Abstract
An effort was made to produce gelatin from Common carp wastes using extracted alkaline protease from Bacillus licheniformis PTCC 1595. Fermentation was performed by submerged media for 48 h and 72 h. The hydrolyzing enzyme was added in 5, 10, 15, 20, and 25 units per gram of wastes powder for hydrolysis. The produced gelatin was compared with commercial bovine gelatin with regard to some rheological and physicochemical properties. The yield of gelatin production was also determined as a result of hydroxyproline extraction from fish wastes. SDS-PAGE was performed for enzyme and gelatins. For enzyme, two bands were achieved with 39 and 10.5 kDa molecular weight which were separated passing through a 15 kDa UF filter. Both gelatins showed β, α1, and α2 chains as basic components, but the fish waste gelatin showed narrow bands. In conclusion, foam expansion and water holding capacity were approximately equal in both gelatin types used for food industry application. The results indicated that using 20 units of enzyme per gram of waste powder was the optimum amount of enzyme application. Further, fish wastes were concluded to be a practical source for gelatin production.
Keywords: Fish gelatin, Microbial protease, Physico-chemical characteristics, Scales and fins
Introduction
Proteases play an important role in industrial biotechnology, particularly in the food and pharmaceutical sectors. Microbial enzymes are the mostly studied and applied in the industry. One of the mostly studied is the alkaline protease produced by Bacillus sp. (Jaswal et al. 2008). Recently, a strong focus has been directed towards microbial proteases that provide benefits to production from an industrial perspective, besides being eco-friendly (Sawant and Nagendran 2014).
Gelatin is a colloidal protein with unique characteristics and numerous applications in the food industries, pharmaceutical, and photography. Gelatin is derived commercially by controlled hydrolysis of collagen in acidic or basic environments (Johnston-Banks 1990; Te Nijenhuis 1997). However, these chemical methods have deleterious effects on the environment and quality of collagen. The purpose of this investigation was to produce gelatin from marine industries wastes by a microbial protease (from Bacillus licheniformis) and compare it with commercial gelatin.
Materials and methods
Chemicals and reagents
Fish wastes were obtained from Fishery Organization, Shiraz, Iran. All the chemicals and reagents were of analytical reagent grade and purchased from Merck Co (Darmstadt, Germany). The microorganism (Bacillus licheniformis, PTCC 1595) was purchased from PTCC.1
Enzyme extraction and partial purification
To prepare 1 L of the submerged culture medium containing 2% soybean meal and 0.04% CaCl2, 0.02% MgCl2 was adjusted to volume 900 mL with 0.1 M Na2HPO4–NaH2PO4 buffer. The pH was adjusted to 7.6 and then sterilized. In order to prevent of Millard reaction, glucose solution (6% w/v) was sterilized separately. The bacterial suspension was inoculated into the fermentation medium then incubated in 37 °C for 48 h and 72 h separately, at 172 rpm. Afterwards, the medium were centrifuged at 5000 rpm, 4 °C, for 20 min (International Universal, UV model, US). Then 7.0 mL of cold acetone (− 20 °C) was added for 3.0 mL of sample to precipitate proteins and stored at − 20 °C for 2 h followed by centrifugation at 4000 rpm, 4 °C, for 20 min. The precipitate was kept at − 20 °C for subsequent studies (Yavari et al. 2013).
Proteolytic activity of protease
Protease activity was determined as described by Klomaklao et al. (2004) with some modifications. 100 µL of enzyme (10 mg/mL) was added into casein solution (3% w/v, pH 8.6) and the mixture was incubated at 37 °C for 30 min. The reaction was stopped by 1.0 mL TCA (20%) fallowed by centrifugation at 7600 rpm for 10 min. The absorption was measured by spectrophotometer (Perkin-Elmer UV–VIS-NIR, US) at 280 nm. One unit of activity was defined as releasing 1 mol of tyrosine per min. Protein concentration was determined by Lowry method (Lowry 1951).
