Abstract
Grapes are well known for their high content of phenolic compounds. Polyphenols are classified into flavonoids and non-flavonoids by their primary chemical structures of hydroxybenzene. Flavonoids mainly consist of anthocyanins, flavonoids, and flavonols whereas non-flavonoids include hydroxycinnamic and hydroxybenzoic acids. In the present study, sixteen phenolic compounds from ten red and nine white grape wine varieties were quantified using high-performance liquid chromatography. Gallic acid, Vanillic acid, Rutin hydrate, Ellagic acid, Chlorogenic acid, Sorbic acid, Catechin hydrate, Epicatechin, p-coumaric acid, Quercetin, Myricetin, Kaempferol, Piceatannol, and Resveratrol were major compounds found in red wine grapes. Among the varieties, Petit Verdot, Cabernet Franc showed maximum quantitative phenolics, whereas Cabernet Sauvignon, Niellucio, Cinsaut, and Syrah showed least quantitative phenolics in grape berries. Phenolic profile of white wine grapes showed lower concentration of phenolics than that of red wine grapes. The variety Gros Meseng showed maximum phenolics followed by Sauvignon, while the variety Colombard and Chenin Blanc showed least phenolics.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3438-x) contains supplementary material, which is available to authorized users.
Keywords: Non-flavonoids; Flavonoids; Hydroxycinnamic acid; Stilbenes, and wine grapes
Introduction
The presence of various phenolic compounds makes grapes as an important source of antioxidants (Andjelkovic et al. 2013). The antioxidant activity of dietary polyphenols is considered to be greater than vitamins and content of polyphenols in grapes reflects as health benefit substances (Banini et al. 2006 and Anastasiadi et al. 2010). Grape (Vitis vinifera L.) is among the fruits with the highest content of phenolic compounds. Phenolic compounds of wine and wine by-products have attracted much interest due to their antioxidant and antimicrobial properties and their potentially beneficial effects for human health (Sun et al. 2002; Baydar et al. 2006). Many workers have reported the importance of phenolic compounds as protector of human health in different ways (Lee et al. 2005; Banini et al. 2006 and Del Pozo-Insfran et al. 2007). Wine is one of the most consumed beverages in the world. Consumption has considerably increased owing to the awareness about health promoting characters in wines which are imparted by various polyphenolic compounds such as flavonoids, anthocyanins, tannins, etc. Phenolic compounds are major important constituents of wines determining the wine quality due to their direct influence on sensory properties of wines such as color, flavor, bitterness, astringency and aroma (Garridio and Borges 2011). Quality of wine grapes used for wine making is one of the major factor contributing to the final quality of the wines (Caceres et al. 2012). To select grape varieties for wine production, emphasis should be given not only on economic yield, resistance to pests and diseases, juice recovery, sugar, and acid content but also to the functional properties like anthocyanins, phenolics, tannins, etc. Not only the content of total anthocyanins, phenolics and tannins, their composition also have a significant role in deciding the wine quality.
Wine grape cultivation in India has been increasing steadily over the years and has potential to grow further shortly. Different varieties of wine grapes are being cultivated to produce wines under tropical conditions of India (Adsule et al. 2012). The wine grape varieties are introduced from different countries. Due to the wide adaptability of grapevines, these are being successfully grown in Indian tropical conditions, and quality parameters of wines made from these grape varieties are complying established norms and found at par with the quality of wines produced in temperate countries. The quality of grapes has been improved tremendously, after the establishment of two pruning and one cropping cultivation practices in this region (Jogaiah et al. 2013). Based on juice yield and must quality parameters the cvs; Shiraz, Cabernet Sauvignon, Merlot and Zinfandel among red varieties and Sauvignon Blanc, Ugni Blanc, Vermentino and Garganega among white varieties are recommended for commercial wine production (Karibasappa and Adsule 2008 and Adsule et al. 2012). Varietal impact on phenolics content and antioxidant activities of wines have been reported by various researchers (Sharma et al. 2012; Silva and Queiroz 2016, Doshi et al. 2015; Patil et al. 2012 and Gagne et al. 2016). However, basic research on quality parameters of wine grapes of different varieties have been studied, but no work is initiated on phenolics profiling of wine grapes grown in hot conditions prevail in India. As the grape growing conditions have its role in deciding phenolics behavior in grapes, the present investigation was carried out for profiling the phenolics in wine grapes. It will lead to quantify phenolic composition at harvest stage and to establish a base for differentiation of wine grape varieties based on the phenolic composition as well as phenolic maturity of grapes.
Materials and methods
Selection of wine grape genotypes
Ten red and nine white wine grape varieties (Table S1) grafted on 110 R rootstock introduced from France were selected for the study. The vineyard is situated near Pune in Maharashtra state at an altitude of 559 m (18.49°N and 73.98°E) with tropical dry climate. The average temperature is ranging between 20 and 28 °C. The vines were planted in the N-S direction in black soil (EC 0.8 dS/m and pH 7.3) spaced at distance of 2.66 × 1.33 m and trained to mini Y trellis with cordons placed horizontally. The vines were drip irrigated during the season. Normal cultural practices were followed for the production of healthy grapes during the season. The experiment was conducted in randomized block design with three replications consisting of ten vines under each replication.
