Abstract
Present study was conducted to evaluate the anti-oxidant and anti-microbial efficiency of porcine blood hydrolysate (PBH) in refrigerated pork batter. PBH produced by alcalase was included into pork batter at different levels viz. PBH1-0.03, PBH2-0.06 and PBH3-0.09% (w/w) and compared with control (C-0%) and positive control (PC-0.02% BHT w/w). The pH increased, whereas water activity, extract release volume and emulsion stability decreased during storage; however, all these parameters were better maintained in the treated groups. Anti-oxidant efficacy of treatments improved in a concentration dependent manner. Peroxide, thiobarbituric acid reacting substances and free fatty acid values were significantly lower than control throughout storage. The colour and microbial quality was better maintained in treatments than C and PC. In microbial challenge test, counts of tested microbes in treatment batter reduced up to 4th day but increased thereafter. Results suggest that PBH can be utilized as a potential component to improve physico-chemical quality, colour, oxidative and microbial stability of meat batter during refrigerated storage.
Keywords: Blood hydrolysate, Lipid-oxidation, Anti-microbial activity, Meat batter, Physico-chemical characteristics
Introduction
Today demand for convenient meat products has escalated the requirement of long term refrigeration of meat batter for on spot preparation. Normally in primal and sub-primal cuts of meat, microbes are usually present on the surface, and oxygen diffuses only to a thin layer of meat chunk, thus the interior is prevented from microbial contamination and lipid-oxidation (Honikel 2014). However, mincing of meat results in break-down of epimysial integrity causing increase in surface area, oxidation reduction potential and distribution of microbes throughout the comminuted meat decreasing shelf life of meat batter. Oxidation of meat is further enhanced by temperature, light, metals and enzymatic activities (Kumar et al. 2015). Lipid-oxidation and microbial growth are key factors for deterioration of meat batter, food poisoning and their wastage, however, inclusion of suitable preservative maintains better food safety, nutritional quality, organoleptic quality and economic value (Jayawardana et al. 2015).
Incorporation of synthetic anti-oxidants and anti-microbial has been extensively used for extending meat batter quality. However, there is rising anxiety about their safety owing to their potential allergic, carcinogenic and genotoxic effects on consumer health. Therefore, customer demand for natural bio-active ingredients has encouraged the meat scientists to discover and develop novel, natural and low-cost anti-oxidant compounds.
Recent studies have recommended that meat by-product proteins hold promise as a potential nutritional, non-toxic source of natural anti-oxidant and anti-microbial bio-active compounds (e.g. peptides, conjugated linoleic acid and coenzyme Q10). Natural anti-microbial and anti-oxidant potential of meat proteins such as rainbow trout by-products (Wald et al. 2016), mechanically deboned chicken residue (Sun et al. 2012), porcine liver protein hydrolysates (Verma et al. 2017) and muscle and by-products (Lafarga and Hayes 2014) have revealed to quench free radicals, chelate transition metals and retard lipid-oxidation and microbial growth in vitro and in vivo.
Utilization of protein hydrolysate has been well studied; however studies on animal protein hydrolysate extracted from by-products are limited. Extraction of protein hydrolysate from meat industry by-products will facilitate revenue generation and would additionally reduce environmental pollution. Hence, the present study was conducted to explore the anti-oxidant and anti-microbial activity porcine blood hydrolysate to extend storage life of meat batter at refrigeration temperature.
Materials and methods
Enzymes and chemicals
Alcalase enzyme, chemicals such as 2,2-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH) were procured from Sigma-Aldrich Chemical Co., USA. Whereas, 2,4,6-tripyridyl-s-triazine (TPTZ) was purchased from MP Biomedical, France. Nitro blue tetrazolium (NBT), NADH and phenozoniummetho sulphate (PMS) were purchased from S. D. Fine Chemicals, India. The freeze dried cultures of various pathogenic and food spoilage micro-organisms’ viz. Escherichia coli, Bacillus cereus, Staphylococcus aureus and Listeria monocytogenes were purchased from Institute of Microbial Technology (IMTECH), Chandigarh-160036, India. Other analytical grade chemicals, media, standards and Low density polyethylene bags (LDPE 200 gauge) required for analysis were procured from standard firms.
Porcine blood hydrolysate powder
Porcine blood was mixed (5% W/V) in phosphate buffer (pH 8.0) and concentration of enzyme (alcalase) and substrate ratio (1:100) for optimal enzymatic hydrolysis. Hydrolysis was accomplished by incubating the samples in stirred water bath at 50 °C for 6 h followed by subsequent inactivation of residual enzymes (85 °C/15 m). Then, the hydrolysate was cooled and centrifuged in a refrigerated centrifuge at 10,000 rpm for 25 min, supernatant was obtained, dried in vacuum oven at 50 ± 2 °C and 600 mmHg vacuum for 12–14 h and pulverized to make fine powder and stored at − 20 °C till use.
Preparation of pork batter
Castrated Large White Yorkshire pigs were purchased from the Instructional Livestock Farm, GADVASU, Ludhiana. Animal welfare and ethical guidelines were followed during slaughtering of pigs in departmental slaughter house and experimental protocol was approved by GADVASU Animal Ethical Committee. Hot deboning was done manually. The deboned meat was chilled over-night in refrigerator, packed in LDPE bags and stored under frozen condition (− 18 °C) till use.
Frozen boneless lean meat was thawed overnight at 4 ± 2 °C, followed by sectioning it into smaller chunks that were passed twice through 6 mm diameter sieve plate for proper mincing. Meat batter containing minced meat pork (83.5%), refined oil (10%), ice (5%), and salt (1.5%) was divided into five groups (C: 0.00%, PBH1: 0.03%, PBH2: 0.06% and PBH3: 0.09%, PC: 0.02% BHT w/w). After proper mixing each group was separately packaged aerobically in LDPE bags and was stored at 4 ± 2 °C under refrigerated conditions. Samples were drawn on 0, 2nd, 4th and 6th day for evaluation.
Physico-chemical parameters
The pH of suspension resulting from blending 10 g sample with 100 mL distilled water for 2 min was measured with a digital pH meter using combined glass electrode. Water activity (Rotronix HYGRO) was determined using potable digital water activity meter at 25 °C. Emulsion stability (ES) was determined using the method described by Townsend et al. (1968) with slight modifications. Extract release volume (ERV) was estimated using the method described by Jay (1964).
Antioxidant assay
2,2-Azinobis (3-ethylbenzthiazoline-6-sulfonic acid) radical scavenging activity
The ABTS+ radical scavenging activity was determined according to Salami et al. (2009). One mL of ABTS+ working solution was well mixed with 10 µL of samples kept in dark at room temperature for 20 min and absorbance was read at 734 nm in multimode reader (Synergy H1 Hybrid Multi-Mode Micro-plate Reader, BioTekUSA).
