Abstract
With the objective of utilizing agro-industrial by-products and enhancing the quality attributes of dehydrated fruits, this study evaluated the effects of edible pectin-based coatings containing disintegrated guava by-products (up to 50% concentration) and drying temperatures (46–74 °C) on the drying kinetics and nutritional properties of dried red guava. Coatings were applied to guava slices prior to hot-air drying. A central composite rotatable design was used to optimize carotenoid and phenolic compound retention. The effects of the edible coating compositions combined with the drying temperatures affected the effectiveness of the film as a barrier to carotenoid oxidation. Total phenolic compound retention, however, was affected only by temperature. Responses were simultaneously optimized, and high carotenoid and total phenolic retentions were obtained at approximately 60 °C with 25% by-product concentration. The effective water diffusivities were mainly affected by temperature. Consequently, the coatings improved nutritional quality without having a major impact on drying times.
Keywords: Psidium guajava L., Diffusion, Bioactive compounds, Carotenoids, Total phenolic compounds, Desirability approach
Introduction
Guava (Psidium guajava L.) is a fruit primarily cultivated in tropical and subtropical areas of the world, including South America, Mexico, Africa and Southeast Asia. It possesses a pleasant aroma and flavor and has excellent nutritional value due to its vitamin, mineral, fiber, and antioxidant contents, which include the carotenoids lycopene and β-carotene in red and pink-flesh varieties (Flores et al. 2015; Rodriguez-Amaya et al. 2007). This fruit is commercially produced in many tropical and subtropical countries. Currently, the international fresh guava trade is limited but processed guava products, such as canned guava and guava beverages, are becoming increasingly popular in many countries. Guava puree is a very important commercial product, and its demand is increasing for use as a flavoring ingredient, which is driven by the food and beverage industries. Even though there is a gap in postharvest technology, a huge potential for the expansion of fresh and processing guava industries is expected in the coming years (Singh 2011). However, guavas are climacteric fruits that present a high metabolic activity after harvest (Vieira et al. 2012). Post-harvest losses are immense for a variety of reasons, including unmet quality standards, excess production, poor distribution logistics, and wasteful food industry processing. In this context, new conservation methods should be studied. For instance, in a dehydrated form, guava can be used in the elaboration of numerous products, adding flavor and nutrients.
Drying, one of the most commonly used methods in the food industry, reduces the water content of a fruit to a level that prevents microbial growth and other reactions. Hot-air drying is commonly applied to fruits and vegetables despite its disadvantages and limitations. These issues include degradation of important compounds such as ascorbic acid (Mrad et al. 2012) or carotenoids (Rodriguez-Amaya 1999) because of high temperatures for long times as well as enzymatic and non-enzymatic oxidation. Because of these problems, several studies have sought practical solutions to minimize the physical and nutritional damage brought about by the drying process.
Another important issue is related to food losses by industries. The food processing industry generates a substantial amount of waste. These by-products, which are difficult to treat properly, often contain a higher nutritional content than the fruit itself. Residues and by-products of fruits and vegetables have been found to be potential sources of natural antioxidants (Kabir et al. 2015; Silva et al. 2014a).
In this sense, new technologies seek to use these residues and by-products during processing to give value to a portion of food that would otherwise be discarded (Silva et al. 2014b). These technologies include edible coatings that are traditionally used in minimal food processing to incorporate active ingredients into fresh-cut fruits in an attempt to improve their appearance, extend the shelf life and enhance their quality and nutritional value (Rojas-Graü et al. 2009). However, in recent literature, there are indications that edible coatings used before the drying processes may have a protective effect in terms of nutrient oxidation and may help improve appearance. Lago-Vanzela et al. (2013) observed a greater carotenoid retention in pre-treated slices than in fresh slices when using starch-based coatings on pumpkin slices. The use of pectin-based coatings has also been used successfully by other authors. Canizares and Mauro (2015) and Garcia et al. (2014) detected an enhancement in ascorbic acid retention in pieces of papayas coated with pectin before air-drying. Pectin has been shown to be a good substance for edible coating development. It has wide use in the food industry as a gelling agent, thickener, texture agent, emulsifier and stabilizer. The functionality of the pectin molecule is determined by numerous factors, including the size and degree of methoxylation of the molecule. Amidated pectin grants thermoreversibility to gels and requires a lower amount of ions to gel (Racape et al. 1989; Thakur et al. 1997; Voragen et al. 1986).
Optimization of a food process can be useful for establishing processing conditions that yield products with desired properties. The Response Surface Methodology technique (Montgomery 2001) is a statistical approach for the modeling and analysis of problems in which the response variable is influenced by one or several factors. The desirability approach (Derringer and Suich 1980) is one of the most widely used methods in the industry for the optimization of multiple response processes (Granato et al. 2010) and is used when more than one answer is important to the product.
With the goal of making better use of agro-industrial by-products and improving the quality of the dried guava, this study investigated the application of pectin-based coatings incorporated with guava by-products prior to hot-air drying and their effects on the drying kinetics and bioactive compound retention.
Materials and methods
Materials
Red guavas (Psidium guajava L.) of the Pedro Sato cultivar were acquired directly from the producer in the greater São José do Rio Preto region of São Paulo State, Brazil. Chemicals reagents were acquired from LabSynth Laboratory Products (Diadema, São Paulo, Brazil). Sigma Aldrich (St. Louis, Missouri, USA) supplied the gallic acid and Folin-Ciocalteu reagent. Food-grade amidated citric pectin (degree of methoxylation: 34%; degree of amidation: 17%) (GRINDSTED® LA 210, Danisco, São Paulo, Brazil) and calcium lactate pentahydrate (PURAC® Synthesis, São Paulo, Brazil) were used to prepare the coating.
Sample preparation
Fruits presenting approximately 10°Brix were selected, washed and wiped dry. The skins and the flesh containing seeds were removed, ground with a blender (Philips Walita®, Varginha, Brazil) and strained, forming a puree of by-products (BP). Pulp was cut into 2.2-cm diameter slices using a sharp-edged tube.
Coating preparation
A 2% (w/w) aqueous pectin solution was prepared at 70 °C. Coatings were obtained by mixing the disintegrated by-products with the 2% pectin solution in different proportions according to the experimental design (Table 1) and maintained at 40 °C using a water bath. The guava slices were placed in perforated baskets, immersed in the coating solution at 40 °C for 1 min and then immersed in a 1% (w/w) calcium lactate aqueous solution for 1 min to cause pectin gelling.
Table 1.
