 2.17 |
45.2q ± 2.99 |
40.8p ± 2.01 |
|
| 90 | IVSD (%) | 79.5b ± 3.69 |
50.1c ± 1.78 |
53.5c ± 1.47 |
45.6b ± 2.78 |
48.6bc ± 2.87 |
80.2b ± 1.99 |
– | 47.2b ± 2.47 |
49.2bc ± 1.11 |
45.2b ± 2.22 |
| EGI | 78.0pq ± 2.22 |
42.1p ± 2.97 |
49.0p ± 1.11 |
46.1p ± 2.97 |
48.2q ± 2.47 |
79.4q ± 2.78 |
– | 51.2pq ± 2.11 |
46.5q ± 2.87 |
43.8q ± 2.78 |
|
| 120 | IVSD (%) | 78.1b ± 1.11 |
– | 54.5c ± 1.97 |
48.5b ± 2.71 |
50.1c ± 3.25 |
79.1b ± 1.55 |
– | – | 50.2bc ± 2.78 |
49.5b ± 3.98 |
| EGI | 79.2pq ± 3.97 |
– | 49.5p ± 2.89 |
47.4pq ± 2.97 |
49.4q ± 1.98 |
80.2q ± 2.78 |
– | – | 49.5qr ± 2.78 |
46.2r ± 2.78 |
|
| 150 | IVSD (%) | 79.4b ± 1.05 |
– | – | 49.9bc ± 2.78 |
51.8c ± 2.47 |
– | – | – | – | – |
| EGI | 80.5pq ± 2.75 |
– | – | 49.2q ± 3.44 |
49.2q ± 1.57 |
– | – | – | – | – | |
| 180 | IVSD (%) | 81.2c ± 3.25 |
– | – | 52.2c ± 2.57 |
53.2cd ± 2.78 |
– | – | – | – | – |
| EGI | 80.5pq ± 2.15 |
– | – | 49.2q ± 2.78 |
49.9q ± 2.57 |
– | – | – | – | – | |
Mean values in the same column with different alphabets differ significantly (p ≤ 0.05) (a,b,c…for IVSD and p,q,r,…for EGI); IVSD in vitro starch digestibility, EGI estimated glycemic index; Sample abbreviations as per Table 1
Influence of storage on starch profile
Results indicated (Fig. 2) that on storage RDS increased significantly at the end of 180 days of storage in Control and OF (SUC) samples from 77.3% and 42.5% to 81.2% and 52.2% respectively. This indicates storage at ambient condition increases the RDS content of the sample, which eventually has an effect on GI of the noodles. As RDS increased during storage, SDS content gradually declined. The decrease in the SDS content may be due to the increase in the RDS content of the samples. Whereas, increase in the SDS content even though there was increase in the RDS content may be due to the increased fiber content and modification process making the sample to release the starch in the slow rate during in vitro digestion. Starch profile of the samples stored at accelerated condition reported that RDS content of the samples increased during storage. Control, DI (9.0), RF (9.0) and OF (SUC) samples showed increased RDS content from 77.3, 39.2, 44.4 and 42.5% to 79.5, 41.2, 46.2 and 43.2% respectively. Whereas, GP (OCT) showed reduction in the RDS content during first 30 days of storage from 41.2 to 39.1% respectively. This may be due to the insoluble and soluble dietary fiber and the modification of its constituents.
Fig. 2.