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
The DPPH radical activity was determined using the method of Brand-Williams et al. (1995) with little modification. One mL of DPPH (100 µM), 0.25 mL of 0.1 M Tris–HCl buffer (pH 7.4) and 25 µL of samples were mixed properly in test tubes and the absorbance was taken (time t = 0 min (t0)) at 517 nm using multimode reader. The above samples were also kept at room temperature under dark condition for measurement of second absorbance after 20 min (t20) and ethanol was taken as blank.
Ferric reducing-antioxidant power (FRAP) and superoxide anionic scavenging activity assay (SASA)
The FRAP assay was determined according to the methodology Benzie and Strain (1999). Freshly prepared 900 µL of FRAP solution (300 mM acetate buffer, pH 3.6: 20 mM FeCl3: 10 mM TPTZ in 40 mM HCl:: 10:1:1) was properly mixed with 100 µL of samples and absorbance was read at 593 nm using multimode reader after a 40 min incubation at 37 °C under dark condition. FRAP values were calculated by comparing change in absorption of test samples with those obtained from increasing concentrations of Fe3+ and expressed as mmol of Fe2+ equivalents per mL of sample. Ferrous sulphate was taken as standard for preparation of standard curve. Superoxide anionic scavenging activity (SASA) was determined according to the method described by Kumar and Chattopadhyay (2007).
Lipid-oxidation parameters
Method described by Witte et al. (1970) was adopted for calculation of TBARS values in meat emulsion. Five gram of sample was triturated with 12.5 mL of precooled 20% trichloroacetic acid (TCA) in 2 M orthophosphoric acid solution for 2 min. The content was then transferred quantitatively to a beaker by rinsing with 12.5 mL of cold distilled water, mixed and filtered through Whatman filter paper No. 1. After that 3 ml of TCA extract was mixed with 3 mL of 2-thiobarbituric acid (TBA) reagent (0.005 M) and placed in a dark cabinet for 16 h. Blank tester was prepared by mixing 3 mL of 10% TCA and 3 ml of 0.005 M TBA reagent. Absorbance (O.D.) was measured at fixed wavelength of 532 nm with using multimode reader.
The method as described by Koniecko (1979) was followed for determination of FFA. Five gram of sample was blended with anhydrous sodium sulphate and dissolved in 30 mL of chloroform for 2 min. Solution was sieved through Whatman filter paper No. 1. About 2 or 3 drops of 0.2% phenolphthalein indicator solution were added to the chloroform extract, which was then titrated against 0.1 N alcoholic potassium hydroxide to get the pink colour end point.
Peroxide value was measured as per procedure described by Koniecko (1979) with suitable modifications. 2.5 g of meat sample was blended with 15 mL chloroform for 2 min in the presence of anhydrous sodium sulphate. The mixture was filtered through Whatman filter paper No. 1 and 12.5 mL aliquot of the filtrate was transferred to conical flask to which 15 ml of glacial acetic acid and 1 mL of saturated potassium iodide solution were added and allowed to stand for 2 min with occasional shaking after which 50 ml of distilled water and 1 mL of fresh 1% starch solution were added. Flask contents were titrated with 0.1 N sodium thiosulphate till the end point was reached.
Colour parameters
Colour indices were recorded using Lovibond Tintometer (Model: RT-300) establish at 2° of cool white light (D65) and lightness (L*), redness (a*), and yellowness (b*) indices were recorded. Initially instrument was readjusted against black and white tile surface supplied with the instrument. Hue and chroma were computed by following formula, (tan−1 (b/a) and (a2 + b2)1/2 respectively.
Microbiological analysis
Microbial quality of stored pork batter samples were evaluated as per methodology illustrated by American Public Health Association (APHA 1984). Total number of microbial growth was calculated by multiplication of number of counted colonies with reciprocal of dilution factor and depicted as log cfu/g.
Microbial challenge testing (MCT)
The MCT was performed procedure described by Abed et al. (2014) with slight changes. Quantity of chilled water (10%) necessary for meat batter formation was equally divided into two parts and one part was used for dissolution of the salt (1.5%) and another part used for dissolve of blood protein hydrolysate powder for respective treatments. Salt solution and refined oil were sterilized in autoclave at 121 psi for 15 min followed by cooling. Similarly PBH solutions were also sterilized by the passing through 0.45 µm membrane filter. The minced meat was uniformly mix with necessary quantity of pre-sterilized salt solution, refined oil, PBH solution and finally with previously standardized concentration of selected live cultures of tested microbes (104–105 cfu/g). Each group of batter was prepared individually for each tested microbes. Each batch of meat batter packed separately under aerobic packaging condition and stored at 4 °C for further study.
Statistical analysis
Total 120 samples were analyzed for each parameter i.g. physico-chemical, anti-oxidant assay, lipid-oxidation, instrumental colour profile and microbial quality (5 groups × 4 storage days’ × 2 subsamples per groups × 3 trials) and 96 samples (4 groups × 4 storage days’ × 2 subsamples per groups × 3 trials) for MCT separately. Two-way analysis of variance (ANOVA) was performed for data analysis using general linear model procedures of SPSS package (SPSS 17.0 for Windows, SPSS Inc., USA) to determine differences between among groups and storage days. Means were compared using Duncan’s multiple range test, at P < 0.05 level of significance.
Result and discussions
Physico-chemical quality parameters
The comprehensive analyses of Table 1 revealed that pH of pork batter varied significantly (P < 0.05) during storage. The increase in pH with storage was significant (P < 0.05) on each day of study for control, but all treatments exhibited slower rate of increase in pH. It was better maintained in PBH2 and PBH3 than C and PBH1 throughout storage. The increase in pH of pork batter during storage might be due to bacterial multiplication leading to accumulation of bacterial volatile basic substances and deamination of meat proteins (Verma et al. 2016). These results were similar to the findings of Jin et al. (2015) who reported increased pH during storage of pork sausage incorporated with mechanically deboned chicken meat hydrolysates.
Table 1.