Content of water, total carotenoid (TC), ascorbic acid (AA) and total phenolic contend (TCP), before and after drying, for each experimental condition of temperature (T) and percentage of guava by-product added to the edible coating (BP)
| Trial | Variables | Moisture contenta | TCb | AA c | TCP without AA correctiond | TCPe | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | Before |
After
|
Before
|
After
|
Before | After | Before | After | Before | After | ||
| 1 | 60 | 25 | 6.89 ± 0.14 | 0.25 ± 0.05 | 710.19 ± 35.01 | 540.57 ± 78.84 | 24.53 ± 1.00 | 9.68 ± 0.10 | 35.17 ± 0.92 | 23.99 ± 1.57 | 15.58 | 17.76 |
| 2 | 50 | 7.3 | 5.99 ± 0.05 | 0.33 ± 0.01 | 500.29 ± 27.10 | 593.46 ± 35.32 | 11.05 ± 1.08 | 11.26 ± 0.40 | 38.50 ± 2.27 | 25.80 ± 0.75 | 27.92 | 16.25 |
| 3 | 60 | 25 | 5.85 ± 0.17 | 0.28 ± 0.03 | 531.03 ± 19.96 | 526.22 ± 40.34 | 11.99 ± 0.28 | 10.48 ± 0.15 | 30.87 ± 1.08 | 27.72 ± 1.22 | 19.54 | 17.06 |
| 4 | 60 | 25 | 6.70 ± 0.07 | 0.19 ± 0.02 | 838.22 ± 65.76 | 808.45 ± 99.32 | 12.52 ± 0.43 | 8.84 ± 0.66 | 31.20 ± 2.40 | 24.41 ± 1.11 | 17.27 | 14.66 |
| 5 | 60 | 0 | 6.87 ± 0.09 | 0.23 ± 0.08 | 768.67 ± 4.09 | 634.74 ± 10.60 | 16.45 ± 0.45 | 8.45 ± 0.11 | 40.75 ± 1.86 | 26.61 ± 1.13 | 26.51 | 16.74 |
| 6 | 70 | 42.7 | 7.23 ± 0.27 | 0.19 ± 0.02 | 913.05 ± 113.76 | 701.66 ± 98.65 | 19.92 ± 0.31 | 9.67 ± 0.10 | 31.80 ± 0.92 | 21.97 ± 1.91 | 14.49 | 12.11 |
| 7 | 60 | 50 | 7.67 ± 0.16 | 0.20 ± 0.00 | 1191.00 ± 93.64 | 839.57 ± 106.09 | 22.13 ± 0.62 | 13.48 ± 1.21 | 28.64 ± 3.15 | 20.15 ± 1.15 | 10.36 | 8.17 |
| 8 | 70 | 7.3 | 7.99 ± 0.03 | 0.26 ± 0.01 | 1228.75 ± 90.15 | 703.03 ± 38.74 | 18.91 ± 0.98 | 12.21 ± 0.29 | 31.34 ± 1.81 | 26.87 ± 0.40 | 13.97 | 14.58 |
| 9 | 50 | 42.7 | 8.42 ± 0.16 | 0.23 ± 0.01 | 964.78 ± 50.11 | 683.99 ± 26.51 | 24.09 ± 1.38 | 13.19 ± 1.15 | 41.07 ± 0.72 | 28.04 ± 0.79 | 18.76 | 14.90 |
| 10 | 60 | 25 | 7.23 ± 0.19 | 0.22 ± 0.02 | 717.06 ± 12.89 | 613.74 ± 80.78 | 16.13 ± 1.10 | 7.59 ± 0.11 | 31.57 ± 1.37 | 22.93 ± 1.21 | 19.20 | 16.60 |
| 11 | 74.1 | 25 | 7.51 ± 0.18 | 0.23 ± 0.02 | 824.59 ± 8.64 | 485.28 ± 9.98 | 16.10 ± 0.94 | 7.54 ± 0.70 | 31.72 ± 1.75 | 19.87 ± 0.45 | 19.36 | 13.77 |
| 12 | 45.9 | 25 | 7.25 ± 0.03 | 0.18 ± 0.01 | 669.79 ± 33.44 | 466.72 ± 48.58 | 14.10 ± 0.30 | 6.03 ± 0.55 | 30.90 ± 1.46 | 14.10 ± 0.76 | 19.95 | 9.32 |
| 13 | 60 | 25 | 6.72 ± 0.09 | 0.24 ± 0.01 | 586.03 ± 18.85 | 558.33 ± 13.06 | 13.23 ± 0.16 | 7.79 ± 0.07 | 28.16 ± 1.27 | 19.22 ± 1.33 | 17.88 | 13.00 |
aExpressed as kg water·kg−1 dry matter; bexpressed as mg lycopene·kg−1 dry matter; cexpressed as g ascorbic acid·kg−1 of dry matter; dexpressed as g gallic acid equivalent (GAE)·kg−1 dry matter, without AA interference correction; eexpressed as g gallic acid equivalent (GAE)·kg−1 dry matter, after applying the correction factor (0.64 g AA g−1 GAE) on ascorbic acid
Hot-air drying
The coated guava slices were weighed in stainless-steel mesh trays and dried in a fixed-bed batch dryer with forced convection of hot-air (1 m s−1) until moisture reached approximately 20% (w/w) (wet basis) using different drying temperatures (T), as shown in Table 1. The trays were weighed every 20 min during the first hour of drying and then every 30 min until the samples reached the desired moisture level.
Analytical procedures
Moisture content
Moisture content was gravimetrically determined in triplicate by drying the samples in a vacuum oven at 60 °C and 10 kPa until a constant weight was reached (AOAC 2005). The results were expressed as kg of water 100 kg−1 of dry matter.
Total carotenoid content
The total carotenoid content (TC) was determined in triplicate according to the methodology proposed by Rodriguez-Amaya and Kimura (2004). Absorbance was measured at 470 nm in a spectrophotometer (Beckman, Fullerton, USA). The results were expressed as mg of lycopene kg−1 of dry matter, as shown in Eq. 1:
| 1 |
where A represents the sample absorbance, V is the total volume of the extract (cm3), is the coefficient of absorbance of 3450 for lycopene in petroleum ether, and m is the dry mass of the sample in kg.
Ascorbic acid content
The ascorbic acid content (AA) of the coated guava slices was analyzed before and after drying in duplicate using the AOAC method (2005) modified by Benassi and Antunes (1988). The results were expressed as g of ascorbic acid kg−1 of dry matter.