Gelatin production
The surface of raw materials were purged by 10% NaCl (twice and each time for 12 h). After rinsing with distilled water, the fins and scales were treated with 0.4 M HCl for 90 min while stirring, for deaminization. Then the mixture was neutralized with water and dried at 30 °C in a vaccume dessicator followed by milling with a laboratory pounder (Panasonic, MJ-W176P, Japan).
Powdered fins and scales were mixed with distilled water (1:10) and the pH was adjusted to 8.6 to add the enzyme at 5, 10, 15, 20, and 25 units per gram of fins and scale powder. Hydrolysis was performed for 48 h in ambient temperature with slow stirring. Subsequently the suspension was rinsed with distilled water three times, followed by heating in 60 °C distilled water to extract gelatin. The extracted solution was passed through a Watman filter paper No.2, and freeze dried (IFD-5012, Dena Vacuum, Iran) (Zhang et al. 2011).
Colour
The colour of gelatin samples was determined according to Afshari-Jouybari and Farahnaky (2011) with slight modifications. The image of each gelatin sample was captured in a wooden box (30 × 30 × 40 cm) with Natural light Source (K = 6500) and a digital camera (Canon Powershot A630 with 8 Mega Pixels) which was placed vertically at a distance of 25 cm from the samples. The values of the corresponding regions were determined by filter/blur/average command in Photoshop software.
pH and proximate analysis
The pH of gelatins was measured by a laboratory glass electrode pH meter (PHT 110, Labtorn, US). The protein, moisture, and ash contents of the gelatins were determined according to the AOAC methods number 984.13 (conversion factor of 5.4), 927.05, and 942.05, respectively (AOAC 2000). All experiments were performed in triplicate.
Foam expansion and foam stability
Foam expansion (FE) and foam stability (FS) of gelatins were examined as described by Shahidi et al. (1995) with slight modifications. Gelatin solutions (2% w/v) were prepared and homogenized at room temperature for 1 min. Samples were transferred into a 50 ml cylinder and the volume of samples were measured at 0, 30, and 60 min after being homogenized. FE was calculated from following equation:
| 1 |
where VT and V0 were the total volume after homogenizing and volume before homogenizing, respectively. To determine the foam stability, which equals to foam expansion after homogenization:
| 2 |
where Vt was the total volume after different times (30 and 60 min). All measurements were performed in triplicate.
Hydroxyproline content
Hydroxyproline content was measured according to the method outlined in ISO (1978). 100 g gelatin was hydrolyzed with 5 mL 6.0 N HCl for 14 h at 105 °C, then neutralized using 6.0 N NaOH. Acetate/citrate buffer was added and total volume was adjusted to 25 mL with 0.3 M NaCl. An aliquot was transferred into a beaker, and 300 µL isopropanol and 600 µL oxidant solution (mixture of acetate/citrate buffer, pH 6.0 and 7% (w/v) chloramine T at a ratio of 4:1 (v/v)) were added and mixed thoroughly. After 5 min, 4 mL Ehrlich’s reagent was added and stirred for 25 min at 60 °C. The absorbance was measured at 660 nm. The hydroxyproline content (mg/g) was calculated using an analytical grade hydroxyproline standard curve.
Extraction yield was calculated in terms of hydroxyproline amount (Eq. 3)
| 3 |
Gel strength
This experiment was carried out according to Gòmez-Guillèn and Montero (2001) with slight modifications. An amount of 7.5 g gelatin was mixed thoroughly with 105 mL distilled water and heated at 42 °C to dissolve the gelatin. Then it was transferred into a 10 °C water bath for 18 h. Eventually, the gel strength was determined as bloom (g), by a texture analyzer (TA.XT2, Mason Technology, Ireland) with 5 KN load cell and a 1.27 cm diamitered probe.