Physico-chemical quality change in pork batter included with PBH hydrolysate by alcalase during refrigerated storage (mean ± SE)
| Groups | 0 day | 2 day | 4 day | 6 day |
|---|---|---|---|---|
| pH | ||||
| C | 5.87 ± 0.01Aa | 5.99 ± 0.01Ab | 6.09 ± 0.01Bc | 6.17 ± 0.01Cd |
| PC | 5.86 ± 0.03Aa | 5.93 ± 0.03Ab | 5.98 ± 0.02Ab | 6.11 ± 0.01Bc |
| PBH1 | 5.87 ± 0.01Aa | 5.97 ± 0.03Ab | 6.03 ± 0.02Ab | 6.13 ± 0.02Bc |
| PBH2 | 5.89 ± 0.02ABa | 5.97 ± 0.01Ab | 6.02 ± 0.02Ab | 6.08 ± 0.02Ac |
| PBH3 | 5.93 ± 0.02Ba | 5.95 ± 0.01Aa | 5.97 ± 0.02Aa | 6.05 ± 0.01Ab |
| Water activity (aW) | ||||
| C | 0.937 ± 0.003ABc | 0.923 ± 0.002Ab | 0.919 ± 0.002Ab | 0.908 ± 0.001Aa |
| PC | 0.934 ± 0.005Ac | 0.927 ± 0.001ABbc | 0.923 ± 0.003ABab | 0.913 ± 0.004ABa |
| PBH1 | 0.939 ± 0.004ABCc | 0.933 ± 0.002BCbc | 0.928 ± 0.003BCb | 0.919 ± 0.002BCa |
| PBH2 | 0.947 ± 0.004BCd | 0.940 ± 0.003CDc | 0.930 ± 0.002BCb | 0.922 ± 0.003CDa |
| PBH3 | 0.949 ± 0.004Cb | 0.945 ± 0.003Db | 0.933 ± 0.002Ca | 0.928 ± 0.003Da |
| Extract release volume (mL) | ||||
| C | 49.50 ± 1.36Ac | 47.17 ± 1.25Abc | 43.67 ± 1.12Aab | 40.33 ± 1.28Aa |
| PC | 50.67 ± 1.15Ab | 48.83 ± 0.60Ab | 44.67 ± 0.49ABa | 43.33 ± 0.67ABa |
| PBH1 | 48.83 ± 1.08Ab | 48.50 ± 1.02Ab | 46.00 ± 1.21ABab | 42.83 ± 1.01ABa |
| PBH2 | 50.17 ± 0.95Ab | 47.00 ± 1.15Aa | 46.33 ± 0.92ABa | 44.67 ± 0.95Ba |
| PBH3 | 49.50 ± 1.12Ac | 46.83 ± 0.93Abc | 47.00 ± 1.17Bab | 45.00 ± 0.89Ba |
| Emulsion stability (%) | ||||
| C | 77.44 ± 1.347Ab | 74.71 ± 1.29Ab | 69.51 ± 1.69Aa | 67.08 ± 0.99Aa |
| PC | 77.74 ± 1.47Ac | 76.73 ± 1.49Abc | 73.60 ± 1.14Cab | 70.60 ± 0.65ABa |
| PBH1 | 77.38 ± 0.59Ab | 75.22 ± 1.62Ab | 71.82 ± 0.73ABa | 69.73 ± 0.93ABa |
| PBH2 | 78.32 ± 0.37Ab | 77.12 ± 0.73Ab | 72.37 ± 0.88ABa | 71.01 ± 0.66Ba |
| PBH3 | 77.52 ± 1.01Ab | 77.12 ± 0.42Ab | 74.44 ± 0.72Ca | 72.91 ± 0.43Ba |
Means values bearing different superscripts (small letters in the same row and capital letters in the same column) indicate differ significantly (P < 0.05) n = 3
C control emulsion without hydrolysate, PC emulsion containing 0.02% BHT, PBH1 emulsion containing 0.03% hydrolysate, PBH2 emulsion containing 0.06% hydrolysate, PBH3 emulsion containing 0.09% hydrolysate
A significant difference (P < 0.05) was observed in water activity of all samples on initial day which might be due to the addition of different levels of PBH. Water activity decreased significantly (P < 0.05) with the progression of storage period (Table 1). Water activity was significantly (P < 0.05) higher in all treated products compared to C and PC throughout storage. This might be due to anti-microbial and anti-oxidant properties of added PBH into pork batter. This finding was also supported by our anti-microbial and anti-oxidant activity of PBH because microbial growth decreases water activity Sperber (1983) and accelerates lipid-oxidation and protein oxidation process. Water activity is positively related to microbial proliferation that leads to production of certain enzymes that assist protein and lipid degradation. Result is also supported by our present finding indicating decrease in the quantity of extract release volume (ERV). Similar gradual decrease in aW values were also reported by Kuo and Chu (2003) in pork product during storage.
Among groups, ERV values varied significantly (P < 0.05) after 2 days of storage while it decreased considerably throughout storage, irrespective of treatment (Table 1). ERV value was significantly (P < 0.05) higher in PBH3 and PBH2 than other groups on day 6. ERV decreases with increase in spoilage due to the alteration in structure of proteins, microbial deterioration of protein and change in meat batter pH (Verma et al. 2018). Reduction in ERV during storage of meat batter has positive correlation with increase in pH and lipid-oxidation parameters. Similar results were also reported by Anandh and Lakshmanan (2014) for buffalo rumen meat.
The PBH incorporation produced significant (P < 0.05) effect on emulsion stability (ES) only by 4th day of storage among groups while with storage it decreased (Table 1). The decrease in ES might be due to microbial decomposition of protein, enzymatic and non-enzymatic lipid-oxidation during refrigerated storage of pork batter. Highest ES was observed in PBH3 by the end of 6th day of storage. The higher ES in the treated groups might be due to higher water holding capacity and better stabilization of lipid molecules in meat batter, which prevents leaching of lipid during cooking. An increase in number of peptide molecules and exposed hydrophobic amino acids residue due to hydrolysis of proteins might have also contributed to improvement of batter formation. Vioque et al. (2000) also reported that hydrolysed rapeseed proteins better improved emulsifying activity and stability of batters than un-hydrolysed protein.
Anti-oxidant activity
On day 0 ABTS+ % inhibition was significantly higher (P < 0.05) in PBH3 than the other groups and the same trend continued throughout storage exemplifying superiority of PBH3 over other groups in scavenging free radicals. In general ABTS+ % inhibition significantly (P < 0.05) decreased with increase in storage across all groups. Decrease in ABTS+ % inhibition and quenching affinity of PBH towards ABTS+ radical values during storage showed positive correlation with concentration of added PBH powder. This might be due to the presence of numerous hydrogen atoms and electron-donating peptides/amino acid residues in the PBH. Chi et al. (2015) also reported that ABTS+ activity of protein hydrolysate of clam blood due to its electron/hydrogen donation activity. These findings are in accordance with results of Rossini et al. (2009) reported that addition of casein peptides in beef homogenates and mechanically deboned poultry meat showed dose dependent significant increase (P < 0.05) in % inhibition of ABTS+ values.
The DPPH scavenging activities among groups varied significantly (P < 0.05) throughout storage (Table 2). In general, DPPH radical scavenging activity followed a decreasing trend with progress of storage period. Previous studies also demonstrated that a number of food-derived peptides or protein hydrolysates are capable of interacting and quenching DPPH radicals. However, it is difficult to make comparison between different studies due to the lack of anti-oxidant standards, variation in protein sources and considerable influence of radical concentration and experimental conditions. Results confirmed that PBH is also a free radical scavenger, particularly of peroxyl radical, which is major propagator of oxidation chain of fat, thereby terminating the chain reaction (Yen et al. 2002).
Table 2.