Total phenolic content
The total phenolic content (TPC) was determined in triplicate using the Folin-Ciocalteu reagent (Asami et al. 2003). The extracts of the samples were prepared using an extraction solution containing 70% acetone (v/v). Absorbance was measured at 720 nm in a spectrophotometer (Beckman, Fullerton, USA). The calibration curve was prepared with a standard gallic acid solution in the range from 80 to 200 µg ml−1 of water. The results were expressed as g of gallic acid equivalent (GAE) kg−1 of dry matter. A correction factor of 0.64 was applied to AA and deduced from the TPC because AA contributes significantly to the response of the Folin-Ciocalteu assay. According to Asami et al. (2003), one gram of AA contributes a proportion of approximately 0.64 g of GAE.
Determination of retention
The bioactive compound retention () of the total carotenoids and total phenolic compounds during the drying process were evaluated according to Murphy et al. (1975) as follows:
| 2 |
where represents bioactive compound retention, i refers to the specific compound, w represents the bioactive compound content, m represents the mass, 0 corresponds to the initial condition before drying and f represents the final condition after drying.
Drying kinetics and effective diffusivity
To describe the drying of the guava slices, the moisture migration was assumed to occur by diffusion in an infinite flat plate whose shrinkage was negligible, and the temperature and the diffusion coefficients were assumed constant over time. Therefore, the water evaporation was modeled according to Fick’s second law:
| 3 |
where is the effective diffusion coefficient (m2 s−1); X is the mass fraction of water (kg water kg−1 dry matter); t is the drying time (s); and z represents the coordinate axis (m) along the thickness slice. The analytical solution of Eq. (3) integrated along the thickness for an infinite flat plate is given in terms of the mean concentration in the plate at time t (Crank 1975):
| 4 |
where is the mean fraction of the water mass in the slice at time t, is the initial water mass fraction (dry basis) and represents the equilibrium water mass fraction on the slice surface (dry basis). MR represents the dimensionless average fraction or residual moisture. Equation (4) was fitted to the experimental data using “Prescribed” software (Silva and Silva 2008) to determine the diffusion coefficient. The fitting efficiency was based on the coefficient of determination (R2) and the mean relative error (P), as defined by Eq. (5) (Lomauro et al. 1985):
| 5 |
where represents the experimental value, the calculated value and n the number of observations.
Empirical models
The drying kinetics were also evaluated by simple empirical drying models suitable for thin-layer drying (Ertekin and Yaldiz 2004; Gupta et al. 2011; Rhim and Lee 2011; Vega-Galvéz et al. 2008), which were the Newton (Eq. 6), Page (Eq. 7), and Henderson–Pabis models (Eq. 8):
| 6 |
| 7 |
| 8 |
where k is the drying rate constant, (s−1) or (s–n), and n and a are fitting constants (dimensionless).
Equations (6–8) were fitted to the experimental data using “Prescribed” software (Silva and Silva 2008) to determine the model constants.
Experimental design and statistical analysis
Preliminary drying experiments were performed to determine the by-product (BP) quantity to be incorporated into the based-pectin coating solution. By-products were mixed with a pectin solution (2%, w/w) according to mass concentrations varying from 0% (w/w) of BP (only pectin) to 100% (w/w) of BP (no pectin) in the total coating solution. Coating solutions composed of more than 50% of BP were difficult to apply to the guava slices, and the thicknesses could not be standardized. Therefore, the BP content was limited to 50%.
To optimize the process based on the ranges selected, a Central Composite Rotatable Design (CCRD) was used (22) with four axial points and five replications of the central point (Montgomery 2001). The two investigated factors were drying temperature (T) and by-product concentration (BP) in the coating pectin-based solution. Drying temperatures varied from 46 to 74 °C, and the by-product concentration varied from 0 to 50% of the total coating solution. Table 1 shows each trial with the combinations of the T (°C) and BP (%) factors and their codified values and , which were obtained by the expressions:
| 9 |
| 10 |
The responses analyzed were total carotenoid retention, ascorbic acid, total phenolic compounds and water diffusivity. The regression model used for describing each response variable (or dependent variable) was the second-order model (Montgomery 2001):
| 11 |
for each i compound. In Eq. (11), and represent linear terms; and are the quadratic terms; is the cross product of the terms; ,, and are the coefficients of the linear terms; and are the coefficients of the quadratic terms, and is the random error.
The effect of the factors on each response was evaluated using analysis of variance (ANOVA) in the software Statistica (Version 7.0, StatSoft Inc, Tulsa, USA). In addition, simultaneous optimization was carried out for bioactive compounds (Montgomery, 2001). The desirability approach proposed by Derringer and Suich (1980) was applied using the software Statistica 7.0 (Statsoft 2007).
The general approach involves transformation of each estimated response variable to an individual desirability value whose values range from 0 to 1. If the response is outside an acceptable region, then , and if the response is at its target (the most desirable value), . Then, the design variables are chosen to maximize the overall desirability of the combined responses, as follows:
| 12 |
where m is the response number.
If the target is located between the lower and upper limits, the transformations are given by:
| 13 |
The values s and t are specified by the user to indicate how important it is for to be close to the target (Montgomery 2001).
Results
Bioactive compounds
Moisture, total carotenoid, ascorbic acid and total phenolics content before and after drying are shown in Table 1 as a function of the temperature (°C) and percentage of red guava (BP) incorporated into the pectin coating. Table 2 presents responses according to the applied Central Composite Rotatable Design (Montgomery 2001).
Table 2.
