Abstract
Blanching was performed to inactivate the enzyme using microwave, steam and hot water blanching methods and effect on the enzymatic activity, chemical properties and physical properties of the sweet corn were studied. The effectiveness of each blanching process was evaluated by measuring the loss of peroxidase activity, which was lost after 60, 90 and 120 s with k-values 0.016, 0.024 and 0.028 s−1 following first order kinetics for microwave, steam and hot water blanching respectively. The total sugar, ascorbic acid, moisture content, kernel mass and geometric diameter changed from 8.40 to 6.30, 7.20 and 7.50 g/100 g; 7.15 to 5.70, 6.10 and 6.60 mg/100 g; 76 to 79.20, 78.20 and 75.30%; 0.47 to 0.53, 0.50 and 0.42 g; 8.00 to 8.50, 8.30 and 7.20 mm at optimum level of blanching during microwave, steam and hot water blanching respectively, indicating higher retention of total sugar and ascorbic acid in microwave blanching. The change in colour, especially increase in brownness was observed during blanching processes. The average R2 for zero-order model was 0.945, suggesting use of model for prediction of physico-chemical parameters during blanching process of sweet corn.
Keywords: Sweet corn, Blanching, Peroxidase activity, Physico-chemical properties
Introduction
Sweet corn (Zea mays L.) is enjoyed as fresh after steaming or boiling. It is also available in frozen and canned form, however frozen sweet corn is being preferred due to its superior quality and convenience in comparison to canned sweet corn. Blanching is referred to as a heat pre-treatment to denature the enzymes before freezing, drying or canning process of fruit and vegetables. The enzymes activities may be inhibited by blanching, which arrest deterioration during frozen storage followed by thawing (Rickman et al. 2007). Blanching is particularly used for preserving the food colour, being usually performed at atmospheric pressure using steam or hot water at higher temperatures for around 1 or 2 min.
Even though enzymes inactivation has been the main reason of blanching, other positive effects were also observed in other aspects, such as decrease in microbial level in frozen fruits and vegetables; enhancement in retention of carotenoids in dried fruits during storage; and cleaning the surface of fruits and vegetable (Lin and Brewer 2005; Nascimento et al. 2009). The blanching is also aimed for texture modification, colour, flavour and nutrition retention, removal of trapped air besides enzymes inactivation. However, loss of water-soluble vitamins in the blanching water is one of the major undesirable effects of blanching.
Blanching is being continued till complete peroxidise inactivation, which generally provide over heating with consequent undesirable quality losses. The spherical shape of fresh agricultural produce often makes blanching difficult due to reach of effective temperature at the centre of produce and to ensure complete enzymatic inactivation. Therefore, most external part of the produce may be over processed due to long process duration at high temperatures (Igual et al. 2013). To overcome problem related to over processing, conventional thermal treatment can be replaced by microwave technology in food industry for a more homogeneous heating in comparatively shorter duration due to greater penetration depth of microwaves (Lau and Tang 2002). These effects also improve product quality significantly and would provide benefits to the industry by decrease in energy costs, water use and clean-up costs (Barrett et al. 2000).
The effect of temperature, duration and methods affect the appropriateness of blanching. The effect of temperature on blanching of various crops was reported. However, the effect of temperature, duration and methods on sweet corn was not reported till date. Therefore, this study was aimed to investigate the influence and kinetics of blanching methods using microwave, boiling water and steaming methods on some functional properties of sweet corn.
Materials and methods
Materials
Fresh sweet corn (Variety: Madhuri) was purchased from local market in Godhra, Gujarat, India. Sweet corn kernels were detached using hand held sweet corn peeler.
Blanching processes
Blanching processes were performed with hot water blanching, steam blanching and microwave blanching for peroxidase inactivation (Barrett et al. 2000; Lin and Brewer 2005). All blanching experiments were performed in triplicates.
Hot water blanching
All the prepared samples of 100 g were soaked in boiling distilled water at 100 °C (Sample:water ratio 1:10 w/w) and blanched for 30, 60, 90, 120 and 150 s followed by draining of water and placing on a blotting paper.
Steam blanching
Samples of 100 g were kept on a stainless steel perforated bowl (diameter 16.0 cm) and placed into vessel containing water for steam generation and blanched in steam for 30, 60, 90, 120 and 180 s followed by draining of water and placing on a blotting paper.
Microwave blanching
One at a time, samples of kernels (100 g) were put into a 1000 ml beaker and microwaved by an oven (Model: LG MC2841SPS, India) working at 2450 MHz–900 W. The beaker was covered with a Pyrex plate to prevent water loss. The samples were placed for blanching in microwave for 40, 50, 60, 70 and 80 s followed by placing on a blotting paper.
Peroxidase enzymatic activity
The samples were analysed for enzymatic activity after each blanching process. A small portion of the sample (1 ± 0.2 g) was taken in a test tube and 10 ml of guaiacol (0.5%) and 10 ml of hydrogen peroxide (0.08%) was added. The test tube was shaken and kept for 3 min and colour was noted after 3 min. Colour of the mixtures changing from colourless to brick red indicated incomplete enzyme inactivation. The substrate solution was transferred into a cuvette and checked absorbance 720 nm in spectrophotometer (Sheu and Chen 1991).
Estimation of kinetic parameters for enzymatic activity
The heat inactivation was described by a first-order reaction (Lappe et al. 2009), and it is described by the following equation:
| 1 |
where residual activity at time t (s) is represented by A/Ao and inactivation rate constant is represented by k (s−1) at a specific temperature.The exponential regression analysis can be used to find inactivation rate constants (k-values).