Change in antioxidant assay of pork batter included with PBH hydrolysate by alcalase during refrigerated storage (mean ± SE)
| Groups | 0 day | 2 day | 4 day | 6 day |
|---|---|---|---|---|
| ABTS (% inhibition) | ||||
| C | 55.48 ± 0.44Ad | 44.72 ± 0.34Ac | 39.73 ± 0.45Ab | 31.06 ± 0.28Aa |
| PC | 84.83 ± 0.14Dd | 82.14 ± 0.27Dc | 80.61 ± 0.45Db | 78.09 ± 0.47Da |
| PBH1 | 81.83 ± 0.26Bd | 78.54 ± 0.24Bc | 74.73 ± 0.13Bb | 72.19 ± 0.17Ba |
| PBH2 | 83.71 ± 0.12Cd | 80.57 ± 0.07Cc | 78.02 ± 0.11Cb | 74.71 ± 0.16Ca |
| PBH3 | 86.57 ± 0.11Ed | 83.54 ± 0.10Ec | 82.30 ± 0.11Eb | 79.23 ± 0.18Ea |
| DPPH (% inhibition) | ||||
| C | 14.93 ± 0.25Ad | 11.31 ± 0.28Ac | 9.51 ± 0.36Ab | 6.88 ± 0.11Aa |
| PC | 42.57 ± 0.43Ec | 40.51 ± 0.41Dbc | 39.00 ± 0.54Eb | 35.51 ± 1.14Da |
| PBH1 | 26.04 ± 0.56Bc | 24.76 ± 0.49Bc | 22.96 ± 0.40Bb | 20.30 ± 0.57Ba |
| PBH2 | 28.59 ± 0.29Cc | 25.91 ± 0.46Bb | 24.74 ± 0.32Cb | 22.00 ± 0.62Ba |
| PBH3 | 32.35 ± 0.25Dc | 31.25 ± 0.51Cc | 29.96 ± 0.33Db | 27.13 ± 0.37Ca |
| SASA (% inhibition) | ||||
| C | 21.35 ± 0.58Ad | 18.49 ± 0.67Ac | 14.36 ± 0.61Ab | 11.18 ± 0.55Aa |
| PC | 28.82 ± 0.06Bd | 27.05 ± 0.54Bc | 25.44 ± 0.59Bb | 23.56 ± 0.41Ba |
| PBH1 | 35.04 ± 0.47Cc | 32.85 ± 0.49Cbc | 29.43 ± 0.41Cab | 26.88 ± 1.28Ba |
| PBH2 | 40.60 ± 0.40Dd | 37.61 ± 0.48Dc | 35.93 ± 0.46Db | 33.84 ± 0.79Ca |
| PBH3 | 44.34 ± 0.58Ed | 41.68 ± 0.43Ec | 40.19 ± 0.53Eb | 38.35 ± 0.44Da |
| FRAP (mM Equivalent to FeSO4·7H2O) | ||||
| C | 10.49 ± 0.16Ad | 8.61 ± 0.15Ac | 7.81 ± 0.13Ab | 6.46 ± 0.11Aa |
| PC | 18.97 ± 0.08Bd | 18.37 ± 0.09Bcd | 17.86 ± 0.07Bb | 15.88 ± 0.04Ba |
| PBH1 | 19.94 ± 0.04Cd | 18.17 ± 0.04Bc | 17.88 ± 0.04Bb | 16.14 ± 0.09Ca |
| PBH2 | 20.23 ± 0.12Dd | 19.74 ± 0.11Cc | 18.87 ± 0.05Cb | 18.39 ± 0.04Da |
| PBH3 | 20.56 ± 0.04Ed | 20.17 ± 0.06Dc | 19.23 ± 0.06Db | 18.84 ± 0.08Ea |
Means values bearing different superscripts (small letters in the same row and capital letters in the same column) indicate differ significantly (P < 0.05) n = 3
C control emulsion without hydrolysate, PC emulsion containing 0.02% BHT; PBH1 emulsion containing 0.03% hydrolysate, PBH2 emulsion containing 0.06% hydrolysate, PBH3 emulsion containing 0.09% hydrolysate
SASA % inhibition was significantly higher (P < 0.05) in PBH3 than others throughout storage showing that PBH3 is potentially nobler in superoxide radical scavenging activity (Table 2). SASA % inhibition significantly (P < 0.05) declined with storage period across all groups. These efficacies were concentration-dependent. These findings exhibited that PBH have good superoxide radical-scavenging activity. Similarly, Onuh et al. (2014) estimated SASA in chicken skin protein hydrolysate and reported higher activity in hydrolysate groups than control.
Ferric ion is well-known stimuli of lipid peroxidation and their reduction helps to retard the peroxidation that subsequently prevents food rancidity (Nasri et al. 2013). The antioxidant activity varied significantly (P < 0.05) among groups throughout storage (Table 2). FRAP values decreased significantly (P < 0.05) for all groups as storage advanced. However in treated samples and PC, the rate of decline in FRAP values was slower than control. Based on our observation, PBH containing groups exhibited reduction in the lipid oxidation than control via scavenging free radicals and FRAP activities. These finding were also in harmony with the result reported by Bernardini et al. (2012) for hydrolysates obtained from bovine brisket sarcoplasmic proteins.
Lipid-oxidation
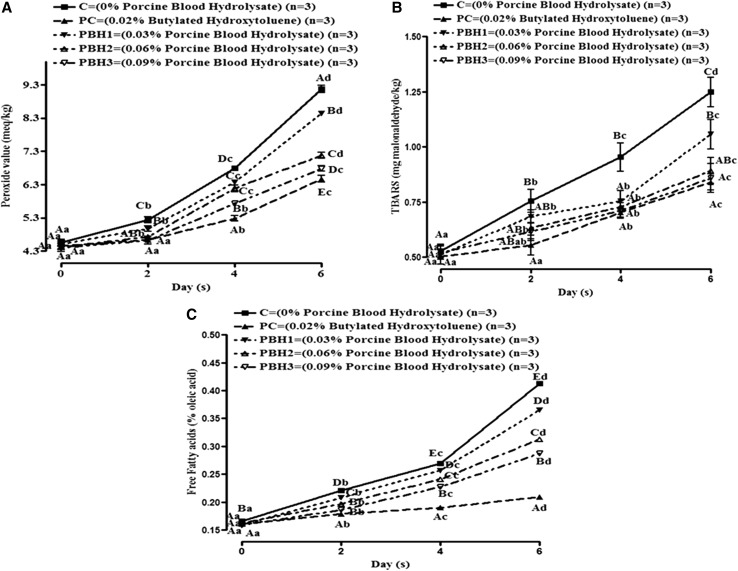
The peroxide value in control pork batter increased continuously during refrigerated storage, probably due to the absence of anti-oxidants added. However, PV value was comparatively lower (P < 0.05) in PBH and BHT incorporated sample (Fig. 1a). PV values increased (P < 0.05) with storage days for all samples. Inhibition activity for PBH3 sample against PV was 26.14% although, BHT imparted slightly superior anti-oxidant efficacy (about 29.52%). The decrease in PV in PBH incorporated groups might be due to radical stabilizing potential, capability to donate hydrogen ion, electron and capacity to chelate transitional metal ions such as Fe2+, Cu2+ and Co2+ etc. The release of non-heme iron and absence of anti-oxidants in pork batter during extended refrigerated storage enhances oxidation process in muscle (Chaijan et al. 2005; Dey and Dora 2014).