Central composite rotatable design arrangement, coded (uncoded) levels of temperature (T) and percentage of guava by-product added to the edible coating (BP), responses based on bioactive compound retention and on effective diffusion coefficients and corresponding R2 and P (%) for the Eq. (4) fittings
| Trial | Coded and uncoded variables | Responses for retention | Response for diffusivity | ||||
|---|---|---|---|---|---|---|---|
|
Y
1
(TC Ret, %) |
Y
2
(TPC Ret, %) |
Y
3
×1010 (m2 s−1) |
R 2 | P (%) | |||
| 1 | 0 (60) | 0 (25) | 75.90 | 113.98 | 9.91 | 0.990 | 22.1 |
| 2 | − 1 (50) | − 1 (7.3) | 118.58 | 58.19 | 8.10 | 0.990 | 25.4 |
| 3 | 0 (60) | 0 (25) | 99.00 | 87.32 | 8.80 | 0.990 | 23.6 |
| 4 | 0 (60) | 0 (25) | 96.64 | 84.88 | 7.76 | 0.989 | 28.5 |
| 5 | 0 (60) | − 1.414 (0) | 82.58 | 63.12 | 8.51 | 0.987 | 11.2 |
| 6 | 1 (70) | 1 (42.7) | 77.10 | 83.55 | 10.31 | 0.981 | 41.4 |
| 7 | 0 (60) | 1.414 (50) | 70.52 | 78.80 | 6.44 | 0.991 | 23.6 |
| 8 | 1 (70) | − 1 (7.3) | 57.20 | 104.37 | 9.07 | 0.980 | 23.3 |
| 9 | − 1 (50) | 1 (42.7) | 70.87 | 79.38 | 5.51 | 0.990 | 13.7 |
| 10 | 0 (60) | 0 (25) | 85.53 | 86.46 | 9.17 | 0.992 | 14.7 |
| 11 | 1.414 (74.1) | 0 (25) | 58.85 | 71.12 | 11.40 | 0.987 | 19.4 |
| 12 | − 1.414 (45.9) | 0 (25) | 69.72 | 46.75 | 5.93 | 0.993 | 10.1 |
| 13 | 0 (60) | 0 (25) | 95.26 | 72.73 | 7.24 | 0.992 | 13.3 |
Y1 = Total carotenoid retention, Y2 = total phenolic retention, Y3 = diffusion coefficients
In the analysis of the total carotenoid retention responses (), the effects of the drying temperature and the interaction between the temperature and by-product concentration were both found to be significant (Table 3). These effects are represented by the significance of the , and terms, as shown in Table 3 and by the regression model (Eq. 11), as follows:
| 14 |
Table 3.
Analysis of variance: standard error (SE) and significance of the effect of temperature and by-product concentration factors on total carotenoids retention (), total phenolic compounds retention () and effective diffusion coefficients, ()
| Variable | TC Ret (Y1) |
TPC Ret (Y2) |
(Y3) |
|||
|---|---|---|---|---|---|---|
| SE | P value | SE | P value | SE ×1011 | P value | |
| Model | 4.794 | < 0.0001 | 6.646 | < 0.0001 | 3.956 | < 0.0001 |
| 3.790 | 0.053** | 5.254 | 0.083** | 3.128 | 0.001* | |
| 3.790 | 0.182 | 5.254 | 0.608 | 3.128 | 0.130 | |
| 4.065 | 0.037* | 5.635 | 0.093** | 3.355 | 0.800 | |
| 4.065 | 0.323 | 5.635 | 0.409 | 3.355 | 0.176 | |
| 5.360 | 0.016* | 7.430 | 0.200 | 4.423 | 0.067** | |
| 0.779 | 0.601 | 0.849 | ||||
*significance level of 5%; **significance level of 10%
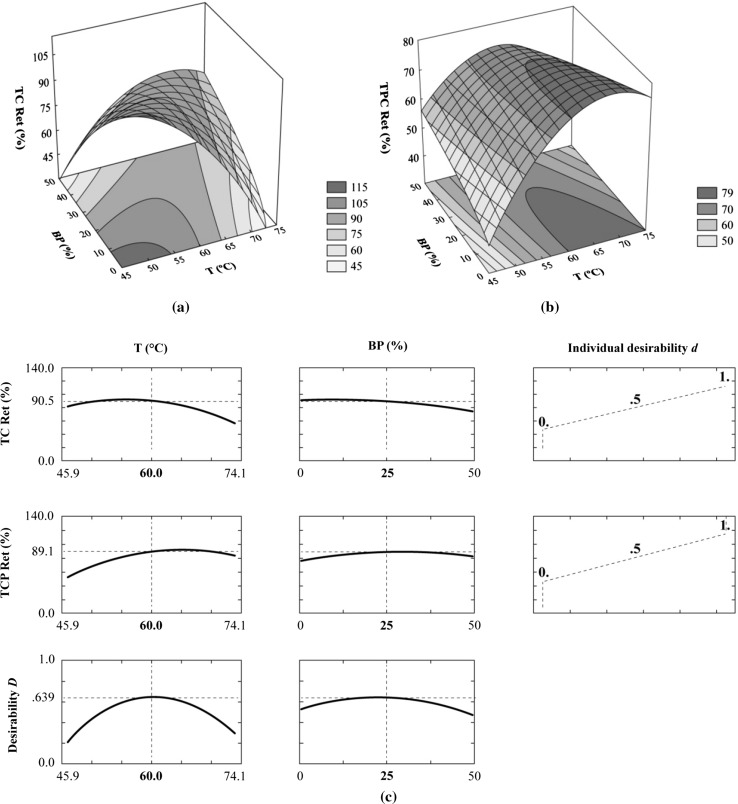
The coefficients of the linear and quadratic terms of the temperature were negative, indicating that the total carotenoid retention tends to be greater at lower drying temperatures. Figure 1a presents a curvature influenced mainly by the effect of the interaction and of the quadratic term . This surface shows that the highest carotenoid retention value corresponds to the lowest BP concentration in the coating solution and the lowest drying temperature. However, as both the drying temperature and BP concentrations simultaneously increase from the optimum region, high values of retention are still observed. Therefore, the temperature and BP concentration at which good retentions were found range between 46 and 60 °C and 0 and 25%, respectively. This may be partly explained by the fact that the ionic gelling of the pectin-based coating was expected to be more effective in coatings with higher percentages of pectin. The presence of large amounts of by-products may form irregular pores that increase the ability of the coating to be permeated by gases. It is known that a lower water content in a pectin coating allows less permeation by gases (Cuq et al. 1995; Gontard et al. 1996). In fact, the effect of the (%) on carotenoid retention was significant only when it interacted with temperature (Table 3). As mentioned before, the higher the BP amount added to the pectin coating, the higher the temperature needed to form a cohesive and efficient film that can act as a barrier to carotenoid oxidation. The high effectiveness of the coating when the temperature and by-product quantities increased simultaneously can be attributed to the drying speed and the increasing solids content, leading to a progressive increase in the thickness and cohesion of the coating. In addition, the temperature and barrier to oxygen are expected to affect the carotenoid degradation in a combined manner. According to Wilberg and Rodriguez-Amaya (1995), during processing and storage of foods, the major cause of carotenoid destruction is enzymatic and non-enzymatic oxidation, which depends on the presence of oxygen and other factors that stimulate oxidation, such as heat and enzymes.
Fig. 1.