Sample collection and preparation
From each variety, one kg berry sample was collected randomly from upper, lower and middle part of the bunches from differently identified vines from each replication. Bunches were harvested at technical maturity when the berries attained total soluble solids (TSS) in the range of 22–24 ºB. The collected berry samples were packed in food grade polythene bags and stored at − 20 °C for further study.
The berries were destemmed and then homogenized. For extraction of the phenolic compounds, 1 g of homogenized sample was drawn into 15 ml polypropylene tube containing 5 ml of 0.1% formic acid in 20% methanol. The mixture was vortexed for 1 min followed by centrifugation at 5000 rpm for 5 min. One ml of supernatant was transferred to a microtube and again centrifuged at 10,000 rpm at 4 °C for 10 min. The supernatant was filtered through 0.2 µm- membrane filter and the filtrate was used for analysis.
Preparation of standard solutions
Individual certified reference standards of all the test compounds were procured from Sigma-Aldrich with greater than 90% purity. Methanol (HPLC grade) was supplied by Sigma-Aldrich, and other reagents were obtained from Thomas Baker. The standard stock solution of each phenolic compound was prepared by dissolving 10 mg individual analyte in 10 ml methanol and stored at − 20 °C. An intermediate working solution of 10 mg/L was prepared by mixing appropriate volume of each stock solution in methanol.
HPLC–DAD analysis
High-performance liquid chromatography (HPLC) with diode array detector (1260 infinity, Agilent Technologies) was used for analysis of phenolic compounds. An Agilent EZ Chrome elite® software was used for instrument control, data acquisition, and data analysis. The chromatographic separation was performed on a Zorbax Eclipse Plus C18 column (4.6 mm × 100 mm, 1.8 µm, Agilent Technologies). The mobile phase consisted of A (0.2% acetic acid in 10% acetonitrile-99%) and B (0.2% acetic acid in acetonitrile-1%) which was maintained at constant flow rate of 0.80 mL/min. The column oven temperature at 30 °C and detector wavelength 280 nm were maintained. The gradient program was used as follows: 0 min, 99% of A; 4 min of 99% of A; 8 min of 80% of A; 10 min of 60% of A; 13 min of 60% of A; 15 min of 99% of A; 20 min of 99% of A. Identification of the compounds was performed based on the reference standards retention time and specific detector wavelength. The quantitation was performed by using external standards calibration method. Total chromatographic runtime was 20 min per injection.
Data analysis
Principal Component Analysis (PCA) was performed using SAS (http://iasri.res.in/design/Analysis%20of%20data/principal_component.html).
Results and discussion
Non-flavonoids
Hydroxybenzoic acid
Non-flavonoids, the class of phenols; contains characteristics compounds such as gallic acid, ellagic acids, sorbic acid and vanillic acid. These compounds were largely found in the grape pulp (Lu and Foo 1999). In the present study, significant differences were recorded for phenolic contents in different wine varieties. Among the various non-flavonoid compounds, gallic acid was the major compound found in red wine grapes ranged from 1.846 mg/kg in Petit Verdot to 0.635 mg/kg in Caladoc variety (Table 1). White wine varieties showed the slightly low concentration of gallic acid as compared to red wine varieties which were ranged from 0.780 mg/kg in Gros Meseng to 0.537 mg/kg in Vermentino variety.
Table 1.
Phenolic compounds from red wine grapes varieties
| Varieties | Cabernet Sauvignon | Cabernet Franc | Merlot | Petit Verdot | Tempra nillo | Niellucio (Sangioves) | Grenache | Caladoc | Cinsaut | Syrah | CD at 5% | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-flavonoids (hydroxybenzoic acid) (mg/kg) | ||||||||||||