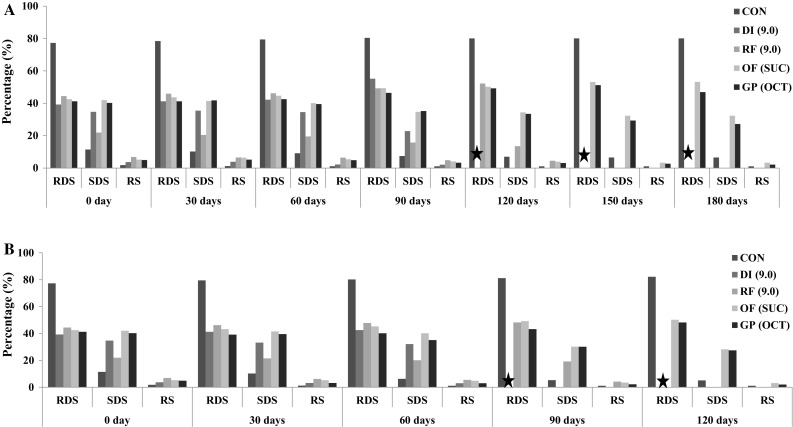
Starch profile of the samples stored at the A: ambient condition and B: accelerated condition. * Analysis was terminated due to unacceptability; Sample abbreviations as per Fig. 1. RDS rapidly digestible starch, SDS slowly digestible starch, RS resistant starch
Influence of storage on estimated glycemic index
In vitro estimated glycemic index analysis was carried out for the samples stored at ambient condition. Results (Table 3) indicated that on storage of the modified samples the EGI value gradually increased. This may be due to the sample deterioration due to oxidation and enzyme activity to modify the glucose releasing property of the noodles (Takizawa et al. 2004; Choo and Aziz 2010). EGI of the samples increased slightly during 30 days of storage from 75.9 and 41.8 to 76.1 and 46.2 for Control and GP (OCT) samples respectively. These results are evident by the IVSD and starch profile analysis. As the storage period proceeds the EGI values of the samples progressively increased and at the end of 180 days of ambient storage the values of Control, OF (SUC) and GP (OCT) samples were 80.5, 49.2 and 49.9 respectively. Even though the EGI increased the values were with in the low GI range. Samples stored at accelerated condition showed EGI values of 76.8, 42.2, 49.5, 42.1 and 39.2 for Control, DI (9.0), RF (9.0), OF (SUC) and GP (OCT) samples respectively. These results indicate that with samples stored under accelerated condition showed increase in the EGI value on 30 days of storage. On 60 days the EGI value further increased. The increase in the EGI is mainly because of the storage condition, due to high humidity content and elevated temperature samples are undergoing some changes in their internal arrangement. Due to this samples are becoming more vulnerable to enzymatic hydrolysis even after modification. Estimated glycemic index of the samples increased slightly due to storage, as the digestibility of starch increased gradually due to storage of foods (Bravo et al. 1998). However, at the end of 120 days of storage EGI values of the samples Control, OF (SUC) and GP (OCT) was 80.2, 49.5 and 46.2 respectively. So, at the end of 120 days samples were still with in the low GI range (< 55). Hence these samples are stable and can be termed as low GI noodles.
Influence of storage on sensory properties
Sensory analysis of samples stored at ambient condition (Fig. 1a–f) indicated that on storage some of the quality attributes slowly deteriorates. Samples stored for 30 days indicated that there was no significant difference in their colour and appearance. Strand quality was also comparable for the sample with the 0 day samples. Attributes such as astringent and bitterness reduced slightly during storage, up to 60 days and disappeared on further storage, which may be positive change for noodle quality. Overall quality of all the samples was slightly reduced at the end of 30 days of storage, but there was drastic decline in the overall quality after 120 days of storage. But all the samples were acceptable by the panellists, with highest score of 12 on 15 cm QDA scale for Control noodles and lowest of 8.4 for GP (OCT) sample. During the period of 180 days of storage samples such as DI (9.0) was eliminated for the analysis on 120 days, because of the presence of off taste during 90 days analysis. In the same way samples such as RF (9.0) was excluded from 150 days analysis. Finally analysis at 180 days was carried out for the samples Control, OF (SUC) and GP (OCT). These three samples had overall quality score of 9.5, 7.5 and 7.3 on 15 cm QDA scale respectively. The analysis was terminated at this stage as the quality of samples started deteriorating with off taste and bitter after taste. So, by this it can be concluded that samples modified with succinic anhydride and octenyl succinic anhydride can be stored and consumed at the end of 180 days with less sample deterioration.