Response surface plot of total carotenoid retention (TC Ret, %), b response surface plot of total phenolic compound retention (TPC Ret, %). c Profiles for predicted values of total carotenoid retention (TC Ret, %) and total phenolic compound retention (TPC Ret, %), individual desirability functions () and overall desirability (D), all of them as a function of the temperature (T, °C) and percentage of guava by-products (BP, %)
It is important to point out that carotenoid retention greater than 100% was observed under the optimal treatment conditions. This has been reported in earlier studies on plant food processing and attributed to incomplete carotenoid extraction from fresh samples in comparison with treated samples (Lago-Vanzela et al. 2013). As mentioned by the authors, this can occur because carotenoids are physically protected or combined with other components in the fresh plant, reducing the extraction efficiency in comparison to that of thermal-processed plant tissues.
Neither temperature nor the residue concentration caused statistically significant differences in the retention of ascorbic acid, which is a very thermal and moisture-sensitive compound. Even though ascorbic acid losses of up to 60% were observed, good levels of this vitamin were maintained in the dried products, as shown in Table 1. Nonsignificant differences are likely related to variables not considered in the model, which could affect the degradation kinetics, such as the dependence on the ascorbic acid concentration or even on moisture. Kurozawa et al. (2014) found two first-order kinetic pathways for ascorbic acid degradation. The first pathway has a higher rate than the second. In addition, it is known that a suitable combination of moisture removal rate and drying temperature can increase the retention of bioactive compounds because faster drying rates reduce the mobility of molecules and, consequently, the reactions between them beyond that of the shorter exposure time to the damaging effect of the temperature. Mrad et al. (2012) found that AA and total phenolic degradation in pears during drying were dependent on time, temperature and moisture content, especially below 2 kg water kg−1 dry matter. Moreover, as pointed out by Kurozawa et al. (2014), molecular mobility in a food matrix has been related to glass transition temperatures, as the rubber states are prone to molecular movement inside the food, which facilitates AA degradation. The authors studied papaya drying and determined the specific moisture level that makes the drying temperature equal to the glass transition temperature of the fruit. Below this moisture level, AA degradation is reduced due to the phase transition from the rubber to the glass state. Ramall and Mascheroni (2012) also observed the effects of the drying temperature and time on AA degradation during drying of pineapples. However, the temperature was more important than drying time, as the maximum retention was found at the lowest temperature, 45 °C. This could also be explained by the low temperature, which maintains the cellular tissue integrity, at least during the first drying period when there is still enough moisture. When the cell membrane integrity is lost, all cellular content is released from the cellular compartments, and bioactive compounds become more susceptible to degradation by oxidation.
With respect to the total phenolic compounds, the analysis using the Folin–Ciocalteau reagent was susceptible to interferences from several compounds (Lester et al. 2012; Prior et al. 2005, Asami et al. 2003), including AA. As guava has a significant amount of AA in comparison to TCP content (Table 1), the 0.64 correction factor was applied to the AA and subtracted from TCP measurements for calculation of TCP retention. This response was described as a function of the coded variables:
| 15 |
Analysis of variance in Table 3 shows that only the temperature had an important influence on the phenolic compound retention.
The response surface for total phenolic compound retention during drying of the coated guava slices shows that the temperatures at which the retention achieved the greatest values ranged between 60 and 74 °C (Fig. 1b). The total phenolic content degradation was also more relevant at low temperatures, probably because of the corresponding lengths of drying.
Many authors have reported the effects of temperature and process time on total phenolic compound degradation, Vega-Galvéz et al. (2012), similar results during drying apple slices at 40, 60, and 80 °C at air speeds between 0.5 and 1.5 m s−1. The greatest retentions at the highest temperature were found only at 1.5 m s−1 air-velocity but not at lower velocities. The authors attributed this to the high drying rates at the interface, retarding heat diffusion into the solid, shortening the drying time and reducing the exposure of the phenolic compounds to thermal and oxidative degradation.
Conversely, some phenolic compounds may be formed during the drying process, as observed by Que et al. (2008) for pumpkin flour dried at 70 °C. These authors suggested that the formation of phenolic compounds may be associated with the availability of precursor molecules of these compounds or even, at least in part, with the increase and accumulation of Maillard-derived melanoidins resulting from the heating process. In addition, lignin binds with other acids that, when broken down at high temperatures, can liberate phenolic acid derivatives (Maillard and Berset 1995).
Finally, both carotenoid and phenolic compound retention were simultaneously maximized by applying the appropriate method (Derringer and Suich 1980). Figure 1c shows the predicted profiles of the response variables TC Ret (%) and TCP Ret (%) as a function of the independent variables T (°C) and BP (%). The predicted optimal process condition that simultaneously satisfied the carotenoid and phenolic compound retention was found at 60 °C and 25% BP (%) and corresponded to a 0.639 global desirability when the desirability functions were linear (s = t = 1). However, upon analyzing the profiles, a range of BP concentrations where high nutrient retentions could be obtained are seen. Therefore, temperatures of approximately 60 °C and BP concentrations from 15 to 25% simultaneously satisfied the requirements for good retention of both total carotenoid and total phenolic compounds. Earlier, Vieira et al. (2012) who found optimal process conditions for osmotic dehydration of guavas through this approach.
Drying kinetics
The effective water diffusion coefficients were obtained by fitting Eq. (4) to the experimental data considering the average slice thicknesses randomly measured for each experimental essay. The coating thickness was assumed to be negligible in comparison to the fruit slice thickness. The diffusion coefficients and corresponding R2 and P (%) values are shown in Table 2. High determination coefficients R2 were obtained (> 0.98). Relative errors P (%) higher than 10 did not represent a good fit (Lomauro et al. 1985). However, these values are justified by the very low water content in the last drying stages, which amplifies the respective relative deviations (Eq. 5).
The drying kinetics results were also described by the empirical models of Newton (Eq. 6), Page (Eq. 7), and Henderson–Pabis (Eq. 8), and the model constants are presented in Table 4. All models were satisfactory, as shown by the R2 and P (%) values in the same table. However, in general, the best R2 and P (%) values were found for the Page model.
Table 4.