| Gallic acid (1.6) | 0.89 | 1.31 | 0.96 | 1.84 | 0.75 | 1.15 | 0.68 | 0.63 | 0.68 | 1.39 | 0.03 | 0.39 |
| Ellagic acid (7.35) | 5.54 | 12.62 | 10.91 | 8.89 | 2.34 | 4.77 | BLQ | 4.58 | 1.76 | 7.99 | 0.02 | 4.10 |
| Vanillic acid (3.9) | 0.52 | 0.44 | 0.54 | 0.26 | 0.11 | 0.07 | BLQ | BLQ | BLQ | BLQ | 0.02 | 0.23 |
| Sorbic acid (3.68) | 0.54 | 0.69 | 0.56 | 0.64 | 0.52 | 0.53 | 0.15 | BLQ | 0.53 | BLQ | 0.02 | 0.26 |
| Non-flavonoids (hydroxycinnamic acid) (mg/kg) | ||||||||||||
| Cafteric acid (1.88) | 0.86 | 12.55 | 2.74 | 9.10 | 1.49 | 1.54 | 7.06 | 4.35 | 5.40 | 2.55 | 0.15 | 3.81 |
| P-Cumaric acid (6.36) | 0.52 | 0.74 | 0.76 | 0.73 | 0.66 | 0.65 | 0.56 | 1.21 | 0.56 | 0.92 | 0.02 | 0.21 |
| Chlorogenic acid (6.71) | BLQ | 1.76 | 1.69 | 3.81 | 0.76 | 0.64 | 1.17 | 0.92 | 0.64 | 1.04 | 0.02 | 1.04 |
| Flavonols (Flavan-3-ols) (mg/kg) | ||||||||||||
| Catechin Hydrate (2.68) | 5.12 | 8.63 | 8.79 | 13.93 | 1.82 | 4.37 | 1.63 | 3.36 | 0.83 | 0.87 | 0.12 | 4.30 |
| Epicatechin (3.47) | 3.53 | 5.55 | 6.42 | 7.44 | 1.09 | 4.94 | 0.23 | 0.91 | 0.59 | 1.19 | 0.04 | 2.72 |
| Qucertinn (10.01) | 3.72 | 9.57 | 8.71 | 8.85 | 3.66 | 5.10 | 3.35 | 5.60 | 4.77 | 1.93 | 0.06 | 2.64 |
| Rutin Hydrate (7.03) | 0.20 | 1.61 | 1.69 | 2.85 | 2.01 | 1.40 | 1.34 | 3.67 | 0.18 | 16.41 | 0.10 | 4.78 |
| Myricitin (11.05) | 1.04 | 0.96 | 0.52 | 0.96 | 1.13 | BLQ | BLQ | BLQ | 1.73 | BLQ | 0.02 | 0.62 |
| Kampherol (12.43) | 0.02 | BLQ | 0.47 | 0.00 | 0.02 | 0.02 | 0.39 | 0.24 | 0.50 | 0.50 | 0.08 | 0.23 |
| Stilbenes (mg/kg) | ||||||||||||
| Picetannol (9.49) | 0.79 | 1.50 | 1.34 | 1.50 | 1.76 | 0.50 | 1.24 | 16.86 | 10.63 | 24.33 | 0.14 | 8.40 |
| Resveretrol (11.49) | 0.55 | 0.54 | 0.53 | 0.55 | 0.64 | 0.27 | 0.13 | 0.58 | 0.56 | 0.52 | 0.02 | 0.16 |
The values in the parenthesis are the retention time in minutes with phenolic compounds
BLQ below limit of quantification
The ellagic acid and vanillic acid the next abundant compounds followed by gallic acid with high antioxidant and anti proliferation activity. The concentration of ellagic acid found in the present study ranged from 12.626 mg/kg in Cabernet Franc to 1.762 mg/kg in Cinsaut in red wine grape varieties, while the concentration of ellagic acid in all the other red wine varieties ranged in between. The concentration of -ellagic acid in white wines was slightly more than that of red wine varieties (Table 1 and 2). Riesling variety showed highest ellagic acid content (17.033 mg/kg) followed by Muscat White (13.341 mg/kg), while least concentration (0.881 mg/kg) was recorded in Sauvignon Blanc. More concentration of vanillic acid was observed in white wine varieties than noted in red wine varieties. Maximum concentration of vanillic acid (0.5 mg/kg) was recorded in Sauvignon Blanc and Gross Mesengwhile minimum i.e. 0.011 mg/kg was observed in Chenin Blanc. In case of red wine grapes, higher concentration i.e. 0.544 mg/kg was recorded in Merlot variety followed by Cabernet Sauvignon (0.515 mg/kg) and least was found in Niellucio variety (0.074 mg/kg) whereas it was below detection limit (BDL) in Grenache, Caladoc, Cinsaut and Syrah varieties. Sorbic acid concentration (0.69 mg/kg) was higher in Cabernet Franc while it was BDL in Caladoc and Syrah. In white grape, it was ranged from 0.36 to 0.13 mg/kg in Chenin Blanc and Colombard, respectively. Variations recorded in the concentration of hydroxybenzoic acids in the present investigations might be due to the genetic makeup of individual variety. Ritter et al. (1994) also noted variations due to the genetic variability of grape vines. Lubomie (2013) reported hydroxybenzoic acids as the most vital phenolics for distinguishing grape wine varieties. The highest content of hydroxybenzoic acids was corresponding to gallic acid, catechin, epicatechin, and quercetin were observed in studied varieties. It was found in same fashion as noted by Shahidi and Naczk (1995).
Table 2.