Fat binding and water holding capacity
Fat-binding capacity (FBC) was determined according to Zayas (1997b) with some modifications. Gelatin (1g) was placed in a centrifuge tube and weighed. Distilled water (100 ml) was added and was held at 25 °C for 60 min. Samples were mixed every 10 min followed by centrifugation at 2100 rpm for 25 min. After removal of the supernatant, the tubes were drained for 25 min on a filter paper at an angle of 45°. Water-holding capacity (WHC) was measured dividing the weight of the tubes and their content, after draining by before adding water.
SDS-PAGE
Samples of gelatin were mixed with the loading buffer (2% SDS, 5% mercaptoethanol, and 0.002% bromophenol blue) by a ratio of 1:4. The samples were heated at 100 °C to denature the proteins. The samples were run as described by Laemmli (1970) in a mini SDS-PAGE system (Bio-Rad Laboratories, Hercules, CA, USA). The stacking and seperating gel were 4% and 7.5%, respectively. After electrophoresis, the gel was stained in a 0.1% coomassie brilliant blue R-250 dessolved in water, methanol, and trichloroacetic acid (5:4:1) and was destained with methanol, water, and acetic acid at the same ratio. For the extracted enzyme, the seperating gel was 10%.
Statistical analysis
The independent t test was performed in order to compare the commercial gelatin and fish waste gelatin. In cases that the data were not normal, the Mann–Whitney test was used. To compare more than two groups with each other, the one-way Anova was used for normal data and the Wallis test for non-parametric data. All tests were performed by the SPSS statistics 21 (P < 0.05).
Results and discussion
Enzyme activity and protein concentration
The enzyme activity at 48 and 72 h of fermentation was 270.70 and 283.70 U/ml, respectively. These values were 258.71 and 273.00 and after 3 weeks following the extraction. The highest enzyme activity reported by Guarv Pant et al. (2015) was 243.28 U/ml after 36 h of fermentation. The difference observed might be due to difference in optimum pH, carbon source or temperature for the assay of the enzyme.
Colour
L* value of fish waste gelatin was significantly higher than the commercial gelatin while b* was higher in commercial samples. The a* value of gelatins was not significantly different (p > 0.05). As shown in Table 1, the commercial gelatin was slightly yellowish as compared with fish waste gelatin.
Table 1.
Composition of Common carp fins and scales, commercial gelatin and fins and scales gelatin
| Composition (%) | Fins and scales | Fins and scales gelatin | Commercial gelatin |
|---|---|---|---|
| pH | 6.99 ± 1.02a | 6.17 ± 0.10a | 5.99 ± 0.13b |
| Ash (%) | 46.16 ± 1.41a | 2.29 ± 0.70b | 0.37 ± 0.10c |
| Moisture (%) | 15.74 ± 0.16a | 7.08 ± 0.20b | 7.04 ± 0.30b |
| Protein (%) | 33.99 ± 0.27b | 90.27 ± 1.70a | 89.97 ± 2.04a |
| L* | – | 54.66 ± 0.57a | 48.33 ± 0.57b |
| a* | – | − 19.33 ± 0.57a | − 15.00 ± 0.00a |
| b* | – | 13.00 ± 0.00b | 27.66 ± 0.57a |
Values are given in mean ± SD from triplicate determinations and similar letters shows lack of significant difference
pH and proximate analysis
As shown in Table 1, the pH of fish waste gelatin is significantly higher than the commercial one, which might be due to the difference between their raw material. Cheow et al. (2007) concluded that the pH of extracted gelatin from Sincroaker skin and Shortfin scad skin was 6.6 and 6.59, respectively.
The protein and moisture contents of gelatins did not show significant differnces. The high values of protein content indicates the high yield of gelatin extraction (Muyonga et al. 2004). As shown in Table 1, the ash content of fish waste gelatin (2.29%) was significantly higher. It is to be noted that the maximum advised amount of ash content in gelatin is 2.6% (Alfaro et al. 2013).