Parameters determined according to the Newton, Page, and Henderson–Pabis models, coefficients of determination (R2) and mean relative error, P (%), as a function of levels of temperature (T) and percentage of guava by-products (BP) added to the pectin coating
| Trial | T (°C) | Newton | Page | Henderson–Pabis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k × 104 (s−1) | R 2 | P (%) | k × 104 (s–n) | n | R 2 | P (%) | k × 104 (s−1) | a | R 2 | P (%) | |||
| 1 | 60 | 25 | 1.61 | 0.999 | 5.47 | 1.82 | 0.9865 | 0.999 | 6.41 | 1.61 | 0.9974 | 0.999 | 5.63 |
| 2 | 50 | 7.3 | 1.35 | 0.999 | 5.26 | 1.69 | 0.9752 | 0.999 | 6.11 | 1.34 | 0.9953 | 0.999 | 5.33 |
| 3 | 60 | 25 | 1.70 | 0.999 | 7.50 | 1.93 | 0.9855 | 0.999 | 8.48 | 1.68 | 0.9930 | 0.999 | 7.92 |
| 4 | 60 | 25 | 1.60 | 0.999 | 12.07 | 1.38 | 1.0162 | 0.999 | 10.11 | 1.61 | 1.0043 | 0.999 | 11.65 |
| 5 | 60 | 0 | 1.77 | 0.998 | 10.00 | 2.65 | 0.9531 | 0.998 | 8.62 | 1.75 | 0.9922 | 0.998 | 9.76 |
| 6 | 70 | 42.7 | 2.31 | 0.997 | 20.47 | 1.04 | 1.0953 | 0.999 | 9.78 | 1.34 | 0.9953 | 0.998 | 18.16 |
| 7 | 60 | 50 | 1.61 | 0.999 | 6.50 | 1.87 | 0.9833 | 0.999 | 7.52 | 1.60 | 0.9930 | 0.999 | 6.81 |
| 8 | 70 | 7.3 | 2.19 | 0.998 | 8.88 | 1.21 | 1.0708 | 0.999 | 4.87 | 2.22 | 1.0125 | 0.998 | 8.10 |
| 9 | 50 | 42.7 | 1.31 | 0.998 | 4.46 | 2.17 | 0.9435 | 1.000 | 3.31 | 1.28 | 0.9845 | 0.999 | 3.47 |
| 10 | 60 | 25 | 1.29 | 0.998 | 3.72 | 2.09 | 0.9465 | 0.999 | 4.82 | 1.26 | 0.9804 | 0.999 | 3.63 |
| 11 | 74.1 | 25 | 2.22 | 1.000 | 2.78 | 1.99 | 1.0125 | 1.000 | 2.24 | 2.22 | 1.0023 | 1.000 | 2.65 |
| 12 | 45.9 | 25 | 1.15 | 0.996 | 6.10 | 2.61 | 0.9099 | 0.999 | 1.48 | 1.11 | 0.9773 | 0.997 | 4.95 |
| 13 | 60 | 25 | 1.69 | 0.999 | 4.85 | 2.80 | 0.9423 | 0.999 | 2.94 | 1.66 | 0.9848 | 0.999 | 3.57 |
The result of the regression model fitting (Eq. 11) to the diffusion coefficients is described by Eq. (16) as a function of the coded variables:
| 16 |
The response surface is presented in Fig. 2a. The linear term of temperature (x1) was the most significant factor positively affecting the effective diffusion coefficients, while the by-product concentration only affected them through the interaction term (x1x2) (Table 3). The surface observed in Fig. 2a showed that at low temperatures, a higher pectin content in the coating resulted in higher water diffusivity. However, as the air-temperature increased, this behavior changed, and the maximum diffusivity values corresponded to the highest concentrations of BP. This suggested that, while temperatures were low, the predominance of pectin in the coating made drying easier because of its great hydrophilicity. However, as the temperature increased, pectin may have exhibited surface hardening because of its fast drying. Therefore, the presence of the by-products mixed with pectin seemed to preclude hardening, probably because the by-products provided heterogeneity to the surface, preventing compact coatings of dry pectin and facilitating the water diffusion. Similar behavior was observed with carotene retention, and the response of which was also significantly influenced by the interaction term (Table 3). Nonetheless, the effects of the temperature on the drying kinetics were always more perceptible than those of the coating composition. Fig. 2b, compares the drying curves for different temperatures and BP concentrations. These curves show the moisture ratio contents (MR) observed, which were calculated according to the Page model (Eq. 7) as a function of the drying time.
Fig. 2.
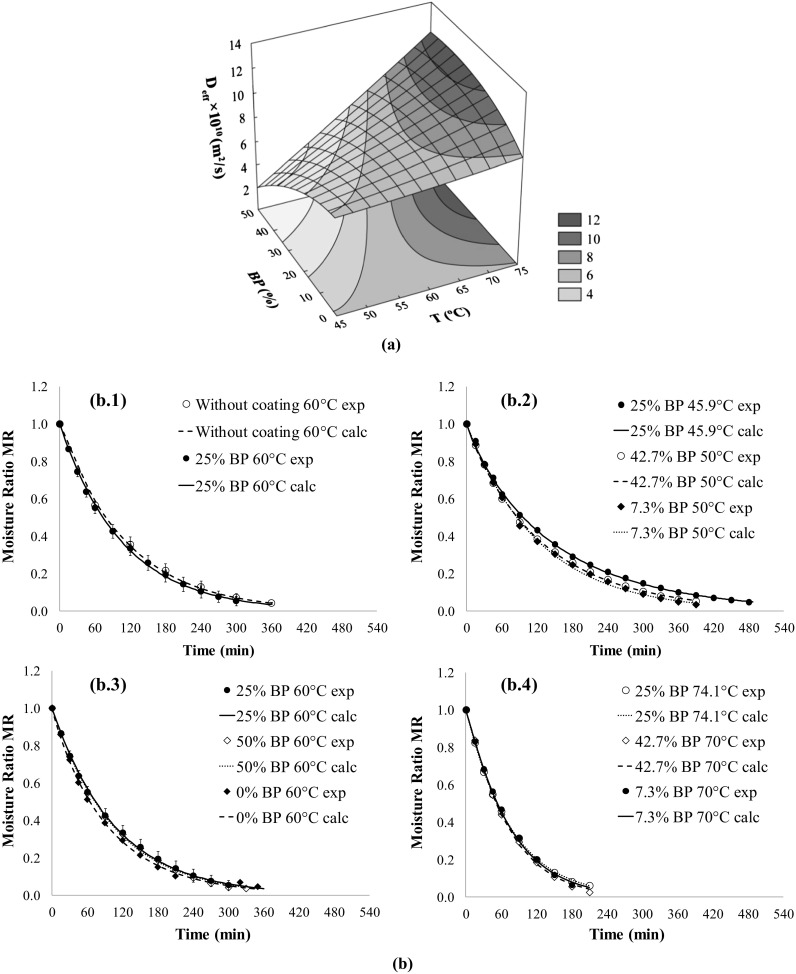
a Response surface plot of diffusion coefficients () as a function of the drying temperature (T, °C) and percentage of guava by-products (BP, %). b Comparison between moisture ratio content (MR) observed and calculated, according to the empirical models as a function of drying time of guava slices subjected to different treatments: b.1 coated with 25% BP and non-coated, dried at 60 °C, b.2 coated with different BP concentrations and dried at lower temperatures (50 °C and 45.9 °C), b.3 coated with different BP concentrations and dried at 60 °C, b.4 coated with different BP concentrations and dried at higher temperatures (70 °C and 74.1 °C)
Figure 2b.1 shows that the presence of 25% BP in the pectin coating (five replicates) only slightly affected the drying kinetics of guava slices at 60 °C in comparison to the drying without coating (duplicate). Figure 2b.2 compares drying curves for lower temperatures, and drying at 50 °C was more effective than drying at 45.9 °C. As discussed above, the coating effects on the drying kinetics at 50 °C were rather less. This low can also be observed in Fig. 2b.3, where drying at 60 °C is compared, as well as in Fig. 2b.4, which presents drying curves at 70 and 74.1 °C. The effects of the temperature are also highlighted in Fig. 2 by the necessary drying time for the samples to reach the pre-determined moisture content.