Phenolic compounds from white wine grapes varieties
| Varieties | Colombard | Gewurztr aminor | Riesling | Sauvignon Blanc | Muscat | Chenin Blanc | Gross Meseng | Vermentino | Viognier | C.D at 5% | S.D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-flavonoids (hydroxybenzoic acid) (mg/kg) | |||||||||||
| Gallic acid (1.6) | 0.55 | 0.55 | 0.59 | 0.72 | 0.59 | 0.57 | 0.71 | 0.53 | 0.61 | 0.02 | 0.07 |
| Ellagic acid (7.35) | 1.76 | 5.93 | 17.93 | 0.88 | 17.03 | 4.51 | 0.89 | 12.69 | 13.34 | 0.02 | 6.95 |
| Vanillic acid (3.9) | 0.27 | 0.28 | 0.23 | 0.50 | 0.23 | 0.01 | 0.47 | 0.05 | 0.13 | 0.08 | 0.17 |
| Sorbic acid (3.68) | 0.13 | 0.29 | 0.29 | 0.31 | 0.29 | 0.36 | 0.31 | 0.17 | 0.17 | 0.02 | 0.08 |
| Non-flavonoids (hydroxycinnamic acid) (mg/kg) | |||||||||||
| Cafteric acid (1.88) | 1.55 | 1.58 | 6.69 | 0.98 | 6.69 | 1.76 | 0.98 | 4.67 | 5.14 | 0.12 | 2.44 |
| P-Cumaric acid (6.36) | 0.05 | 0.50 | 0.28 | 0.47 | 0.28 | 0.51 | 0.53 | 0.22 | 0.38 | 0.09 | 0.16 |
| Chlorogenic acid (6.71) | BLQ | 0.87 | 0.83 | 0.74 | 0.83 | 0.65 | 0.74 | BLQ | BLQ | 0.02 | 0.08 |
| Flavonols (Flavan-3-ols) (mg/kg) | |||||||||||
| Catechin Hydrate (2.68) | 1.77 | 4.38 | 2.25 | 2.69 | 2.25 | 1.576 | 2.73 | 1.24 | 1.82 | 0.09 | 0.92 |
| Epicatechin (3.47) | 0.93 | 1.26 | 1.79 | 2.87 | 1.79 | 0.81 | 2.88 | 0.93 | 1.42 | 0.02 | 0.79 |
| Qucertinn (10.01) | 0.75 | 2.55 | 3.45 | 1.94 | 3.45 | 2.86 | 1.94 | 4.02 | 2.39 | 0.02 | 0.99 |
| Rutin Hydrate (7.03) | 0.47 | 1.43 | 2.58 | 1.09 | 2.58 | 1.02 | 1.09 | 0.85 | 1.24 | 0.07 | 0.73 |
| Myricitin (11.05) | 0.00 | 0.94 | 0.10 | 0.18 | 0.13 | 0.92 | 0.18 | 0.09 | 0.53 | 0.04 | 0.37 |
| Kampherol (12.43) | 0.35 | 0.52 | 0.25 | 0.23 | 0.25 | 0.21 | 0.23 | 0.08 | 0.29 | 0.02 | 0.12 |
| Stilbenes (mg/kg) | |||||||||||
| Picetannol (9.49) | 0.15 | 0.56 | 0.55 | 0.36 | 0.55 | 0.61 | 0.36 | 0.56 | 0.61 | 0.02 | 0.16 |
| Resveretrol (11.49) | 0.04 | 0.24 | 0.34 | 0.11 | 0.34 | 0.11 | 0.11 | 0.11 | 0.13 | 0.02 | 0.11 |
The values in the parenthesis are the retention time in minutes with phenolic compounds
BLQ below limit of quantification
Hydroxycinnamic acids
Hydroxycinnamic acids represent another important group of phenolic compounds in wine grapes. These generally originate from hydroxycinnamic tartaric esters (Cheynier et al. 2010). Among the hydroxycinnamic acids, caftaric acid and p-cumaric acid are important compounds. Higher concentrations of caftaric acid were found in red wine varieties than in white wine varieties. The concentration varied significantly between the specific wine varieties and ranged from 9.102 mg/kg in Petit Verdot to 0.808 mg/kg in Cabernet Sauvignon. In white wines, the concentration was ranged from 6.691 mg/kg in Riesling to 0.989 mg/kg in Sauvignon Blanc. The results obtained for p-cumaric acids are distinct and having concentration in different trend as observed in both type wine grape varieties (Table 2). The highest concentration of p-cumaric acid was recorded in Caladoc (1.218 mg/kg) followed by Merlot (0.762 mg/kg), and least was recorded in Colombard (0.052 mg/kg). The variation in the concentration of caftaric acid and p-cumaric acid obtained in the present investigation could be due to the genetics of grapevine. Since all the varieties do not attain maturity at a time, the variability in weather conditions after veraison to harvest might have been more responsible for these parameters. Similar studies were reported by Ritter et al. (1994) who used the ratio of caftaric acid and p-coumaric acids in grapes when identifying and deterring the origin of grapevine varieties. The concentrations of caftaric acid and p-cumaric acids in different varieties were diverse and these observations are in agreement with Pour et al. (2007) who reported concentration ranged between 0.5 and 10.6 mg/L. Hydroxycinnamic acid (Caftaric acid and p-Cumaric acid) could be the important biochemical markers of varietal discrimination due to their significant differences in the concentration in both red and white varieties.
The concentration of chlorogenic acid in red wine verities varied from 3.813 mg/kg in Petit Verdot to 0.647 mg/kg in Niellucio and Cinsaut, whereas, it could not be detected in Cabernet Sauvignon. The concentration of chlorogenic acid in white wine varieties was ranged between 3.885 mg/kg (Gros Meseng) to 0.659 mg/kg (Chenin Blanc) and was below the detectable limits in Colombard, Muscat White, Vermentino and Viognier varieties. In general, the higher Chlorogenic acid concentration was observed in red wine grapes as compared to white wine grapes.