Fig. 1.
Change in lipid oxidation parameters (a–c) of pork batter included with PBH hydrolysate by alcalase during refrigerated storage (Mean ± SE). Means values bearing small letters days wise and capital letters groups wise indicate differ significantly (P < 0.05)
Thiobarbituric acid reactive substance (TBARS) values of all samples increased throughout storage (Fig. 1b). The increase in TBARS indicates formation of secondary lipid oxidation products. The Highest TBARS value was noted in control due to higher rate of fat oxidation during entire storage than other groups. Moreover, the release of ferric and other pro-oxidant compounds from meat batter during storage also catalyzed lipid-oxidation in control. Although PC exhibited greatest (32.60%) lipid inhibition; among hydrolysates, lipid-peroxidation inhibition varied in a dose dependent manner where PBH3 showed the highest (31.30%) anti-oxidant activity. Findings were in line with the results of Oliveira et al. (2014) that documented inhibition of meat lipid-oxidation by soy protein hydrolysates and Sakanaka and Tachibana (2006) who studied egg-yolk protein hydrolysates and their effects on lipid-oxidation in beef and tuna homogenates.
FFAs are formed during storage due to the enzymatic or microbial degradation of fat. Measurement of FFAs content gives information about the level of rancidity of lipid during storage. Figure 1c shows that the FFA content was higher in control than all the treatments and positive control. The FFA values increased significantly (P < 0.05) among all treatments and control with the advancement of storage days. The treated samples had lower FFA content as compared to control, which might be due to the anti-oxidant properties of PBH in the treated groups. The anti-oxidant effect of protein hydrolysate is due to the presence of various radical scavenging activities, metal chelating ability and ferric ion chelation. The treatments showed a dose dependent behavior for FFA values. These results were in agreement with the finding of Dey and Dora (2014) in croaker fillet preserved with sodium erythroborate and shrimp waste protein hydrolysates.
Colour parameter
The colour parameters (Table 3) of pork batter incorporated with PBH varied significantly (P < 0.05) during storage. Lightness values remained comparable among groups during storage except last day. The lightness value decrease in treatments and positive control were lower than control, which might be due to the ability of the porcine blood protein hydrolysate and positive control to maintain the colour of the product by retarding the oxidation reaction. The decrease in the L* values might also be due to the native red colour of blood hydrolysate as well as due to interaction of lipid hydro-peroxide with pigments and other macromolecules in pork batter causing discoloration. It might also be attributed to the moisture loss during storage, because moisture content is linearly related with lightness values, and thus concentration of haem pigment resulting in decreased L* value. Similar finding was given by Dey and Dora (2014) for croaker fillet prepared by incorporation of shrimp waste hydrolysate during storage.
Table 3.
Changes in instrumental colour profiles of pork batter included with PBH hydrolysate by alcalase during refrigerated storage (mean ± SE)
| Groups | 0 day | 2 day | 4 day | 6 day |
|---|---|---|---|---|
| Lightness (L* value) | ||||
| C | 47.60 ± 1.01Ac | 45.27 ± 1.00Abc | 42.92 ± 1.12Ab | 38.12 ± 1.14Aa |
| PC | 47.94 ± 1.54Ab | 46.96 ± 0.82Ab | 44.74 ± 0.65Aab | 43.07 ± 0.90Ba |
| PBH1 | 45.60 ± 1.87Aa | 43.68 ± 1.77Aa | 43.71 ± 1.59Aa | 41.40 ± 1.51ABa |
| PBH2 | 44.33 ± 1.22Aa | 43.62 ± 1.21Aa | 43.15 ± 0.81Aa | 42.76 ± 1.18Ba |
| PBH3 | 44.41 ± 1.50Aa | 44.16 ± 1.34Aa | 43.72 ± 1.26Aa | 42.70 ± 1.42Ba |
| Redness (a* value) | ||||
| C | 4.39 ± 0.21Ac | 3.86 ± 0.23Abc | 3.27 ± 0.32Aab | 2.98 ± 0.19Aa |
| PC | 4.53 ± 0.12Ab | 4.42 ± 0.31ABb | 3.71 ± 0.10Aa | 3.39 ± 0.10Aa |
| PBH1 | 5.19 ± 0.14Bb | 4.96 ± 0.16BCb | 4.36 ± 0.13Ba | 4.09 ± 0.20Ba |
| PBH2 | 5.37 ± 0.10Bb | 4.83 ± 0.25BCa | 4.51 ± 0.07Ba | 4.38 ± 0.21BCa |
| PBH3 | 5.88 ± 0.19Cb | 5.47 ± 0.17Cb | 5.26 ± 0.29Cab | 4.69 ± 0.11Ca |
| Yellowness (b* value) | ||||
| C | 13.87 ± 0.44Ab | 11.79 ± 0.43Ab | 10.42 ± 0.36Aa | 9.45 ± 0.36Aa |
| PC | 13.04 ± 0.53Ab | 12.67 ± 0.49Ab | 11.70 ± 0.35Aab | 10.99 ± 0.33Ca |
| PBH1 | 12.82 ± 0.50Ac | 11.96 ± 0.50Abc | 11.03 ± 0.41Aab | 9.93 ± 0.23ABa |
| PBH2 | 12.57 ± 0.29Ab | 12.09 ± 0.46Ab | 11.54 ± 0.43Aab | 10.82 ± 0.26BCa |
| PBH3 | 12.37 ± 0.64A | 11.95 ± 0.49A | 11.42 ± 0.45A | 11.28 ± 0.4C |
| Hue angle | ||||
| C | 14.56 ± 0.44Ac | 12.41 ± 0.42Ab | 10.94 ± 0.35Aa | 9.92 ± 0.36Aa |
| PC | 13.81 ± 0.53Ac | 13.43 ± 0.49Abc | 12.28 ± 0.36Bab | 11.51 ± 0.33BCa |
| PBH1 | 13.85 ± 0.50Ac | 12.97 ± 0.50Abc | 11.86 ± 0.40ABab | 10.74 ± 0.24ABa |
| PBH2 | 13.67 ± 0.29Ac | 13.05 ± 0.46Abc | 12.40 ± 0.43Bab | 11.68 ± 0.26Ca |
| PBH3 | 13.72 ± 0.64Ab | 13.16 ± 0.48Aab | 12.62 ± 0.45Bab | 12.22 ± 0.41Ca |
| Chroma | ||||
| C | 72.41 ± 0.21D | 71.88 ± 0.24C | 72.73 ± 0.32B | 72.30 ± 0.19B |
| PC | 70.73 ± 0.12CD | 70.70 ± 0.31BC | 72.28 ± 0.10B | 72.84 ± 0.10B |
| PBH1 | 67.79 ± 0.14BC | 67.24 ± 0.16AB | 68.36 ± 0.13A | 67.62 ± 0.20A |
| PBH2 | 66.80 ± 0.10AB | 68.01 ± 0.25AB | 68.51 ± 0.07A | 67.95 ± 0.21A |
| PBH3 | 64.37 ± 0.19A | 65.25 ± 0.17A | 65.08 ± 0.30A | 67.31 ± 0.11A |
Means values bearing different superscripts (small letters in the same row and capital letters in the same column) indicate differ significantly (P < 0.05) n = 3
C control emulsion without hydrolysate, PC emulsion containing 0.02% BHT; PBH1 emulsion containing 0.03% hydrolysate, PBH2 emulsion containing 0.06% hydrolysate, PBH3 emulsion containing 0.09% hydrolysate
Redness values increased significantly (P < 0.05) with incorporation of PBH than control and PC. The increase in redness values in treatments might be attributed to blood hydrolysate inherent colour. The intensity of redness values also varied according to hydrolysate concentration in pork batter and it decreased throughout storage. This might be due to lipid-oxidation, protein oxidation of myoglobin and accumulation of met-myoglobin with storage. Dekkers et al. (2011) also reported loss of redness values in mahi–mahi red muscle due to oxidation. However, a* value was higher in treated samples than control throughout the storage period and rate of decrease in a* value was significantly (P < 0.05) lower in treated products than control. Similar results were reported by Jin et al. (2015) for meat sausages on the addition of mechanically deboned chicken meat hydrolysates, the redness (a*) values of product remained significantly (P < 0.05) higher than control, before and after storage. This indicated that incorporation of the blood protein hydrolysate might be effective in controlling chemical rancidity as well as red colour deterioration.