The less effect of edible coatings on the drying kinetics suggests that the main resistance to water diffusion was the fruit itself. This corroborated previous work of coatings based on pectin that have shown the less influence of these films on the drying kinetics of papayas (Canizares and Mauro 2015; Lago-Vanzela et al. 2013). In fact, the average value of all diffusion coefficients determined at 60 °C (Table 2), was (8.2 ± 1.3) × 10−10 m2 s−1, when was values obtained during drying of samples without coating, at 60 °C i.e., (7.8 ± 1.4) × 10−10 m2 s−1 (data not shown). This showed that the drying efficiency did not change appreciably due to the pectin coatings and the by-product concentration.
Conclusion
Total carotenoid retention and total phenolic retention were significantly affected by the drying temperature and the concentration of guava by-products incorporated in the pectin-based coating. When both carotenoid and total phenolic responses were simultaneously optimized, the best retention values were found, i.e., approximately 60 °C and 25% by-product.
It was concluded that the effects of the edible coating compositions combined with the drying temperatures can affect the permeation properties of the coatings, the drying times and, consequently, the retention of nutrients. Diffusivities, in turn, were mainly influenced by the drying temperatures, while the coatings or the by-product concentration only slightly affected them without causing a major impact on the drying times. Therefore, this technology is promising for developing dried products with desirable nutritional characteristics by using fruit by-product-pectin coatings.
Acknowledgements
The authors are grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES) for the scholarship, the São Paulo Research Foundation (FAPESP, Process 2014/11514-8) for financing and the Purac Synthesis (Brazil) and Danisco (Brazil) for their contributions.
References
- AOAC . Official methods of analysis of the association of official analytical Chemists. 18. Arlington: AOAC; 2005. [Google Scholar]
- Asami DK, Hong Y-J, Barrett DM, Mitchell AE. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J Agric Food Chem. 2003;51:1237–1241. doi: 10.1021/jf020635c. [DOI] [PubMed] [Google Scholar]
- Benassi MT, Antunes AJ. A comparisom of meta-phosphoric and oxalic acids as extractant solutions for the determination of vitamin C in selected vegetables. Braz Arch Biol Technol. 1988;31:507–513. [Google Scholar]
- Canizares D, Mauro MA. Enhancement of quality and stability of dried papaya by pectin-based coatings as air-drying pretreatment. Food Bioprocess Tech. 2015;8:1187–1197. doi: 10.1007/s11947-015-1483-2. [DOI] [Google Scholar]
- Crank J. The mathematics of diffusion. 2. Oxford: Clarendon Press; 1975. [Google Scholar]
- Cuq B, Gontard N, Guilbert S. Edible films and coatings as active layers. In: Rooney ML, editor. Active food packaging. Glasgow: Chapman and Hall; 1995. pp. 111–142. [Google Scholar]
- Derringer G, Suich R. Simultaneous-optimization of several response variables. J Qual Technol. 1980;12:214–219. doi: 10.1080/00224065.1980.11980968. [DOI] [Google Scholar]
- Ertekin C, Yaldiz O. Drying of eggplant and selection of a suitable thin layer drying model. J Food Eng. 2004;63:349–359. doi: 10.1016/j.jfoodeng.2003.08.007. [DOI] [Google Scholar]
- Flores G, Wu S-B, Negrin A, Kennelly EJ. Chemical composition and antioxidant activity of seven cultivars of guava (Psidium guajava) fruits. Food Chem. 2015;70:327–335. doi: 10.1016/j.foodchem.2014.08.076. [DOI] [PubMed] [Google Scholar]
- Garcia CC, Caetano LC, Silva KS, Mauro MA. Influence of edible coating on the drying and quality of papaya (Carica papaya) Food Bioprocess Tech. 2014;7:2828–2839. doi: 10.1007/s11947-014-1350-6. [DOI] [Google Scholar]
- Gontard N, Thibault R, Cuq B, Guilbert S. Influence of relative humidity and film composition on oxygen and carbon dioxide permeabilities of edible films. J Agric Food Chem. 1996;44:1064–1069. doi: 10.1021/jf9504327. [DOI] [Google Scholar]
- Granato D, Catro IA, Ellenderson LN, Masson ML. Physical stability assessment and sensory optimization of a dairy-free emulsion using response surface methodology. J Food Sci. 2010;75(3):S149–S155. doi: 10.1111/j.1750-3841.2010.01514.x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Cox S, Abu-Ghannam N. Effect of different drying temperatures on the moisture moisture and phytochemical constituents of edible Irish brown seaweed. LWT-Food Sci Technol. 2011;44(5):1266–1272. doi: 10.1016/j.lwt.2010.12.022. [DOI] [Google Scholar]
- Kabir F, Tow WW, Hamauzu Y, Katayama S, Tanaka S, Nakamura S. Antioxidant and cytoprotective activities of extracts prepared from fruit and vegetable wastes and by-products. Food Chem. 2015;167:358–362. doi: 10.1016/j.foodchem.2014.06.099. [DOI] [PubMed] [Google Scholar]
- Kurozawa LE, Terng L, Hubinger MD, Park KJ. Ascorbic acid degradation of papaya during drying: effect of process conditions and glass transition phenomenon. J Food Eng. 2014;123:157–164. doi: 10.1016/j.jfoodeng.2013.08.039. [DOI] [Google Scholar]