Flavonols
Flavan-3-ols
Flavonoids such as catechin hydrate and epicatechin are one of the major classes of phenolics in grapes. However, their concentration is negligible in pulp and skin. These compounds are mainly extracted from grape seeds during winemaking (Gambuti et al. 2004). Catechin and epicatechin analyzed from berries of red wine varieties ranged from 0.828 mg/kg in Cinsaut to 13.935 mg/kg in Petit Verdot. A similar trend for catechin content was also observed in white wine. However, their concentration was slightly lower compared to red wine grape varieties. Among the white wine grapes, Gros Meseng contained highest catechin concentration (10.304 mg/kg) whereas the least concentration of catechin was recorded in Vermentino. Among the red wine varieties, a higher concentration of epicatechin was recorded in Petit Verdot (7.44 mg/kg) followed by Merlot (6.42 mg/kg) while in Grenache exhibited the lowest amount (0.20 mg/kg). The concentration of epicatechin in white wine was slightly lower than the red wine varieties ranging from 5.66 mg/kg in Gros Meseng to 0.80 mg/kg in Chenin Blanc. The variations in the concentration of catechin and epicatechin may be due to exposure of bunches to sunlight and the warm climate (Fernandez et al. 2007; Conde et al. 2007; Raul et al. 2009). Flavan-3-ols are mainly found in grapes seeds. Two different factors may determine their final concentration found in grapes i.e., the concentration of these compounds in the grape seeds and the total weight of seeds in the grape. Similarly, Raul et al. (2009) found a higher concentration of flavan-3-ol in seeds than the berries. This indicates that the total number of seeds in grapes plays an important role in the total content of catechin and epicatechin in whole grape berry.
Flavonols were mainly characterized by quercetin, rutin hydrate, and kaempferol. The quercetin concentration varied in all the red and white grape varieties. In general, the concentration of quercetin was higher in red wine grapes than in white wine grapes. The similar trend was observed with kaempferol. However, the concentration in both the white and red varieties was very less. In red wine varieties, Kaempferol ranged from 0.64 to 0.13 mg/kg while in white wine varieties, it varied between 0.529 and 0.083 mg/kg. Among the red wine varieties, rutin hydrate was maximum in Syrah (6.41 mg/kg) followed by Caladoc (5.60 mg/kg) whereas the least amount was recorded in Cinsaut (0.17 mg/kg). The concentration of rutin hydrate in white wine grapes ranged from 5.863 mg/kg in Gros Meseng to 0.13 mg/kg in Viognier. Myricetin, another important flavonol was found in less abundance in white wine varieties than red wine grapes. Among the red varieties, Cinsaut produced the highest content of myricetin (1.72 mg/kg) compared to least in Merlot (0.52 mg/kg) while, the concentration could not be detected in Niellucio, Grenache, Caldoc, and Syrah. In white wine grapes, myricetin concentration ranged between 0.941 mg/kg in Gewurztraminer to 0.004 mg/kg in Colombard. It is well known that the biosynthetic pathway involved in polyphenols production in plant tissue are greatly influenced by sunlight, temperature, skin thickness, and other cultural practices followed during the growing season (Vinci et al. 2008; Price et al. 1995; McDonald et al. 1998 and Downey et al. 2006). Varache-Lembege et al. (1996) while working on phenolics in grapes affected by sunlight and temperature reported quercetin, myricetin and kaempferol concentration was higher in the sun-exposed clusters than the shaded clusters. Under tropical conditions, during the ripening stage the temperature increases above 35 °C with humidity less than 30%, results in the exposure of bunches under sunlight.
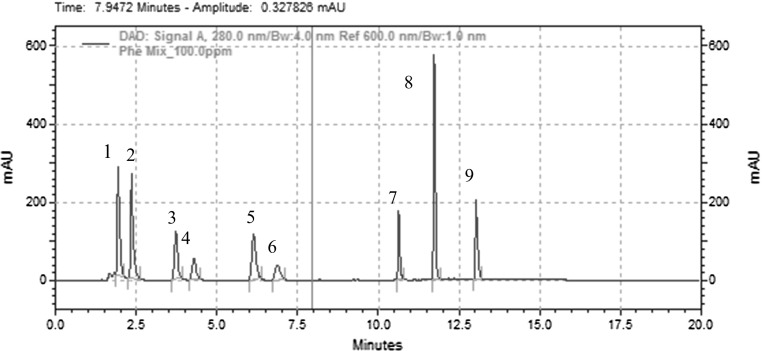
In studied wine grape varieties, piceatannol ranged from 16.86 mg/kg in Caladoc to 0.52 mg/kg in Niellucio. The berries of red varieties contained a higher proportion of piceatannol than the white wine grapes. In white wine varieties, the concentration ranged from 0.61 mg/kg in Muscat White to the lowest amount of in Colombard (0.15 mg/kg). Taware et al. (2010) reported increased piceatannol accumulation in leaves and berries at post infection stage. Hence, the variation in the concentration of piceatannols may be due to the capacity of the vine to develop immune system against pathogens (Figs. 1 and 2).
Fig. 1.
Chromatograms of phenolics standard at concentration level 100 µg/mL (1: Gallic acid; 2: Chlogenic acid; 3: Caftaric acid; 4: Vanilic acid; 5: Catechin; 6: Epicatechin; 7: Quercetin; 8: Kampherol and 9: Resveratrol
Fig. 2.