Half-life (t1/2) value of inactivation is given by the expression:
| 2 |
The time required for 90% reduction in initial activity is represented by D-value. Following expression was used for determination of D-value (Espachs-Barroso et al. 2006).
| 3 |
Physico-chemical properties
Ascorbic acid
Ascorbic acid content of samples was determined using the 2,6-dichlorophenol-indophenol method (Ranganna 1986). Accordingly, 10 g of sweet corn kernels was transferred to volumetric flask and volume was made up to 100 ml by adding 3% metaphosphoric acid solution. After 30 min, the suspension was filtered through Whatman No. 1 filter paper. From the filter, 10 ml of aliquot was taken and titrated against standardized 2, 6 dichloride indophenols dye solution.
Total sugar
Total sugars were determined by the method of “Lane and Eynon” (1934). The sample (5 g) in a volumetric flask 40 ml of water was added and neutralized with 1 N NaOH. 2 ml of 66% lead acetate solution was added and kept for 10 min. Excess lead acetate was precipitated by necessary amount of 20% potassium oxalate, made up to the volume with water, filtered and 50 ml of filtered sample was taken in a 250 ml conical flask to which 50 ml water and 5 g of citric acid was added, boiled gently for 10 min to complete the inversion of sucrose, transferred to 250 ml volumetric flask and neutralized with 1 N NaOH. The volume was made up to the mark and taken in burette. 10 ml of mixed Fehling’s solution was taken in 250 ml conical flask. Little quantity of the sample was run into flask and heated to boil moderately for 2 min. Three drops of methylene blue solution were added and completed the titration until the indicator was completely decolourized. Brick red colour of the solution indicates the end point.
Total soluble solid (TSS)
All the samples were blended for 1 min and centrifuged at 2000 rpm for 15 min. Few drops of the supernatant were kept on prism of hand refractometer for the direct reading of total soluble solid (Vigneault et al. 2007).
Moisture content
The hot air oven was used for determination of moisture content and samples were kept at 105 ± 1 °C for 24 h (Karababa and Coskuner 2007).
Kernel mass
A digital weight balance (M/s Shimadhu Corporation, Japan) with precision of ± 0.1 g was used to measure the mass of randomly selected 100 kernels.
Geometric mean diameter
The three major perpendicular dimensions of the 100 randomly selected sweet corn kernels were measured using a digital calliper (M/S Mitutoyo Measuring Instruments (Suzhou) Co. Ltd., China) having precision of ± 0.02 mm. The geometric mean diameter (Dg) of the sweet corn kernels was estimated by following expression (Mohsenin 1980), where L, W and T are the largest dimension, the largest dimension perpendicular to L and the largest dimension perpendicular to L and W respectively:
| 4 |
Colour
Colour measurements were performed with a Chroma Meter (Make: Minolta, Osaka, Japan, model-NH310) on CIE Lab scale. The instrument was calibrated prior to measurements with a white standard. The experimental data of L*, a* and b* were represent spectrum range from 0 (black) to 100 (white), from negative (green) to positive (red) and from negative (blue) to positive (yellow) respectively.
Total colour difference was calculated using following Eq. (5), where subscript “o” refers to the colour reading of untreated sweet corn kernel. Untreated sweet corn kernel was used as the reference and a larger ΔE denotes greater colour change from the reference material.
| 5 |
where L* is degree of lightness to darkness, Lo* is initial value of L*, a* is degree of redness to greenness, ao* is initial value of a*, b* is degree of yellowness to blueness and bo* is initial value of b*.
Hue is regarded as color appearance parameters and defines degree of stimulus, whereas chroma is the colorfulness relative to the brightness of a similarly illuminated area. These parameters represent combined effect of colour values. The hue angle was estimated using experimental data as (arc tan (b*/a*) + 180), and saturation index and chroma was expressed as (a*2 + b*2)1/2 for providing more information regarding spatial colour distribution (Hashim et al. 2012).
Browning index (BI) is a measure of purity of brown colour and provide information regarding browning (Mohammadi et al. 2008).
| 6 |
where
| 7 |
Estimation of kinetics parameter of bio-chemical and physical properties
The Zero-order [Eq. (8)] and First-order [Eq. (9)] kinetics models were used to evaluate the kinetic degradation of bio-chemical and physical properties during blanching process.
| 8 |
| 9 |
where C is the measured response at time t, C0 is the initial value of response, t is the blanching time as independent variable (s), k0 and k1 are zero-order kinetic constant (s−1) and first-order kinetics constant (s−1) respectively. To check goodness of model fitting three primary parameters were used to evaluate the adjustment of fit to the models which included the coefficient of determination (R2), the root means square error (RMSE) and the reduce Chi square (χ2) using eq.
| 10 |
| 11 |
| 12 |
where exp, i is the ith experimental data, pre, i is the ith predicted data, N is the number of observations and Z is the number of constants.
Data analysis
The regression analysis was performed using Origin-Pro 9.2 (Origin Lab Corporation, Northampton, MA, USA) tool. All experimental data were analysed using one-way analysis of variance (ANOVA). A p level < 0.05 was used, where values were described as being significant.