Foam expansion and foam stability
The FE of fish wastes gelatin (124.23 ± 3.90%) was not significantly different from the commercial gelatin (129.76 ± 3.30%), but the FS of commercial gelatin in both 30 and 60 min (123.46 ± 2.60% and 117.30 ± 3.50% respectively) was significantly higher than the fish wastes gelatin (118.26 ± 2.90% and 113.00 ± 2.90% respectively).
FE is affected by the source of protein, intrinsic characteristics of protein, chemical composition of protein, the conformation of protein molecules in water/air interfacial, the molecular weight of proteins, and protein–protein interactions in the matrix (Wilde and Clurk 1996; Zayas 1997a; Van der vano et al. 2002; Mutilangy et al. 1996).
Hydroxyproline content
The hydroxyproline content of fish wastes gelatin was determined as a function of enzyme concentration (Table 2). The results showed that the enzyme concentration has a direct relation with the hydroxyproline content and subsequently the extraction yield. This was continued until 20 U/g powdered fins and scales, and thereafter no significant increase was observed. It is suggested that the protease hydrolyzes some covalent bonds and strong crosslinks in collagen molecules and increase the extraction yield (Galea et al. 2000; Jongjareonark et al. 2006).
Table 2.
Total hydroxyproline and yields of gelatin extracted from the Common carp fins and scales at different levels of enzyme concentrations
| Enzyme concentration (U/g powdered fins and scales) | Exteracted hydroxyproline (mg/g) | Gelatin exteraction yield (%) |
|---|---|---|
| 5 | 2.25 ± 0.05d | 13.51 ± 0.64d |
| 10 | 4.61 ± 0.09c | 27.71 ± 0.75c |
| 15 | 5.89 ± 0.09b | 34.80 ± 0.73b |
| 20 | 7.71 ± 0.16a | 46.30 ± 0.91a |
| 25 | 8.70 ± 0.04a | 52.28 ± 0.87a |
Values are given in mean ± SD from triplicate determinations and similar letters shows lack of significant difference
Gel strength
The commercial gelatin had a markedly higher gel strength (269.00 ± 2.09 g). The commercial gelatins have a range of gel strength between 100 and 300 g, but it is idealistic to have a gelatin within the range of 250–260 g bloom value (Holzer 1996). Fortunately, according to the previous statement, the fish wastes gelatin had an acceptable gel strength (250.80 ± 0.95 g).
Gel strength is a function of amino acid composition and the proportions of alfa and beta chains and high molecular weights leads to higher gel strength (Cho et al. 2004).
Fat binding and water holding capacity
Fish wastes gelatin had a significantly higher FBC (302.08 ± 1.80%) than the commercial gelatin (251.20 ± 1.82%), and their WHC was 205.44 ± 2.30% and 218.34 ± 14.60%, respectively, which did not show significant difference. The higher FBC was likely due to the higher amounts of hydrophobic amino acids like leucine, and isoleucine in fish wastes gelatin. The WHC was approximately similar in both gelatin types, probably because of the equal amounts of hydrophilic amino acids. Balti et al. (2011) noted the same results for cattlefish skin gelatin where in the FBC and WHC were 300 and 190, respectively.
SDS-PAGE
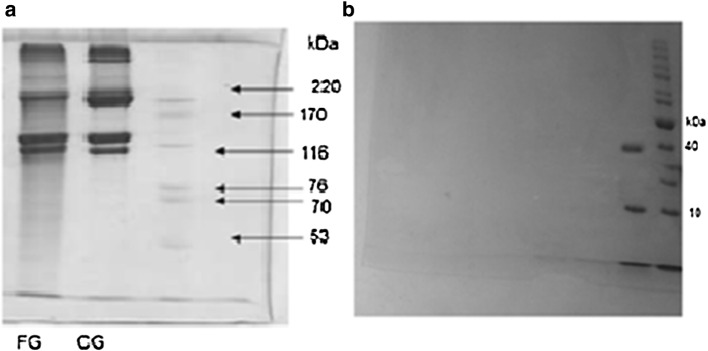
Electrophoresis experiment showed two bands at 39 and 10.5 kDa for enzyme (Fig. 1). After passing through UF filter, sample was reloaded into the electrophoresis gel. The 10.5 kDa band was the protease and the impure band (39 kDa) was probably residues from the culture medium of fermentation. This was in accordance with Yavari et al. (2013), who purified the protease by ion exchange chromatography and obtained a band beneath the 14.4 kDa range.