Results in Table 3 indicates that during refrigerated storage, b* values (yellowness) for control decreased more rapidly in control than treatments. After 2 days of refrigerated storage, yellowish colour of pork batter with respect to control decreased significantly (P < 0.05), while the pork batter treated with BHT and PBH maintained the yellowish colour. At the end of storage, b* value of control was lower (P < 0.05) than treatments. However b* values were better maintained in PBH3 than control and it also remained comparable for PBH2 and PC throughout study. Dey and Dora (2014) also reported lower b* values than control for croaker fillet prepared by inclusion of shrimp waste hydrolysate.
Hue angle, which measures saturation of light also varied significantly (P < 0.05) among groups on 4th and 6th days of storage study. However, it decreased significantly (P < 0.05) in all samples with storage. Highest values were estimated for PBH3 and lowest for control at 6th day storage. Chroma was derived from b* and a* values mathematically. Chroma values differed significantly (P < 0.05) among groups and higher values were calculated for control and PC followed by PBH2 > PBH1 > PBH3 on day zero. Though it varied during entire storage study but on last day of storage, it was comparable among all treated samples, whereas C was comparable to PC.
Microbial quality
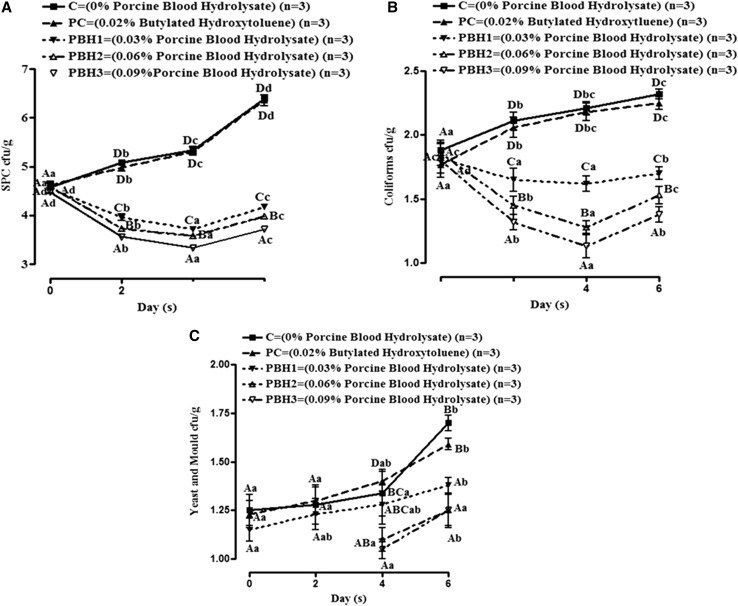
Microbiological changes in pork batter incorporated with PBH during refrigeration storage (4 ± 1 °C) are presented in Fig. 2. SPC was comparable in all samples on 0 day irrespective of PBH or BHT addition Fig. 2a. However SPC count decreased in treated samples up to 4th day of storage, thereafter it increased on further storage. However, in C and PC, it followed an increasing trend throughout storage. This could be due to additive effect of primary cellular damage resulted from iron chelating capacity and consequently elongation of lag phase/lysis of selected microbes by smaller peptides present in PBH (Verma et al. 2018). Similarly, hydrolysates obtained from controlled peptic hydrolysis of bovine hemoglobin by gastrointestinal proteases have also proved to be anti-bacterial (Adje et al. 2011). Coliforms, increased significantly in control and PC with storage Fig. 2b. On 0 day of storage, coliforms counts were comparable in all groups however, coliforms decreased in treated meat batter up to 4th day and thereafter it increased. Further it was observed that coliform counts decreased in treated meat batter in dose dependent manner. On the initial day of storage, Yeast and Mould counts were not detected in PBH3 and PBH2 Fig. 2c. Yeast and Mould counts increased during the storage study among all the groups. Reduction in Yeast and Mould counts in pork batter with PBH could be attributed to the anti-fungal properties of the protein hydrolysate. The anti-fungal activity of PBH might be due to the interruption of cell wall and cell membrane synthesis, and chelation of essentials minerals (Verma et al. 2018). Liu et al. (2008) isolated an anti-fungal protein, CgPep33, with an inhibitory action on several fungi mycelial growth, from digested oyster (Crassostrea gigas) using alcalase and bromelin enzyme.
Fig. 2.