Results and discussion
Peroxidase activity
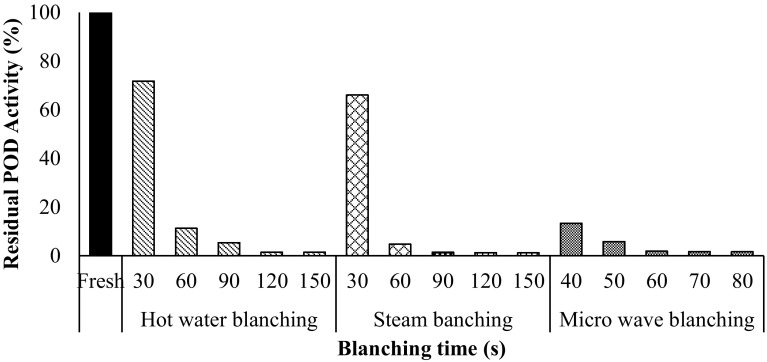
The effects of blanching treatment (Hot water blanching, Steam blanching, Microwave blanching) on enzymatic activities are presented in Fig. 1. Residual activity of peroxidase enzyme in sweet corn reduced with increase in time of hot water, steam and microwave blanching treatment. More than 95% of peroxidase (POD) activity was lost within the first 120, 90 and 60 s in hot water, steam and microwave blanching respectively. It is recommended to reduce the indicator enzyme activity by 90% for deciding the appropriateness of blanching methods and optimization of quality of non-pasteurized product (Bahceci et al. 2005). It can be further observed from Fig. 1 that optimum level for hot water blanching, steam blanching and microwave blanching were taken 120, 90 and 60 s respectively.
Fig. 1.
Variation in peroxidase (POD) activity of sweet corn during blanching
Table 1 shows the kinetic parameters of the enzymes peroxidase (POD) activity. The experimental data fitted well to first order equation for all treatments studied with R2 (0.92, 0.91 and 0.75), χ2 (0.01, 0.02 and 0.05) and RMSE (0.11, 0.12 and 0.20) for hot water blanching, steam blanching and microwave blanching respectively. The k, t(1/2) and D value were 0.016, 0.024 and 0.028 s−1; 43.87, 28.61 and 24.50 s; 145.73, 95.03 and 81.39 s in hot water, steam and microwave blanching respectively, indicating minimum D value and t(1/2) values in case of microwave blanching.
Table 1.
Thermal inactivation parameters of peroxidase (POD) activity
| Treatment | Inactivation rate constant | Half-life | Decimal reduction time | Parameters for first order model | ||
|---|---|---|---|---|---|---|
| k (s−1) | t(1/2) (s) | D (s) | R2 | χ2 | RMSE | |
| Hot water blanching | 0.016 | 43.87 | 145.73 | 0.92 | 0.01 | 0.11 |
| Steam blanching | 0.024 | 28.61 | 95.03 | 0.91 | 0.02 | 0.12 |
| Microwave blanching | 0.028 | 24.50 | 81.39 | 0.75 | 0.05 | 0.20 |
Effect of blanching on physico-chemical properties
Total sugar
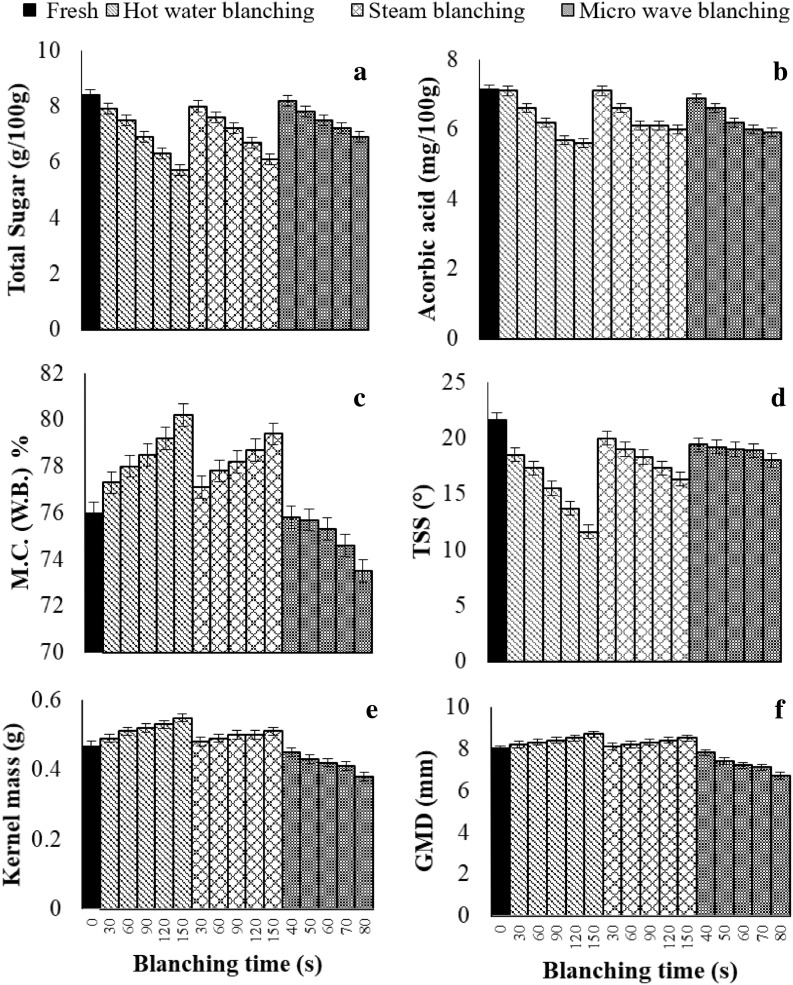
Total sugar content of untreated sweet corn is extremely important and its content depends on variety, harvesting time and post-harvest practises (Park et al. 1994). The initial content of total sugar was 8.40 ± 0.67 g/100 g in untreated sweet corn. Total sugar significantly decreased from 8.40 to 5.70 ± 0.54, 6.10 ± 0.46 and 6.90 ± 0.49 g/100 g in hot water blanching, steam blanching and microwave blanching respectively (p < 0.05). However, total sugar at optimum level of blanching reduced to 6.30 ± 0.23, 7.20 ± 0.34 and 7.50 ± 0.29 g/100 g in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively (Fig. 2a).
Fig. 2.