Sensory analysis of the samples stored at accelerated condition (Fig. 1g–l) indicated that there was a significant decrease in the surface colour of the sample due to storage in the high humidity and elevated condition. Strands were distinct and firmness increased slightly during 30 days of storage, this may be due to the loss of moisture during storage, which directly affects the cooking behaviour of the product. Further storage resulted in dullness of the surface colour of the samples. Firmness of the samples increased compared to initial samples. Chewiness reduced significantly due to storage, because of the moisture loss there was loss of interaction in the sample matrix leading to reduction in chewiness. Overall quality score of the samples in each month reduced gradually and some samples were eliminated at the end of each month starting from 90 days. Control, OF (SUC) and GP (OCT) were analysed on 120 days and the analysis was terminated at that point of time. This is due to the perceiving of slight off taste by the panellists and also reducing scores for the other quality parameters. At the end of the study these three samples had an overall quality score of 10, 8 and 7.9 respectively.
Influence of storage on microstructural characteristics
Microstructural analysis of the samples stored till 180 days were analysed (Fig. 3). Micrographs indicate that with modification and storage at ambient condition there lays a major internal change, leading to changes in the cooking quality and also starch digestibility. These changes in the sample may be main reason for the increase in the EGI of the samples. There are no significant changes in Control sample at 0 days and 180 days of storage. Matrix shows slight deformation due to storage condition and time. In case of OF (SUC) disruption of internal structure can be seen evidently for 180 days of storage compared to 0 day sample. This can be supported by cooking loss and In-vitro digestibility analyses. As the number of storage days increased starch and protein matrix disrupted and release of starch will be easier and hence the digestibility increased (Alsaffar 2010). The same trend was followed in the case of GP (OCT) sample, here the disruption is significantly higher compared to 0 days sample and also OF (SUC) samples. These results were also comparable to the solid leach-out during cooking and In-vitro digestibility analyses.
Fig. 3.
Micrographs of samples stored for 180 days at ambient condition. DM disrupted matrix, FM fiber matrix, SG starch granules; Sample abbreviations as per Fig. 1
Conclusion
Samples such as OF (SUC) and GP (OCT) modified with succinic anhydride and octenyl–succinic anhydride can be stored for 180 days with the GI value not exceeding 55. Samples indicated good textural properties and sensory characteristics. Thus these noodles with modified ingredients can be termed as low glycemic index noodles, which are suitable for the population with diabetes with its beneficial effects.
References
- American Association of Cereal Chemists (AACC) (2000) Approved methods of the AACC, 10th edn. AACC method 44-15A, one stage moisture air oven method; AACC method 08-01, Ash–Basic method; AACC method 46-13, Micro-Kjeldahl method; AACC method 22-10A; AOAC 991.43; dietary fiber, AACC method 16-50; pasta cooking time-66–50, AACC, AACC 2, method 54–21. St. Paul, Minnesota
- Alsaffar AA. Effect of thermal processing and storage on digestibility of starch in whole wheat grains. J Cereal Sci. 2010;52:480–485. doi: 10.1016/j.jcs.2010.08.002. [DOI] [Google Scholar]
- Bharath Kumar S, Prabhasankar P. Chemically modified wheat flours in noodle processing: effect on in vitro starch digestibility and glycemic index. Food Meas. 2015;9:575–585. doi: 10.1007/s11694-015-9266-z. [DOI] [Google Scholar]
- Bharath Kumar S, Prabhasankar P. Modified low glycemic index ingredients in noodle processing: rheology and microstructural characteristics. Akad Gıda. 2017;15:211–221. doi: 10.24323/akademik-gida.345247. [DOI] [Google Scholar]