Chromatograms of phenolics in sample (Cabernet Sauvignon) (1: Gallic acid; 2: Chlogenic acid; 3: Caftaric acid; 4: Vanilic acid; 5: Catechin; 6 and Epicatechin; 7
Stilbenes
Stilbenes are major phenolic compounds possessing antifungal activity which enables the plant to fight against pathogen attack (Bavaresco and Fregoni 2001). Resveratrol is the most important compound belonging to stilbenes group of phenolics (Lubomie 2013). Higher concentration of Resveratrol content (0.64 mg/kg) was found in Tempranillo followed by Caladoc (0.58 mg/kg) while the lowest amount of resveratrol was found in Grenache (0.13 mg/kg). In the white wine grapes, the highest amount of resveratrol was found in Gros Meseng (0.63 mg/kg) while the least in Colombard (0.041 mg/kg). In general, the resveratrol content was more in red wine than in the white wine varieties. Sharma et al. (2012) reported that the more resveratrol content in red wines and white wines showed low levels of phenolic compounds and some phenolics viz.; epicatechin gallate, resveratrol, and kaempferol were found absent. Resveratrol is mainly contained in the skins of grapes (Schmandke 2002) due to this reason resveratrol was absent from white wines as no skin and seeds involve in wine making. However, Goldberg et al. (1995) found the similar content of resveratrol in red and white grapes. The variations in results obtained in this study may be due to the large number of factors influencing its synthesis. Contradictory to the present results, Jeandet et al. (1995) reported no significant correlation between the enzymes involved in their synthesis. Vincenzi et al. (2013) reported that resveratrol is not synthesized in grapes but is the result of the isomerization of the trans-isoforms promoted by UV light during extraction.
The concentration for various phenolics varied among different wine grapes. Petit Verdot and Cabernet Franc showed maximum quantitative phenolic content, whereas, Cabernet Sauvignon, Niellucio, Cinsaut and Syrah showed least phenolics in the grapes. In general, the phenolic content of red wine grapes was higher than the white wine grapes. In the present study, total phenols varied among the varieties. The obtained results supports the findings of Singh et al. (2016) who reported that total phenolic content (TPC) and antioxidant activity (ABTS and DPPH) ranged from 354.9 to 1639.7 mg GAE/100 g, 2.6–5.5 and 3.0–6.3 mM TE/g, respectively for different fruits, Özcan et al. (2017) also reported variation in phenolic contents and bioactive compounds in different grape varieties.
Principal component analysis
To differentiate the wine grape varieties based on their phenolic content, the varietywise phenolic concentration data were analysed using Principal Component Analysis (PCA). PCA was used for visualization of the differences between red wine grapes and white wine grapes in two-dimensional spaces. Based on the correlation matrix, multivariate analysis was carried out using principal component analysis to determine the relationship among the phenolic compounds of red and white wine grapes. A clear differentiation between red wines and white wines was shown in Fig. 3. The two principal components (PC) account for 60.40% of total variance of the data. White wine grapes were situated in the positive part of PC2 (21.40%) and negative part of PC1 (39.10%). Some red wine grapes located in the positive part of both the principal components whereas other red wine grapes situated in a negative part of both the principal components. The PCA provided clearcut differentiation between white (W1–W9) and red wine grapes (R1–R10). In red wine grapes, R1–R6 falling in positive part whereas R7–R10 in negative part (Fig. 3). The differentiation of wine varieties using statistical analysis can be used for selection of appropriate wine grapes for the preparation of specific quality of wines. In the present study, the principal component analysis allowed the separation of wine varieties according to polyphenols content. The performance of variety in each group is different about the phenolic compound available. Daudt and de Oliveira (2013) also worked on PCA for separation of wine according to origin and maceration and reported that each maceration type is different according to the wine. Based on the PCA, the red wine varieties R7–R10 were found significantly different from varieties R1–R6 for total phenolics. However, white wine varieties W1 to W9 were found in a single group showing close to each other for phenolic compounds (Fig. 3). Silva and Queiroz (2016) recorded the differences in principal component analysis grape variety Touriga Nacional from the other varieties due to their high contents in anthocyanins, non-coloured phenolics and organic acids. Similarly, Gagne et al. (2016) reported large differences between the berry compositions of wine grape varieties by using Principal component analysis.
Fig. 3.
Differentiation of white and red wine varieties based on phenolic composition
Conclusion
On the basis of phenolic compounds and their profiling, wine grape varieties may be differentiated. On the basis of data obtained from present study it may be concluded that basic character of variety responsible for expression of the main phenolic compounds in grape berries. The expressions have been recorded in the form of phenolic variations. The generated information may be useful for optimizing the wine making process to produce quality wine from tropical climatic conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to the Director General of Agriculture, Food Processing, and Territorial Policies of the Ministry of Agriculture and Fisheries, Government of France for providing the planting material to carry out research work on the evaluation of wine varieties under Pune condition. The Director, ICAR-NRC Grapes, Pune also deserves for sincere thanks for providing the guidance and required facilities for carrying out the research.