Variation in physico-chemical parameter of sweet corn during blanching
The loss of water soluble sugars might be correlated with the leaching of sugar during blanching (Song et al. 2003). The decrease in total sugar during blanching of hot water was also reported by Neri et al. (2011). The decrease of total sugar during microwave blanching may be attributed to the reaction between reducing sugars and primary amino group at higher temperature attained during the blanching. Higher retention of total sugar by microwave blanching in comparison to hot water blanching and steam blanching for carrot was also reported by Oliveira and Silva (1992).
Ascorbic acid
Ascorbic acid content decreased during processing as compare to untreated samples. An initial content of ascorbic acid was 7.15 ± 0.68 mg/100 g in untreated sweet corn. Ascorbic acid significantly decreased from 7.15 to 5.60 ± 0.24, 6.00 ± 0.42 and 5.90 ± 0.21 mg/100 g in hot water blanching, steam blanching and microwave blanching (p < 0.05). However, ascorbic acid at optimum level of blanching reduced to 5.70 ± 0.25, 6.10 ± 0.19 and 6.60 ± 0.31 mg/100 g in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively. It can be further noted from the (Fig. 2b) that minimum loss of ascorbic acid occurred in microwave blanching at optimum time.
The lower retention of sweet corn in hot water blanching could be attributed to the readily loss of nutrients through leaching, since it is known that blanching by immersion in hot water produces higher ascorbic acids losses than blanching with steam (Arroqui et al. 2001; Vallejo et al. 2002). The superior nutritional quality of microwaves blanched samples is evident due to avoidance of leaching losses during processing.
Moisture content
Moisture content of sweet corn during blanching varied with the blanching time. The initial moisture content was about 76 ± 1.04% in untreated sweet corn. Moisture content significantly increased from 76 to 80.20 ± 0.96 and 79.40 ± 0.84% in hot water blanching and steam blanching respectively and significantly decreased to 73.50 ± 0.91% in microwave blanching (p < 0.05). Higher moisture content in hot water and steam blanching is evident due to absorbing moisture, whereas lower moisture content in microwave blanching is a result of losing moisture due heating of sweet corn during blanching operation. However, moisture content at optimum level of blanching was changed to 79.20 ± 0.81, 78.20 ± 0.64 and 75.30 ± 0.81% in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively. It can be further noted from the Fig. 2c that minimum change of moisture content occurred in microwave blanching at optimum time.
Total soluble solid
An initial content of TSS was about 21.8 ± 1.23°brix for untreated sweet corn. TSS significantly decreased from 21.8 ± 0.89 to 11.6 ± 0.81 and 16.3 ± 0.78°brix in hot water blanching, steam blanching respectively and did not significantly change in microwave blanching. However, TSS at optimum level of blanching reduced from 21.8 ± 1.23 to 13.7 ± 0.82 and 18.3 ± 0.76°brix in hot water blanching for 120 s, steam blanching for 90 s respectively. It can be further noted from the Fig. 2d that minimum change in TSS occurred in microwave blanching at optimum time. Total soluble solids are contributed by sugars which are water soluble. Similar loss of TSS in hot water blanching by leaching of nutrients and other water soluble components during blanching was also reported by De Corcuera et al. (2004). The reduction in total soluble solids during microwave blanching may also be due to reaction between reducing sugars and primary amino group at higher temperature attained during the blanching.
Kernel mass
The initial kernel mass was 0.47 ± 0.12 g for untreated sweet corn. The kernel mass significantly increased from 0.47 to 0.55 ± 0.11 and 0.51 ± 0.09 g in hot water blanching, steam blanching and significantly decreased to 0.38 ± 0.14 g in microwave blanching respectively (p < 0.05). Higher kernel mass in hot water and steam blanching was evident due to absorbing moisture by the kernels, whereas lower kernel mass in microwave blanching may be a result of losing moisture due to heating of sweet corn during blanching operation. However, kernel mass at optimum level of blanching changed to 0.53 ± 0.07, 0.50 ± 0.09 and 0.42 ± 0.05 g in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively. It can be further noted from the Fig. 2e that minimum change in kernel mass occurred in microwave blanching at optimum time.
Geometric mean diameter
The initial geometric mean diameter was about 8.00 ± 0.16 mm for untreated sweet corn. The geometric diameter significantly increased from 8.00 to 8.70 ± 0.06 and 8.50 ± 0.08 mm in hot water blanching, steam blanching and significantly decreased to 6.70 ± 0.11 mm in microwave blanching respectively (p < 0.05). Higher geometric mean diameter in hot water and steam blanching is evident due to enlargement of dimensions due absorbing moisture, whereas lower geometric mean diameter in microwave blanching is a result of contraction of dimensions due to losing moisture during heating of sweet corn. However, geometric diameter at optimum level of blanching was changed to 8.5 ± 0.06, 8.3 ± 0.08 and 7.2 ± 0.10 mm in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively. It can be further noted from the Fig. 2f that minimum change in geometric diameter occurred in microwave blanching at optimum time.