Changes in microbiological quality parameter (a–c) of pork batter included with PBH hydrolysate by alcalase during refrigerated storage (Mean ± SE). Means values bearing small letters days wise and capital letters groups wise indicate differ significantly (P < 0.05)
Microbial challenge test (MCT)
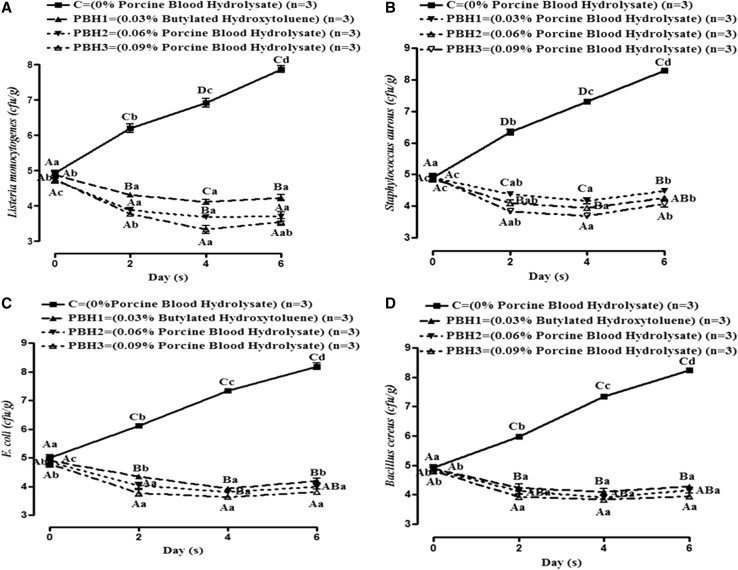
Effect of different concentrations of PBH incorporated in meat batter on the inoculated test microorganisms was observed (Fig. 3). MCT was conducted to measure the survivability of known food spoilage microbes (L. monocytogens, S. aureus, E. coli and B. cereus) in the presence of PBH. The MCT counts varied significantly (P < 0.05) among groups with storage study. MCT values also increased significantly (P < 0.05) with storage for control groups however, it decreased in treatments up to 4th day and increased on 6th day. Results indicated that cfu/g values decreased with increase in concentration of PBH. The decrease in MCT count in treated pork batter might be due to the interaction of the peptides with the microbial cell membrane which caused leakage of cellular content, inhibition of protein synthesis and binding of some essential minerals (Verma et al. 2017; Lafarga and Hayes 2014). Similar results have also been reported by Verma et al. (2017) for food spoilage microbes during in vitro study. Robert et al. (2015) and Sila et al. (2014) also obtained anti-microbial peptides from fish proteins and confirmed anti-microbial activity towards food spoilage microbes such as E. coli, Micrococcus luteus, Bacillus cereus and Listeria monocytogenes. However the activity of protein hydrolysates depends on the type, composition, size and molecular weight of peptides and concentration of hydrolysate, type of microorganism, and dose of target micro-organism inoculated in meat.
Fig. 3.
Changes in microbial counts (a–d) in pork batter included with PBH hydrolysates by alcalase during microbial challenge test at refrigeration temperature storage (Mean ± SE). Means values bearing small letters days wise and capital letters groups wise indicate differ significantly (P < 0.05)
Conclusion
Porcine blood hydrolysate has potential to replace synthetic anti-oxidants in meat batter, as it exhibited substantial efficacy in reducing lipid-oxidation and microbial growth. The inclusion of PBH at 0.09% (w/w) concentration significantly improved the physico-chemical properties, anti-oxidant assay, oxidative stability/lipid-oxidation, instrumental colour profile and microbial quality of meat batter. Therefore, PBH can be utilized as a potential source of natural anti-oxidant and anti-microbial agents/compounds that can extend shelf-life (refrigerated storage) of meat batter without adversely affecting its quality. Furthermore, the use of PBH in preserving meat batter would also satisfy the consumers demand for natural, safe and healthy food ingredients.
Acknowledgements
The first author is thankful to Department of Science and Technology, Ministry of Science and Technology, Government of India for financial assistance provided in the form of Inspire Fellowship (JRF-P).
Compliance with ethical standards
Conflict of interest
There is no conflict of interest.
References
- Abed NE, Kaabi B, Smaali MI, Chabbouh M, Habibi K, Mejri M, Marzouki MN, Ahmed SBH. Chemical composition, antioxidant and antimicrobial activities of Thymus capitata essential oil with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Evid Based Complement Altern Med. 2014 doi: 10.1155/2014/152487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adje EY, Balti R, Kouach M, Dhulster P, Guillochon D, Nedjar-Arroume N. Obtaining antimicrobial peptides by controlled peptic hydrolysis of bovine hemoglobin. Int J Biol Macromol. 2011;49:143–153. doi: 10.1016/j.ijbiomac.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Anandh MA, Lakshmanan V. Storage stability of smoked buffalo rumen meat product treated with ginger extract. J Food Sci Technol. 2014;51:1191–1196. doi: 10.1007/s13197-012-0622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APHA . Compendium of methods for microbiological examination of foods. 2. Washington: American Public Health Association; 1984. [Google Scholar]
- Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol. 1999;299:15–27. doi: 10.1016/S0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- Bernardini DR, Mullen AM, Bolton D, Kerry J, O’Neill E, Hayes M. Assessment of the angiotensin-I-converting enzyme (ACE-I) inhibitory and antioxidant activities of hydrolysates of bovine brisket sarcoplasmic proteins produced by papain and characterisation of associated bioactive peptidic fractions. Meat Sci. 2012;90:226–235. doi: 10.1016/j.meatsci.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chaijan M, Benjakul S, Visessanguan W, Faustman C. Changes of pigments and colour in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chem. 2005;93:607–617. doi: 10.1016/j.foodchem.2004.10.035. [DOI] [Google Scholar]
- Chi C-F, Hu F-Y, Wang B, Li T, Ding G-F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J Funct Foods. 2015;15:301–313. doi: 10.1016/j.jff.2015.03.045. [DOI] [Google Scholar]
- Dekkers E, Raghavan SK, Hordur G, Kristinsson Maurice R, Marshall MR. Oxidative stability of mahimahi red muscle dipped in tilapia protein hydrolysates. Food Chem. 2011;124:640–645. doi: 10.1016/j.foodchem.2010.06.088. [DOI] [Google Scholar]
- Dey SS, Dora KC. Antioxidative activity of protein hydrolysate produced by alcalase hydrolysis from shrimp waste (Penaeus monodon and Penaeus indicus) J Food Sci Technol. 2014;51:449–457. doi: 10.1007/s13197-011-0512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honikel KO. Minced meat. In: Dikeman M, Devine C, editors. Encyclopedia of meat sciences. 2. London: Elsevier; 2014. pp. 422–424. [Google Scholar]
- Jay JM. Beef microbial quality determined by extract release volume (ERV) Food Technol. 1964;18:1637–1641. [Google Scholar]