Fig. 1.
Electrophoretic pattern 7.5% seperating gel for gelatins CG caw gelatin, FG fish wastes gelatin (a) and 10% seperating gel for protease (b)
Both gelatins showed β, α1, and α2 chains as basic components, which makes them both categorized as type I (Balti et al. 2011), but the fish waste gelatin showed narrow bands. Also, the beta chain intensity in commercial gelatin was higher than fish wastes gelatin, and the ratio of α1/α2 in commercial and fish wastes gelatins was close to 2 and 1, respectively.
Conclusion
An alkaline protease was extracted from a GRAS bacteria (Bacillus licheniformis PTCC1595) and was used to produce gelatin from common carp fish scales and fins. Functional and physicochemical properties of commercial gelatin and fish waste gelatin and their electrophoresis patterns, showed that the fish waste gelatin can serve as a good replacement for commercial gelatin. This study revealed that fish waste gelatin can be considered as an appropriate option to be used as a gelling agent and ingredient in some food products.
Acknowledgements
Shiraz University Research Council Grant No: 96-GR-VT-11 supported this research. Special thanks for Seafood Processing Research Group and Ms. Tavana, Biochemistry Lab School of Veterinary Medicine, Shiraz University.
Author contributions
A. Mirzapour Kouhdasht, M. Moosavi-Nasab, and M. Aminlari designed the study, interpreted the results and collected test data, and drafted the manuscript.
Footnotes
.Persian Type Culture Collection.
References
- Afshari-Jouybari H, Farahnaky A. Evaluation of Photoshop software potential for food colorimetry. J Food Eng. 2011;106:170–175. doi: 10.1016/j.jfoodeng.2011.02.034. [DOI] [Google Scholar]
- Alfaro ADT, Fonseca GG, Balbinot E, Machado A, Prentice C. Physical and chemical properties of wami tilapia skin gelatin. Food Sci Technol. 2013;33:592–595. doi: 10.1590/S0101-20612013005000069. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 17. Washington: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Balti R, Jridi M, Sila A, Souissi N, Nedjar-Arroume N, Guillochon D, Nasr M. Extraction and functional properties of gelatin from the skin of cuttlefish (Sepia officinalis) using smooth hound crude acid protease-aided process. Food Hydrocoll. 2011;25:943–950. doi: 10.1016/j.foodhyd.2010.09.005. [DOI] [Google Scholar]
- Cheow CS, Norizah MS, Kyaw ZY, Howell NK. Preparation and characterisation of gelatins from the skins of sin croaker (Johnius dussumieri) and shortfin scad (Decapterus macrosoma) Food Chem. 2007;10:386–391. doi: 10.1016/j.foodchem.2006.01.046. [DOI] [Google Scholar]
- Cho SM, Kwak KS, Park DC, Gu YS, Ji CI, Jang DH. Processing optimization and functional properties of gelatin from shark (Isurus oxyrinchus) cartilage. Food Hydrocoll. 2004;18:573–579. doi: 10.1016/j.foodhyd.2003.10.001. [DOI] [Google Scholar]
- Galea CA, Dalrymple BP, Kuypers R, Blakeley R. Modification of the substrate specificity of Porcine Pepsin for the enzymatic production of bovine hide gelatin. Protein Sci. 2000;9:1947–1959. doi: 10.1110/ps.9.10.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gòmez-Guillèn MC, Montero P. Extraction gelatin from megrim (Lepidorhombus boscii) skins with several organic acids. J Food Sci. 2001;66:213–216. doi: 10.1111/j.1365-2621.2001.tb11319.x. [DOI] [Google Scholar]
- Holzer D (1996) Patent and Trademark Office. No. 5,484,888, Washington DC