Colour
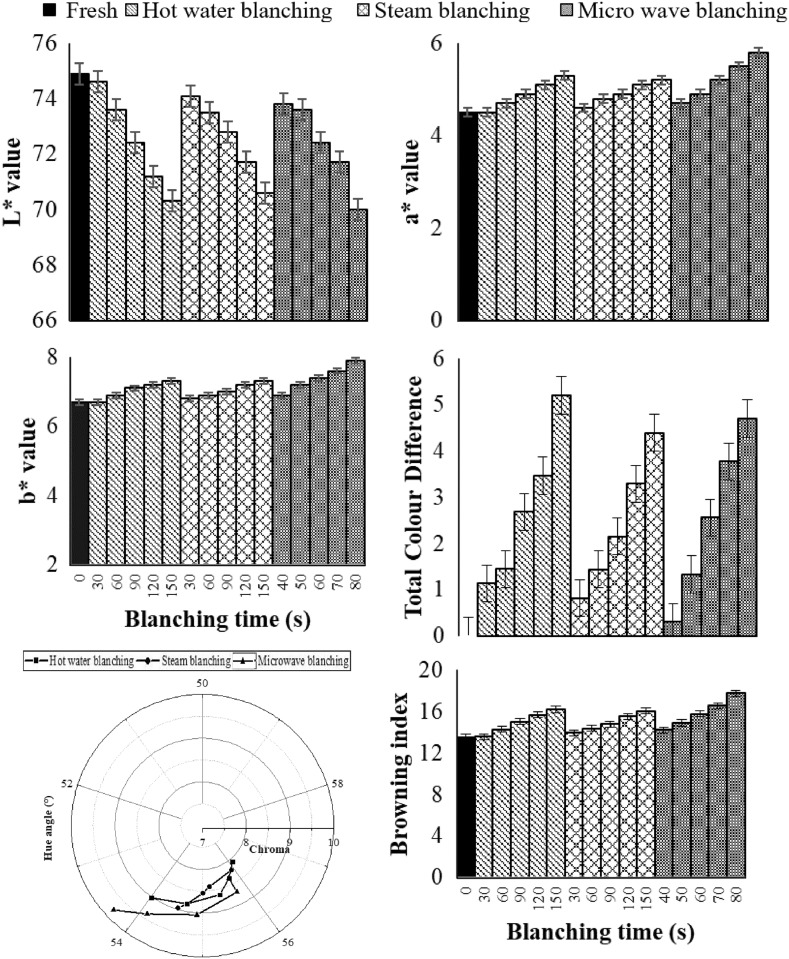
The L* value significantly decreased from 74.9 ± 0.6 to 70.3 ± 0.7, 70.6 ± 0.5 and 70.0 ± 0.6 in hot water blanching, steam blanching and microwave blanching respectively (p < 0.05). However, L* value at optimum level of blanching reduced to 71.2 ± 0.7, 71.8 ± 0.8 and 72.4 ± 0.8 in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively (Fig. 3a). The higher L* values in fresh samples indicated lighter colour of samples (Thakur et al. 2015, Parmar et al. 2017). The darkness of colour during blanching may be associated to higher pigment content that contributes to higher antioxidant capacity (Thakur et al. 2017) and non-enzymatic browning at higher temperatures. The a* value significantly increased from 4.5 ± 0.8 to 5.8 ± 0.6, 5.2 ± 0.7 and 5.3 ± 0.4 in hot water blanching, steam blanching and microwave blanching respectively (p < 0.05). However, a* value at optimum level of blanching reduced to 5.1 ± 0.2, 4.9 ± 0.4 and 5.2 ± 0.5 in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively (Fig. 3b). This increase in a* value may associated to the red pigments (Thakur et al. 2017) formed during the blanching. Similar results at higher temperature treatment were also reported by Deylami et al. (2016). The b* value significantly increased from 6.7 ± 0.9 to 7.3 ± 0.6, 7.3 ± 0.7 and 7.9 ± 0.6 in hot water blanching, steam blanching and microwave blanching respectively (p < 0.05). However, Colour b* at optimum level of blanching increased to 7.2 ± 0.7, 7.0 ± 0.3 and 7.4 ± 0.4 in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively (Fig. 3c). Total colour difference significantly increased to 5.2 ± 0.2, 4.4 ± 0.3 and 4.7 ± 0.5 in hot water blanching, steam blanching and microwave blanching respectively (p < 0.05). The smallest colour difference ΔE, which can be assessed by human eye is 1.0, indicating noticeable change in colour was observed. However, ΔE at optimum level of blanching reduced to 3.7 ± 0.4, 2.1 ± 0.6 and 2.7 ± 0.2 in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively (Fig. 3d). However, the difference in total colour difference index in various blanching treatments at optimum level was significant (p < 0.05). Chroma significantly increased from 8.1 ± 0.2 to 9.0 ± 0.3, 8.9 ± 0.8 and 9.8 ± 0.7 in hot water blanching, steam blanching and microwave blanching respectively (p < 0.05). The increase in chroma value indicates higher vividness of colours attained during heat treatment, which is evident due to the non-enzymatic browning during the blanching at higher temperatures. The chroma at optimum level of blanching was reduced to 8.8 ± 0.5, 8.5 ± 0.6 and 9.0 ± 0.2 in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively (Fig. 3e). However, the difference in chroma in various blanching treatments at optimum level was not significant. Similar results for mangosteen at higher temperature treatment were also reported by Deylami et al. (2016). The hue angle value corresponds to whether the object is red, orange, yellow, green, blue or violet. The initial hue angle of sweet corn was yellow region (hue angle between 0° and 90°) of the colour solid dimensions. Hue angle significantly decreased from 56.1 ± 0.6 to 54.0 ± 0.2, 54.5 ± 0.7 and 53.7 ± 0.2 in hot water blanching, steam blanching and microwave blanching respectively (p < 0.05). Hue angle at optimum level of blanching reduced to 54.7 ± 0.8, 55.0 ± 0.9 and 54.9 ± 0.6 in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively (Fig. 3e). However, the difference in hue angle in various blanching treatments at optimum level was not significant. The 0°–60° values of hue angle indicates red to yellow colour region. The decrease in hue angle upon heating indicates shifting of colour towards more reddishness in sweet corn kernels (Shahabi et al. 2013). Browning index significantly increased from 13.5 ± 0.7 to 16.2 ± 0.6, 16.0 ± 0.3 and 17.7 ± 0.2 in hot water blanching, steam blanching and microwave blanching respectively (p < 0.05). The increase in browning index is evident due to non-enzymatic browning at higher temperatures during blanching operations. However, browning index at optimum level of blanching increased to 15.6 ± 0.7, 14.7 ± 0.6 and 15.7 ± 0.4 in hot water blanching for 120 s, steam blanching for 90 s and microwave blanching for 60 s respectively (Fig. 3f) and the difference in browning index in various blanching treatments at optimum levels was not significant.