- Jayawardana BC, Liyanage R, Lalantha N, Iddamalgoda S, Weththasinghe P. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT Food Sci Technol. 2015;64:1204–1208. doi: 10.1016/j.lwt.2015.07.028. [DOI] [Google Scholar]
- Jin SK, Choi JS, Choi YJ, Lee SJ, Lee SY, Hur SJ. Development of sausages containing mechanically deboned chicken meat hydrolysates. J Food Sci. 2015;80:S1563–S1567. doi: 10.1111/1750-3841.12920. [DOI] [PubMed] [Google Scholar]
- Koniecko R. Handbook for meat Chemists. Wayne: Avery Publishing Group, Inc.; 1979. pp. 53–55. [Google Scholar]
- Kumar A, Chattopadhyay S. DNA damage protecting activity and antioxidant potential of pudina extract. Food Chem. 2007;100:1377–1384. doi: 10.1016/j.foodchem.2005.12.015. [DOI] [Google Scholar]
- Kumar Y, Yadav DN, Ahmad T, Narsaiah K. Recent trends in the use of natural antioxidants for meat and meat products. Compr Rev Food Sci Food Saf. 2015;14:796–812. doi: 10.1111/1541-4337.12156. [DOI] [Google Scholar]
- Kuo CC, Chu CY. Quality characteristic of Chinese sausages made from PSE pork. Meat Sci. 2003;64:441–449. doi: 10.1016/S0309-1740(02)00213-9. [DOI] [PubMed] [Google Scholar]
- Lafarga T, Hayes M. Bioactive peptides from meat muscle and by-products: generation, functionality and application as functional ingredients. Meat Sci. 2014;98:227–239. doi: 10.1016/j.meatsci.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Liu ZY, Dong SY, Xu J, Zeng MY, Song HX, Zhao YH. Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostreagigas) with alcalase and bromelin. Food Control. 2008;19:231–235. doi: 10.1016/j.foodcont.2007.03.004. [DOI] [Google Scholar]
- Nasri R, Younes I, Jridi M, Trigui M, Bougatef A, Nedjar-Arroume N, et al. ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: effect on meat lipid oxidation. Food Res Int. 2013;54:552–561. doi: 10.1016/j.foodres.2013.07.001. [DOI] [Google Scholar]
- Oliveira CF, Coletto D, Correa APF, Daroit DJ, Toniolo R, Cladera-Olivera F, Brandelli A. Antioxidant activity and inhibition of meat lipid oxidation by soy protein hydrolysates obtained with a microbial protease. Int Food Res J. 2014;21:775–781. [Google Scholar]
- Onuh JO, Girgih AT, Aluko RE, Aliani M. In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chem. 2014;150:366–373. doi: 10.1016/j.foodchem.2013.10.107. [DOI] [PubMed] [Google Scholar]
- Robert M, Zatylny-Gaudin C, Fournier V, Corre E, Le Corguille G, Bernay B, Henry J. Molecular characterization of peptide fractions of a Tilapia (Oreochromis niloticus) by-product hydrolysate and in vitro evaluation of antibacterial activity. Process Biochem. 2015;50:487–492. doi: 10.1016/j.procbio.2014.12.022. [DOI] [Google Scholar]
- Rossini K, Norena CPZ, Cladera-Olivera F, Brandelli A. Casein peptides with inhibitory activity on lipid oxidation in beef homogenates and mechanically deboned poultry meat. LWT Food Sci Technol. 2009;42:862–867. doi: 10.1016/j.lwt.2008.11.002. [DOI] [Google Scholar]
- Sakanaka S, Tachibana Y. Active oxygen scavenging activity of egg-yolk protein hydrolysates and their effects on lipid oxidation in beef and tuna homogenates. Food Chem. 2006;95:243–249. doi: 10.1016/j.foodchem.2004.11.056. [DOI] [Google Scholar]
- Salami M, Yousefi R, Ehsani MR, Razavi SH, Chobert JM, Haertle T. Enzymatic digestion and antioxidant activity of the native and molten globule states of camel α-lactalbumin: possible significance for use in infant formula. Int Dairy J. 2009;19:518–523. doi: 10.1016/j.idairyj.2009.02.007. [DOI] [Google Scholar]
- Sila A, Nedjar-Arroume N, Hedhili K, Chataigne G, Balti R, Nasri M, Dhulster P, Bougatef A. Antibacterial peptides from barbel muscle protein hydrolysates: activity against some pathogenic bacteria. LWT Food Sci Technol. 2014;55:183–188. doi: 10.1016/j.lwt.2013.07.021. [DOI] [Google Scholar]
- Sperber WH. Influence of water activity on foodborne bacteria—a review. J Food Prot. 1983;46:142–150. doi: 10.4315/0362-028X-46.2.142. [DOI] [PubMed] [Google Scholar]
- Sun Y, Daodong P, Yuxing G, Li J. Purification of chicken breast protein hydrolysate and analysis of its antioxidant activity. Food Chem Toxicol. 2012;50:3397–3404. doi: 10.1016/j.fct.2012.07.047. [DOI] [PubMed] [Google Scholar]
- Townsend WE, Witnauer LP, Riloff JA, Swift CE. Comminuted meat emulsions. Differential thermal analysis of fat transition. Food Technol. 1968;22:319–323. [Google Scholar]
- Verma AK, Pathak V, Singh VP, Umaraw P. Storage study of chicken meatballs incorporated with green cabbage (Brassica olerecea) at refrigeration temperature (4 ± 1 °C) under aerobic packaging. J Appl Anim Res. 2016;44:409–414. doi: 10.1080/09712119.2015.1091328. [DOI] [Google Scholar]
- Verma AK, Chatli MK, Kumar P, Mehta N. Antioxidant and antimicrobial activity of protein hydrolysate extracted from porcine liver. Indian J Anim Sci. 2017;87:711–717. [Google Scholar]
- Verma AK, Chatli MK, Mehta N, Kumar P. Assessment of physico-chemical, antioxidant and antimicrobial activity of porcine blood protein hydrolysate in pork emulsion stored under aerobic packaging condition at 4 ± 1 °C. LWT Food Sci Technol. 2018;88:71–79. doi: 10.1016/j.lwt.2017.10.002. [DOI] [Google Scholar]
- Vioque J, Sanchez-Vioque R, Clemente A, Pedroche J, Millan F. Partially hydrolyzed rapeseed protein isolates with improved functional properties. J Am Oil Chem Soc. 2000;77:447–450. doi: 10.1007/s11746-000-0072-y. [DOI] [Google Scholar]
- Wald M, Schwarz K, Rehbein H, Bubmann B, Beermann C. Detection of antibacterial activity of an enzymatic hydrolysate generated by processing rainbow trout by-products with trout pepsin. Food Chem. 2016;205:221–228. doi: 10.1016/j.foodchem.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Witte VC, Krause GF, Bailey ME. A new extraction method for determining 2-Thiobarbituric acid values of pork beef during storage. J Food Sci. 1970;35:582–585. doi: 10.1111/j.1365-2621.1970.tb04815.x. [DOI] [Google Scholar]
- Yen GC, Chang YC, Chen JP. Antioxidant activity of mycelia from Aspergillus candidus. J Food Sci. 2002;67:567–572. doi: 10.1111/j.1365-2621.2002.tb10639.x. [DOI] [Google Scholar]