References
- Adsule PG, Sharma AK, Upadhyay A, Sawant IS, Jogaiah S, Upadhyay AK, Yadav DS. Grape research in India: a review. Progress Hortic. 2012;44(20):180–193. [Google Scholar]
- Anastasiadi M, Pratsinis H, Kletsas D, Skaltsounis AL, Haroutounian SA. Bioactive non-coloured polyphenols content of grapes, wines and vilification by-products: evaluation of the antioxidant activities of their extracts. Food Res Int. 2010;43:805–813. doi: 10.1016/j.foodres.2009.11.017. [DOI] [Google Scholar]
- Andjelkovic M, Radovanović B, Radovanović A, Andjelkovic AM. Changes in polyphenolic content and antioxidant activity of grapes cv. vranac during ripening. S Afr J Enol Vitic. 2013;34(2):147–155. [Google Scholar]
- Banini AE, Boyd LC, Allen JC, Allen HG, Sauls DL. Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition. 2006;22:1137–1145. doi: 10.1016/j.nut.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Bavaresco L, Fregoni M. Physiological role and molecular aspects of grapevine stilbene compounds. In: Roubelakis-Angelakis KS, editor. Molecular biology and biotechnology of the Grapevine. Dordrecht: Kluwer; 2001. pp. 153–182. [Google Scholar]
- Baydar NG, Sagdç O, Ozkan G, Çetin ES. Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera L.) seed extracts. Int J Food Sci Technol. 2006;41:799–804. doi: 10.1111/j.1365-2621.2005.01095.x. [DOI] [Google Scholar]
- Caceres A, Pena NA, Obreque SE, Loez SR, Canals JM. Phenolic compositions of grapes and wines from cultivar Cabernet Sauvignon produced in Chile and their relationship to commercial value. J Agric Food Chem. 2012;60(35):8694–8702. doi: 10.1021/jf301374t. [DOI] [PubMed] [Google Scholar]
- Cheynier V, Schneider R, Salmon J, Fulcrand H. Chemistry of wine. In: Mander L, Liu HW, editors. Comprehensive natural products II. Oxford: Elsevier; 2010. pp. 1119–1172. [Google Scholar]
- Conde C, Silva P, Fontes N, Dias ACP, Tavares RM, Sousa MJ, Agasse A, Delrot S, Geros H. Biochemical changes throughout grape berry development and fruit and wine quality. Food. 2007;1(1):1–22. [Google Scholar]
- Daudt CE, de Oliveira A. Phenolic compounds in Merlot wines from two wine regions of Rio Grande do Sul, Brazil. Food Sci Technol Campinas. 2013;33(2):355–361. doi: 10.1590/S0101-20612013005000045. [DOI] [Google Scholar]
- Del Pozo-Insfran D, Del Follo-Martinaz A, Talcott ST, Brenes CH. Stability of co-pigmented anthocyanins and ascorbic acid in muscadine grape juice processed by high hydrostatic pressure. J Food Sci. 2007;72:247–253. doi: 10.1111/j.1750-3841.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- Doshi P, Adsule PG, Banerjee K, Oulkar D. Phenolic compounds, antioxidant activity and insulinotropic effects of extracts prepared from grape (Vitis vinifera L.) byproducts. J Food Sci Technol. 2015;52(1):181–190. doi: 10.1007/s13197-013-0991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey MO, Dokoozlian NK, Krstic MP. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: a review of recent research. Am J Enol Vitic. 2006;3:257–268. [Google Scholar]
- Fernandez K, Kennedy JA, Agosin E. Characterization of Vitis vinifera L. cv. Carmenere grape and wine proanthocyanidins. J Agric Food Chem. 2007;55(9):3675–3680. doi: 10.1021/jf063232b. [DOI] [PubMed] [Google Scholar]
- Gagne MP, Angers P, Pedneault K. phenolic compounds profile of berries and wines from five fungus-resistant grape varieties. Ann Food Process Preserv. 2016;1(5):1003. [Google Scholar]
- Gambuti A, Strollo D, Ugliano M, Lecce L, Moio L. Trans-resveratrol, quercetin, (+) -catechin, and (−) -epicatechin content in South Italian monovarietal wines: relationship with maceration time and marc pressing during winemaking. J Agric Food Chem. 2004;52:5747–5751. doi: 10.1021/jf0354895. [DOI] [PubMed] [Google Scholar]
- Garridio J, Borges F. Wine and grape polyphenols: a chemical perspective. Food Res Int. 2011;44:3134–3148. doi: 10.1016/j.foodres.2011.08.010. [DOI] [Google Scholar]
- Goldberg DM, Yan J, Ng E, Diamandis EP, Karumanchiri A, Soleas GJ, Waterhouse AL. A global survey of trans-resveratrol concentrations in commercial wines. Am J Enol Vitic. 1995;46:159–165. [Google Scholar]
- http://iasri.res.in/design/Analysis%20of%20data/principal_component.html. Accessed on 15 July 2016
- Jeandet P, Sbaghi M, Bessis R, Meunier P. The potential relationship of stilbene (resveratrol) synthesis to anthocyanin content in grape berry skins. Vitis. 1995;34:91–94. [Google Scholar]