Fig. 3.
Variation in colour of sweet corn during blanching
Kinetics of biochemical and physical properties
Zero order model and first order model fitted well in varies physico-chemical parameter due to blanching operation (Table 2). The average values of R2, χ2 and RMSE were 0.945, 0.007 and 0.1146 for zero-order and 0.944, 1.164 and 1.950 for first-order respectively. The average value of R2 is higher along with lower χ2 and RMSE in zero-order equation in comparison to first-order equation. Therefore, zero-order equation is recommended for the prediction of all physico-chemical parameters in the study.
Table 2.
Kinetic parameters of zero-order and first-order reactions of physico-chemical properties
| Treatment | Zero order | First order | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ko | Co | R2 | χ2 | RMSE | K1 | C1 | R2 | χ2 | RMSE | |
| Total sugar | ||||||||||
| HW | − 0.0187 | 8.5400 | 0.9949 | 0.0022 | 0.0566 | − 0.0028 | 8.7321 | 0.9880 | 1.8433 | 1.7595 |
| SM | − 0.0157 | 8.5300 | 0.9915 | 0.0028 | 0.06164 | − 0.0022 | 8.6618 | 0.9825 | 1.2681 | 1.4587 |
| MW | − 0.0320 | 9.4400 | 0.9961 | 0.0005 | 0.02828 | − 0.0043 | 9.6883 | 0.9983 | 2.0271 | 1.9531 |
| Ascorbic acid | ||||||||||
| HW | − 0.0130 | 7.4100 | 0.9676 | 0.0087 | 0.1010 | − 0.0021 | 7.4885 | 0.9727 | 0.9344 | 1.1633 |
| SM | − 0.0090 | 7.1900 | 0.8399 | 0.0218 | 0.1667 | − 0.0014 | 7.2119 | 0.8477 | 0.4484 | 0.7952 |
| MW | − 0.0260 | 7.8800 | 0.9548 | 0.0052 | 0.0800 | − 0.0041 | 8.0610 | 0.9605 | 1.5014 | 1.5298 |
| TSS | ||||||||||
| HW | − 0.0580 | 20.5400 | 0.9918 | 0.0162 | 0.2245 | − 0.0039 | 21.4473 | 0.9766 | 9.2224 | 6.2207 |
| SM | − 0.0303 | 20.9100 | 0.9968 | 0.0015 | 0.0735 | − 0.0017 | 21.0868 | 0.9941 | 2.1738 | 3.0131 |
| MW | − 0.0290 | 20.6400 | 0.7250 | 0.0171 | 0.2526 | − 0.0016 | 20.7362 | 0.7206 | 0.7883 | 1.8036 |
| Moisture content | ||||||||||
| HW | 0.0233 | 76.5400 | 0.9855 | 0.0009 | 0.12 | 0.0003 | 76.5636 | 0.9867 | 0.3375 | 2.2739 |
| SM | 0.0183 | 76.5900 | 0.9912 | 0.0004 | 0.0735 | 0.0002 | 76.6036 | 0.9913 | 0.2162 | 1.82 |
| MW | − 0.0570 | 78.4000 | 0.8955 | 0.0051 | 0.2753 | − 0.0008 | 78.4856 | 0.8934 | 0.8082 | 3.5607 |
| Kernel weight | ||||||||||
| HW | 0.0005 | 0.4741 | 0.9823 | 0.0001 | 0.0026 | 0.0010 | 0.4747 | 0.9805 | 0.0159 | 0.0432 |
| SM | 0.0003 | 0.4723 | 0.9542 | 0.0001 | 0.0022 | 0.0005 | 0.4725 | 0.9522 | 0.0056 | 0.0244 |
| MW | − 0.0016 | 0.514 | 0.9552 | 0.0003 | 0.0049 | − 0.0038 | 0.5260 | 0.9473 | 0.0608 | 0.0645 |
| Geometric diameter | ||||||||||
| HW | 0.0038 | 8.0476 | 0.9796 | 0.0004 | 0.0282 | 0.0004 | 8.0667 | 0.9757 | 0.0736 | 0.3459 |
| SM | 0.0026 | 8.04 | 0.9411 | 0.0004 | 0.0282 | 0.0003 | 8.0421 | 0.9403 | 0.0335 | 0.2330 |
| MW | − 0.025 | 8.74 | 0.9585 | 0.0037 | 0.0734 | − 0.0034 | 8.8962 | 0.9590 | 1.2675 | 1.4824 |
| Colour L* | ||||||||||
| HW | − 0.042 | 76.3100 | 0.7777 | 0.0630 | 0.0960 | − 0.0006 | 76.3717 | 0.7813 | 1.2219 | 4.3181 |
| SM | − 0.034 | 75.1100 | 0.9726 | 0.0042 | 0.2445 | − 0.0005 | 75.1542 | 0.9744 | 0.7777 | 3.4175 |
| MW | − 0.095 | 78 | 0.9401 | 0.0079 | 0.3391 | − 0.0013 | 78.2389 | 0.9373 | 2.3165 | 6.0173 |
| Colour a* | ||||||||||
| HW | 0.0023 | 4.4300 | 0.9423 | 0.0007 | 0.0245 | 0.0005 | 4.4340 | 0.9438 | 0.0362 | 0.1802 |
| SM | 0.0023 | 4.4300 | 0.9423 | 0.0007 | 0.0245 | 0.0005 | 4.4340 | 0.9438 | 0.0362 | 0.1802 |
| MW | 0.0190 | 3.8200 | 0.9704 | 0.0022 | 0.0469 | 0.0038 | 3.9398 | 0.9766 | 0.8039 | 0.8214 |
| Colour b* | ||||||||||
| HW | 0.0323 | 35.1100 | 0.9272 | 0.0191 | 0.3844 | 0.0008 | 35.2146 | 0.9353 | 1.3282 | 3.0631 |
| SM | 0.0200 | 36.3600 | 0.9698 | 0.0029 | 0.1496 | 0.0005 | 36.3931 | 0.9707 | 0.5023 | 1.9137 |
| MW | 0.0550 | 36.4600 | 0.9720 | 0.0022 | 0.1319 | 0.0014 | 36.5769 | 0.9695 | 1.3910 | 3.1939 |
| Colour ΔE | ||||||||||
| HW | 0.03755 | − 0.8433 | 0.998 | 0.011 | 0.075 | 0.02184 | 0.2504 | 0.875 | 1.178 | 0.7438 |
| SM | 0.03008 | − 0.2849 | 0.983 | 0.101 | 0.1696 | 0.01401 | 0.5828 | 0.987 | 1.657 | 0.9102 |
| MW | 0.1017 | − 3.311 | 0.952 | 0.313 | 0.3227 | 0.0392 | 0.2287 | 0.982 | 1.593 | 0.9642 |
| Colour chroma | ||||||||||