- ISO (1978) Meat and meat products-determination of L-hydroxyproline content (reference method). International Organization for Standardization 3496-1978
- Jaswal RK, Kocher GS, Virk MS. Production of alkaline protease by Bacillus circulans using agricultural residues: a statistical approach. Indian J Biotechnol. 2008;7:356–360. [Google Scholar]
- Johnston-Banks FA. Gelatin, handbook of food gels. London: Elsevier; 1990. [Google Scholar]
- Jongjareonark A, Benjakul S, Visessanguan W, Prodpran T, Tanka M. Characterization of edible films from skin gelatin of brown stripe, red snapper and bigeye snapper. Food Hydrocoll. 2006;20:492–501. doi: 10.1016/j.foodhyd.2005.04.007. [DOI] [Google Scholar]
- Klomaklao S, Benjakul S, Visessanguan W. Comparative studies on proteolytic activity of splenic extract from three tuna species commonly used in Thailand. J Food Biochem. 2004;28:355–372. doi: 10.1111/j.1745-4514.2004.05203.x. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH. The Lowry protein assay. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mutilangy WAM, Panyam D, Kilara A. Functional properties of hydrolysates from proteolysis of heat—denatured whey protein isolate. J Food Sci. 1996;61:270–275. doi: 10.1111/j.1365-2621.1996.tb14174.x. [DOI] [Google Scholar]
- Muyonga JH, Cole CGB, Doudo KG. Extraction and physic-chemical characterization of Nile perch (lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004;18:581–592. doi: 10.1016/j.foodhyd.2003.08.009. [DOI] [Google Scholar]
- Pant G, Prakash A, Pavani JVP, Bera S, Deviram GVNS, Kumar A, Prasuna RG. Production, optimization and partial purification of protease from Bacillus subtilis. J Taibah Univ Sci. 2015;9:50–55. doi: 10.1016/j.jtusci.2014.04.010. [DOI] [Google Scholar]
- Sawant R, Nagendran S. Protease: an enzyme with multiple industrial applications. World J Pharm Sci. 2014;30:568–579. [Google Scholar]
- Shahidi F, Han X-Q, Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus) Food Chem. 1995;53:285–293. doi: 10.1016/0308-8146(95)93934-J. [DOI] [Google Scholar]
- Te Nijenhuis K. Thermoreversible networks: viscoelastic properties and structure of gels. Advances polymer science. Berlin: Springer; 1997. pp. 160–193. [Google Scholar]
- Van der vano C, Gruppen H, de Bont DBA, Vorgaen AGH. Correlations between biochemical characteristics and foam forming and stabilizing ability of whey and casein hydrolysates. J Agric Food Chem. 2002;50:2938–2946. doi: 10.1021/jf011190f. [DOI] [PubMed] [Google Scholar]
- Wilde PJ, Clurk DC. Foam formation and stability. Methods of testing protein functionality. London: Springer; 1996. pp. 110–152. [Google Scholar]
- Yavari M, Pazuki GR, Vossoughi M, Mirkhani SA, Seifkordi AA. Partitioning of alkaline protease from Bacillus licheniformis (ATCC 21424) using PEG–K2 HPO 4 aqueous two-phase system. Fluid Ph Equilib. 2013;337:1–5. doi: 10.1016/j.fluid.2012.09.012. [DOI] [Google Scholar]
- Zayas JF. Water holding capacity of proteins. Functionality of proteins in food. Berlin: Springer; 1997. pp. 76–133. [Google Scholar]
- Zayas JF. Oil and fat binding properties of proteins. Functionality of proteins in food. Berlin: Springer; 1997. pp. 228–259. [Google Scholar]
- Zhang F, Xu S, Wang Z. Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food Bioprod Process. 2011;89:185–193. doi: 10.1016/j.fbp.2010.05.003. [DOI] [Google Scholar]