- Jogaiah S, Oulkar DP, Vijapure AN, Maske SR, Sharma AK, Somkuwar RG. Influence of canopy management practices on fruit composition of wine grape cultivars grown in the semi-arid tropical region of India. Afr J Agric Res. 2013;8(26):3462–3472. doi: 10.5897/AJAR12.7307. [DOI] [Google Scholar]
- Karibasappa GS, Adsule PG. Evaluation of wine grape genotypes by National Research Centre for Grapes at their farm at Pune, Maharashtra, India. Acta Hort. 2008;785:497–504. doi: 10.17660/ActaHortic.2008.785.65. [DOI] [Google Scholar]
- Lee JH, Johnson JV, Talcott ST. Identification of ellagic conjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. J Agric Food Chem. 2005;53:6003–6010. doi: 10.1021/jf050468r. [DOI] [PubMed] [Google Scholar]
- Lu Y, Foo LY. The polyphenol constituents of grape pomace. Food Chem. 1999;65:1–8. doi: 10.1016/S0308-8146(98)00245-3. [DOI] [Google Scholar]
- Lubomie L. Varietal differentiation of white wines on the basis of phenolic compounds profile. Czech J Food Sci. 2013;31(2):172–179. doi: 10.17221/270/2012-CJFS. [DOI] [Google Scholar]
- McDonald MS, Hughes M, Burns J, Lean MEJ, Matthews D, Crozier A. Survey of the free and conjugated myricetin and quercetin content of red wines of different geographical origins. J Agric Food Chem. 1998;46:368–375. doi: 10.1021/jf970677e. [DOI] [PubMed] [Google Scholar]
- Özcan MM, Al Juhaimi F, Gülcü M, Uslu N, Geçgel Ü, Ghafoor K, Dursun N. Effect of harvest time on physico-chemical properties and bioactive compounds of pulp and seeds of grape varieties. J Food Sci Technol. 2017;54(8):2230–2240. doi: 10.1007/s13197-017-2658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil SH, Banerjee K, Oulkar D, Jogaiah S, Sharma AK, Dasgupta S, Adsule PG, Deshmukh MB. Phenolic composition and antioxidant activity of Indian wines. Bull de l’OIV. 2012;84:517–545. [Google Scholar]
- Pour NM, Kohler HJ, Schmitt A, Patz CD, Dietrich H. Polyphenolic composition of German white wines and its use for the identification of cultivar. Mitteilungen Klosterneuburg. 2007;57:146–152. [Google Scholar]
- Price SF, Breen PJ, Valladao MBT. Watson Cluster sun exposure and quercetin in Pinot-Noir grapes and wine. Am J Enol Vitic. 1995;46:187–194. [Google Scholar]
- Raul F, Guerreroa AL, Miguel P, Belen P, Rocio GB, Ángel GI, Carmelo GB, Emma CV. Phenolic characterisation of red grapes autochthonous to Andalusia. Food Chem. 2009;112:949–955. doi: 10.1016/j.foodchem.2008.07.014. [DOI] [Google Scholar]
- Ritter G, Gotz H, Dietrich H. Untersuchung der phenolischen substanzen in Rheingauer Riesling weinen. Vitic Enol Sci. 1994;49:71–77. [Google Scholar]
- Schmandke H. Resveratrol and piceid in grapes and soybeans and products made from them. Ernährungs-Umsch. 2002;49:349–352. [Google Scholar]
- Shahidi F, Naczk M. Food phenolics: sources, chemistry, effects, applications. Pennsylvania: Technomic Publishing Co; 1995. Wine; pp. 136–148. [Google Scholar]
- Sharma AK, Navale SV, Aute SV, Karibasappa GS, Oulkar DP, Adsule PG. Changes in phytochemicals during fermentation of wine grapes. Int J Food Ferment Technol. 2012;2(1):19–25. [Google Scholar]
- Silva LR, Queiroz M. Bioactive compounds of red grapes from Dão region (Portugal): evaluation of phenolic and organic profile. Asian Pac J Trop Biomed. 2016;6(4):315–321. doi: 10.1016/j.apjtb.2015.12.015. [DOI] [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53(11):4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Chu YF, Wu X, Liu RH. Antioxidant and antiproliferative activities of common fruits. J Agric Food Chem. 2002;50:7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- Taware PB, Dhumal KN, Oulkar DP, Patil SH, Banerjee K. Phenolic alterations in grape leaves, berries and wines due to foliar and cluster powdery mildew infections. Int J Pharma Biol Sci. 2010;1:1–14. [Google Scholar]
- Varache-Lembege M, Waffo P, Decendit A, Devaux G, Deffieux G, Merillon JM, (1996) Polyhydroxy stilbenes from Vitis vinifera L. cells: inhibitory effect on human platelet aggregation and molecular modeling. Presented at the 18th International Conference on Polyphenols; Polyphenols Communications 96, Bordeaux (France), July 15–18
- Vincenzi S, Tomasi D, Gaiotti F, Lovat L, Giacosa S, Torchio FS, Río S, Rolle L. Comparative study of the resveratrol content of twenty-one italian red grape varieties. S Afr J Enol Vitic. 2013;34(1):30–35. [Google Scholar]
- Vinci G, Sara L, Maria E, Isabella N, Donatella R. Influence of environmental and technological parameters on phenolic composition in red wine. J Commod Sci Technol. 2008;47(1–4):245–266. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.