| HW | 0.00792 | 7.8657 | 0.992 | 5E −04 | 0.0302 | 0.00093 | 7.8859 | 0.989 | 0.287 | 0.6779 |
| SM | 0.00641 | 8.012 | 0.989 | 5E −04 | 0.0281 | 0.00075 | 8.0268 | 0.99 | 0.187 | 0.5511 |
| MW | 0.03576 | 6.9113 | 0.998 | 2E −04 | 0.021 | 0.00395 | 7.1345 | 0.999 | 2.056 | 1.7444 |
| Colour hue angle | ||||||||||
| HW | − 0.0175 | 56.761 | 0.975 | 0.001 | 0.1195 | − 0.0003 | 56.781 | 0.973 | 0.263 | 1.728 |
| SM | − 0.0109 | 56.044 | 0.906 | 0.002 | 0.1482 | − 0.0002 | 56.049 | 0.908 | 0.103 | 1.0756 |
| MW | − 0.057 | 58.268 | 0.939 | 0.004 | 0.2064 | − 0.001 | 58.374 | 0.939 | 1.088 | 3.5615 |
| Browning index | ||||||||||
| HW | 0.02317 | 12.85 | 0.952 | 0.017 | 0.2210 | 0.00156 | 12.947 | 0.948 | 1.075 | 1.7082 |
| SM | 0.01902 | 13.336 | 0.991 | 0.002 | 0.0754 | 0.00127 | 13.406 | 0.99 | 1.072 | 1.7054 |
| MW | 0.08675 | 10.638 | 0.989 | 0.005 | 0.1276 | 0.00546 | 11.383 | 0.995 | 7.836 | 4.2914 |
Conclusion
The study revealed that 120, 90, 60 s were needed for optimum level of hot water blanching, stem blanching and microwave blanching for sweet corn.The peroxidase activity data fitted well to first order equation for all treatments. The microwave blanching has minimum D value and t(1/2) values for inactivation of peroxidase enzyme. The maximum retention of total sugar, ascorbic acid, moisture content, kernel mass and geometric diameter were 7.50 g/100 g, 6.60 mg/100 g, 75.30%, 0.42 g and 7.20 mm in microwave blanching. Total change in colour, chroma, hue angle and browning index showed increase in brown colour during blanching process. The average R2, χ2 and RMSE value for zero-order model are 0.945, 0.007 and 0.1146, indicates the applicability of the model for prediction of physico-chemical parameters during blanching of sweet corn.
Acknowledgements
Authors are thankful for Anand Agricultural University for providing the facility for conducting the research.
References
- Arroqui C, Rumsey TR, Lopez A, Virseda P. Effect of different soluble solids in the water on the ascorbic acid losses during blanching of potato tissue. J Food Eng. 2001;47:123–126. doi: 10.1016/S0260-8774(00)00107-2. [DOI] [Google Scholar]
- Bahceci KS, Serpen A, Gokmen V, Acar J. Study of lipoxygenase and peroxidase as indicator enzymes in green beans: change of enzyme activity, ascorbic acid and chlorophylls during frozen storage. J Food Eng. 2005;66:187–192. doi: 10.1016/j.jfoodeng.2004.03.004. [DOI] [Google Scholar]
- Barrett DM, Garcia EL, Russel GF, Ramirez E, Shirazi A. Blanch time and cultivar effects on quality of frozen and stored corn and broccoli. J Food Sci. 2000;65:534–540. doi: 10.1111/j.1365-2621.2000.tb16043.x. [DOI] [Google Scholar]
- De Corcuera JIR, Cavalieri RP, Powers JR. Encyclopaedia of agri, food and biological engineering. New York City: Marcel Dekker; 2004. Blanching of foods; pp. 1–5. [Google Scholar]
- Deylami MZ, Rahman RA, Tan CP, Bakar J, Olusegun L. Effect of blanching on enzyme activity, color changes, anthocyanin stability and extractability of mangosteen pericarp: a kinetic study. J Food Eng. 2016;178:12–19. doi: 10.1016/j.jfoodeng.2016.01.001. [DOI] [Google Scholar]
- Espachs-Barroso A, Van Loey A, Hendrickx M, Martín-Belloso O. Inactivation of plant pectin methylesterase by thermal or high intensity pulsed electric field treatments. Innov Food Sci Emerg Technol. 2006;7:40–48. doi: 10.1016/j.ifset.2005.07.002. [DOI] [Google Scholar]
- Hashim N, Janius RB, Baranyai L, Rahman RA, Osman A, Zude M. Kinetic model for colour changes in bananas during the appearance of chilling injury symptoms. Food Biopro Technol. 2012;5:2952–2963. doi: 10.1007/s11947-011-0646-z. [DOI] [Google Scholar]
- Igual M, Sampedro F, Martínez-Navarrete N, Fan X. Combined osmo dehydration and high pressure processing on the enzyme stability and antioxidant capacity of a grapefruit jam. J Food Eng. 2013;114:514–521. doi: 10.1016/j.jfoodeng.2012.09.006. [DOI] [Google Scholar]
- Karababa E, Coskuner Y. Moisture dependent physical properties of dry sweet corn kernels. Int J Food Prop. 2007;10:549–560. doi: 10.1080/10942910601003981. [DOI] [Google Scholar]