- Bravo L, Siddhuraju P, Saura-Calixto F. Effect of various processing methods on the in vitro starch digestibility and resistant starch content of Indian pulses. J Agric Food Chem. 1998;46:4667–4674. doi: 10.1021/jf980251f. [DOI] [Google Scholar]
- Bui LT, Small DM. Riboflavin in Asian noodles: the impact of processing, storage and the efficacy of fortification of three product styles. Food Chem. 2009;114:1477–1483. doi: 10.1016/j.foodchem.2008.11.048. [DOI] [Google Scholar]
- Chakraborty M, Hareland GA, Manthey FA, Berglund LR. Evaluating quality of yellow alkaline noodles made from mechanically abraded sprouted wheat. J Sci Food Agric. 2003;83:487–495. doi: 10.1002/jsfa.1403. [DOI] [Google Scholar]
- Choo CL, Aziz NAA. Effects of banana flour and β-glucan on the nutritional and sensory evaluation of noodles. Food Chem. 2010;119:34–40. doi: 10.1016/j.foodchem.2009.05.004. [DOI] [Google Scholar]
- Duncan BD. Multiple range and multiple F-tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:S33–S50. [PubMed] [Google Scholar]
- Gacula MC. The design of experiments for shelf life study. J Food Sci. 1975;40:399–403. doi: 10.1111/j.1365-2621.1975.tb02211.x. [DOI] [Google Scholar]
- Goni I, Garcia-Alonso A, Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nutr Res. 1997;17:427–437. doi: 10.1016/S0271-5317(97)00010-9. [DOI] [Google Scholar]
- Han JA, BeMiller JN. Preparation and physical characteristics of slowly digesting modified food starches. Carbohydr Polym. 2007;67:366–374. doi: 10.1016/j.carbpol.2006.06.011. [DOI] [Google Scholar]
- Hutchings JB (1994) Food color and appearance. Chapman and Hall, Blackie Academic and Professional, Great Britain and London
- Kongkachuichai R, Kounhawej A, Chavasit V, Charoensiri R. Effects of various iron fortificants on sensory acceptability and shelf-life stability of instant noodles. Food Nutr Bull. 2007;28:165–172. doi: 10.1177/156482650702800205. [DOI] [PubMed] [Google Scholar]
- Konik CM, Mikkelsen LM, Moss R, Gore PJ. Relationships between physical starch properties and yellow alkaline noodle quality. Starch. 1994;46:292–299. doi: 10.1002/star.19940460804. [DOI] [Google Scholar]
- Kruger JE, Matsuo RB, Dick JW. Pasta and noodle technology. Eagan: American Association of Cereal Chemists; 1996. [Google Scholar]
- Pangloli P, Collins JL, Penfield MP. Storage conditions affect quality of noodles with added soy flour and sweet potato. Int J Food Sci Technol. 2000;35:235–242. doi: 10.1046/j.1365-2621.2000.00333.x. [DOI] [Google Scholar]
- Parveen S, Chakravarty A. Sensory evaluation and acceptability of noodles prepared from different food items. Asian J Home Sci. 2014;9:339–341. [Google Scholar]
- Polydera AC, Stoforos NG, Taoukis PS. Comparative shelf life study and vitamin C loss kinetics in pasteurised and high pressure processed reconstituted orange juice. J Food Eng. 2003;60:21–29. doi: 10.1016/S0260-8774(03)00006-2. [DOI] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. India: Tata McGraw-Hill Education; 1986. [Google Scholar]
- Rho KL, Seib PA, Chung OK, Deyoe CW. Noodles. VII. Investigating the surface firmness of cooked oriental dry noodles made from hard wheat flours. Cereal Chem. 1988;65:320–326. [Google Scholar]
- Takizawa FF, Silva GDOD, Konkel FE, Demiate IM. Characterization of tropical starches modified with potassium permanganate and lactic acid. Braz Arch Biol Technol. 2004;47:921–931. doi: 10.1590/S1516-89132004000600012. [DOI] [Google Scholar]
- Thompson LU, Yoon JH, Jenkins DJ, Wolever TM, Jenkins AL. Relationship between polyphenol intake and blood glucose response of normal and diabetic individuals. Am J Clin Nutr. 1984;39:745–751. doi: 10.1093/ajcn/39.5.745. [DOI] [PubMed] [Google Scholar]
- Ugarcic-Hardi Z, Peric L, Strelec I, Koceva D. Comparison of colorimetric and spectrophotometric methods for colour determination in pasta. Z Lebensm Unters Forsch A. 1999;208:383–387. doi: 10.1007/s002170050434. [DOI] [Google Scholar]