- Lago-Vanzela ES, do Nascimento P, Fontes EAF, Mauro MA, Kimura M. Edible coatings from native and modified starches retain carotenoids in pumpkin during drying. LWT-Food Sci Technol. 2013;50:420–425. doi: 10.1016/j.lwt.2012.09.003. [DOI] [Google Scholar]
- Lester GE, Lewers KS, Medina MB, Saftner RA. Comparative analysis of strawberry total phenolics via Fast Blue BB versus Folin–Ciocalteu: assay interference by ascorbic acid. J Food Comp Anal. 2012;27:102–107. doi: 10.1016/j.jfca.2012.05.003. [DOI] [Google Scholar]
- Lomauro CJ, Bakshi AS, Labuza TP. Evaluation of food moisture sorption isotherm equations. Part I: fruit, vegetables and meat products. LWT-Food Sci Technol. 1985;18:111–117. [Google Scholar]
- Maillard M-N, Berset C. Evolution of antioxidant activity during kilning: role of insoluble bound phenolic acids of barley and malt. J Agric Food Chem. 1995;43:1789–1793. doi: 10.1021/jf00055a008. [DOI] [Google Scholar]
- Montgomery DC. Design and analysis of experiments. 5. New York: Wiley; 2001. [Google Scholar]
- Mrad ND, Boudhrioua N, Kechaou N, Courtois F, Bonazzi C. Influence of air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears. Food Bioprod Process. 2012;90:433–441. doi: 10.1016/j.fbp.2011.11.009. [DOI] [Google Scholar]
- Murphy EW, Criner PE, Gray BC. Comparisons of methods for calculating retentions of nutrients in cooked foods. J Agric Food Chem. 1975;23:1153–1157. doi: 10.1021/jf60202a021. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Que F, Mao L, Fang X, Wu T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int J Food Sci Tech. 2008;43:1195–1201. doi: 10.1111/j.1365-2621.2007.01590.x. [DOI] [Google Scholar]
- Racape E, Thibault JF, Reitsma JCE, Pilnik W. Properties of amidated pectins. 2. Poly-electrolyte behavior and calcium-binding of amidated pectins and amidated pectic acids. Biopolymers. 1989;28(8):1435–1448. doi: 10.1002/bip.360280809. [DOI] [Google Scholar]
- Ramall LA, Mascheroni RH. Quality evaluation of pineapple fruit during drying process. Food Bioprod Process. 2012;90:275–283. doi: 10.1016/j.fbp.2011.06.001. [DOI] [Google Scholar]
- Rhim J-W, Lee JH. Drying kinetics of whole and sliced shiitake mushrooms (Lentinus edodes) Food Sci Biotechnol. 2011;20(2):419–427. doi: 10.1007/s10068-011-0059-9. [DOI] [Google Scholar]
- Rodriguez-Amaya DB. Changes in carotenoids during processing and storage of foods. Arch Latinoam Nutr. 1999;49:38S–47S. [PubMed] [Google Scholar]
- Rodriguez-Amaya DB, Kimura M. Harvest plus handbook for carotenoid analysis. Washinton DC: Food Policy Research Institute (IFPRI); 2004. pp. 13–37. [Google Scholar]
- Rodriguez-Amaya DB, Porcu MM, Azevedo-Meleiro CH. Variation in the carotenoid composition of fruits and vegetables along the food chain. Acta Hortic. 2007;744:387–394. doi: 10.17660/ActaHortic.2007.744.44. [DOI] [Google Scholar]
- Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O. Edible coatings to incorporate active ingredients to fresh-cut fruits: a review. Trends Food Sci Technol. 2009;20:438–447. doi: 10.1016/j.tifs.2009.05.002. [DOI] [Google Scholar]
- Silva WP, Silva CMDPS (2008) Prescribed adsorption–desorption, Version 2.2, online. http://zeus.df.ufcg.edu.br/labfit/Prescribed.htm. Accessed Feb 2016
- Silva LMR, Figueiredo E, Ricardo NMPS, Vieira IGP, Figueiredo RW, Brasil IM. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014;143:398–404. doi: 10.1016/j.foodchem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Silva LM, Hill LE, Figueiredo E, Gomes CL. Delivery of phytochemicals of tropical fruit by-products using poly (dl-lactide-co-glycolide) (PLGA) nanoparticles: synthesis, characterization, and antimicrobial activity. Food Chem. 2014;165:362–370. doi: 10.1016/j.foodchem.2014.05.118. [DOI] [PubMed] [Google Scholar]
- Singh SP. Guava (Psidium guajava L.) In: Elhadi Y, editor. Postharvest biology and technology of tropical and subtropical fruits. 3. Cambridge: Woodhead Publishing Limited; 2011. pp. 213–245. [Google Scholar]
- Thakur BR, Singh RK, Handa AK. Chemistry and uses of pectin—a review. Crit Rev Food Sci Nutr. 1997;37(1):47–73. doi: 10.1080/10408399709527767. [DOI] [PubMed] [Google Scholar]
- Vega-Galvéz A, Miranda M, Bilbao-Sainz C, Uribe E, Lemus-Mondaca R. Empirical modeling of drying process for apple (CV. Grannny Smith) slices at different temperatures. J Food Sci Technol. 2008;32(6):972–986. [Google Scholar]
- Vega-Galvéz A, Ah-Hen K, Chacana M, Vergara J, Martinéz-Monzó J, García-Segovia P. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012;132:51–59. doi: 10.1016/j.foodchem.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Vieira GS, Pereira LM, Hubinger MD. Optimization of osmotic dehydration process of guavas by response surface methodology and desirability function. Int J Food Sci Tech. 2012;47:132–140. doi: 10.1111/j.1365-2621.2011.02818.x. [DOI] [Google Scholar]
- Voragen AGJ, Schols HA, Pilnik W. Determination of the degree of methylation and acetylation of pectins by H.P.L.C. Food Hydrocoll. 1986;1(1):65–70. doi: 10.1016/S0268-005X(86)80008-X. [DOI] [Google Scholar]
- Wilberg VC, Rodriguez-Amaya DB. HPLC quantization of major carotenoids of fresh and processed guava, mango and papaya. LWT-Food Sci Technol. 1995;28:474–480. doi: 10.1006/fstl.1995.0080. [DOI] [Google Scholar]