- Lane JH, Eynon L. Determination of reducing sugars by Fehling’s solution with methylene blue indicator. London: N. Rodger; 1934. [Google Scholar]
- Lappe R, Cladera-Olivera F, Dominguez APM, Brandell A. Kinetics and thermodynamics of thermal inactivation of the antimicrobial peptide cerein 8A. J Food Eng. 2009;91:223–227. doi: 10.1016/j.jfoodeng.2008.08.025. [DOI] [Google Scholar]
- Lau MH, Tang J. Pasteurization of pickled asparagus using 915 MHz microwaves. J Food Eng. 2002;51:283–290. doi: 10.1016/S0260-8774(01)00069-3. [DOI] [Google Scholar]
- Lin S, Brewer MS. Effects of blanching method on the quality characteristics of frozen peas. J Food Qual. 2005;28:350–360. doi: 10.1111/j.1745-4557.2005.00038.x. [DOI] [Google Scholar]
- Mohammadi A, Rafiee S, Emam-Djomeh Z, Keyhani A. Kinetic models for colour changes in kiwifruit slices during hot air drying. World J Agric Sci. 2008;4:376–383. [Google Scholar]
- Mohsenin NN. Thermal properties of foods and agricultural materials. New York: Gordon and Breach Science Publishers; 1980. [Google Scholar]
- Nascimento P, Fernandes NS, Mauro MA, Kimura M. Beta-carotene stability during drying and storage of cassava and sweet potato. Acta Hortic. 2009;841:363–366. doi: 10.17660/ActaHortic.2009.841.45. [DOI] [Google Scholar]
- Neri L, Hernando IH, Pérez-Munuera I, Sacchetti G, Pittia P. Effect of blanching in water and sugar solutions on texture and microstructure of sliced carrots. J Food Sci. 2011;76:23–30. doi: 10.1111/j.1750-841.2010.01906.x. [DOI] [PubMed] [Google Scholar]
- Oliveira FA, Silva CL. Freezing influences diffusion of reducing sugars in carrot cortex. J Food Sci. 1992;57:932–934. doi: 10.1111/j.1365-2621.1992.tb14326.x. [DOI] [Google Scholar]
- Park SU, Cha SW, Son YH, Son YK. Changes of sugar content by different storage duration in sweet corn and super sweet corn. Korean J Crop Sci. 1994;39:79–84. [Google Scholar]
- Parmar N, Singh N, Kaur A, Thakur S. Comparison of color, anti-nutritional factors, minerals, phenolic profile and protein digestibility between hard-to-cook and easy-to-cook grains from different kidney bean (Phaseolus vulgaris) accessions. J Food Sci Technol. 2017;54:1023–1034. doi: 10.1007/s13197-017-2538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. New York: Tata McGraw-Hill Education; 1986. [Google Scholar]
- Rickman JC, Barrett DM, Bruhn CM. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. Vitamins C and B and phenolic compounds. J Sci Food Agric. 2007;87:930–944. doi: 10.1002/jsfa.2825. [DOI] [Google Scholar]
- Shahabi M, Rafiee S, Mohtasebi SS, Hosseinpour S. Image analysis and green tea color change kinetics during thin-layer drying. Food Sci Technol Int. 2013;20(6):465–476. doi: 10.1177/1082013213492524. [DOI] [PubMed] [Google Scholar]
- Sheu SC, Chen AO. Lipoxygenase as blanching index for frozen vegetable soybeans. J Food Sci. 1991;56:448–451. doi: 10.1111/j.1365-2621.1991.tb05300.x. [DOI] [Google Scholar]
- Song JY, An GH, Kim CJ. Color, texture, nutrient contents, and sensory values of vegetable soybeans [Glycine max (L.) Merrill] as affected by blanching. Food Chem. 2003;83(1):69–74. doi: 10.1016/S0308-8146(03)00049-9. [DOI] [Google Scholar]
- Thakur S, Kaur A, Singh N, Virdi AS. Successive reduction dry milling of normal and waxy corn: grain, grit, and flour properties. J Food Sci. 2015;80:C1144–C1155. doi: 10.1111/1750-3841.12895. [DOI] [PubMed] [Google Scholar]
- Thakur S, Singh N, Kaur A. Characteristics of normal and waxy corn: physicochemical, protein secondary structure, dough rheology and chapatti making properties. J Food Sci Technol. 2017;54:3285–3296. doi: 10.1007/s13197-017-2775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo F, Tomas-Barberan F, Garcia-Viguera C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur Food Res Technol. 2002;215:310–316. doi: 10.1007/s00217-002-0560-8. [DOI] [Google Scholar]
- Vigneault C, Goyette B, Gariépy Y, Cortbaoui P, Charles MT, Raghavan VG. Effect of ear orientations on hydrocooling performance and quality of sweet corn. Postharvest Biol Technol. 2007;43:351–357. doi: 10.1016/j.postharvbio.2006.09.013. [DOI] [Google Scholar]