Abstract
The effects of different drying temperatures between 40 and 80 °C on bioactive constituents and antioxidant activity of edible sub Antarctic brown seaweed, Durvillaea antarctica were studied. Dietary fibre, amino acids profile, pigments (chlorophyll and carotenoids), vitamin E, total phenolics and total flavonoids as well as antioxidant activity were determined, beside a measurement of the chromatic coordinates. The brown seaweed D. antarctica had a high content of dietary fibre and was rich in essential amino acids and drying between 40 and 80 °C did not influence significantly dietary fibre content nor the level of essential amino acids that remained around 44%. However, a significant degradation of the chlorophyll pigments was observed with the lowest level of the initial chlorophyll content occurring at 60 °C (59%). Total carotenoids content was stable during drying between 50 and 70 °C. Vitamin E showed no significant loss during drying at any of the assayed temperatures, which could be due to its occurrence within the lipid fraction. Drying at 40 °C imparted a darker brown colour to the seaweed, while a lighter brown colour was acquired as drying temperature increased. The greatest loss in total phenolics content occurred at 60 °C, while total flavonoids content showed a significant reduction, which declined as drying temperature increased. According to the experimental results, phenolics and flavonoids could be considered as an important source of bioactive compounds with relatively high antioxidant activity. Thus D. antarctica may satisfy the requirements for development of functional foods.
Keywords: Brown seaweed Durvillaea, Bioactive components, Amino acids, Antioxidant activity, Functional food
Introduction
Durvillaea antarctica is an edible brown seaweed with a sub Antarctic distribution and endemic to the southern hemisphere. In the Chilean coast, it can be found from Coquimbo to Cape Horn (Mansilla et al. 2012). It can measure up to 15 m and has a dark greenish-brown or yellowish-brown colour, consisting of two parts: the fronds of the plant called cochayuyo or chamisso, with a typical width between 3 and 12 cm, and the stem (known as hulte) that is usually consumed as a fresh product (Uribe et al. 2017). As reported by Chile Central Bank in 2015, an average of 2000 tons of cochayuyo has been exported annually between 2010 and mid-2015, with a peak in FOB value of 5,261,665 US$ in 2014. Nutritional and chemical compositions of some edible Chilean seaweeds have great fluctuations, which depend on species, habitat, degree of maturity and environmental conditions (Astorga-España and Mansilla 2014; Ortiz et al. 2006). Edible seaweeds contain a wide variety of components with biological activities, such as antioxidant and anticoagulant properties, associated to phlorotannins and polysaccharides (Liu et al. 2012), antiviral, anti-tumour and hypocholesterolemic and hypolipidemic properties (Løvstad-Holdt and Kraan 2011), as well as antibacterial activity (Gupta et al. 2010). However, prior to industrial processing fresh seaweeds are usually dried, which significantly effects their nutritional, functional and biological properties (Chan et al. 1997; Wong and Cheung 2001). Seaweeds also have high water content that are responsible for rapid deterioration, so that drying is a prerequisite for a potential industrial use, allowing an easy handling during transport.
Traditionally brown seaweeds are sun-dried and are therefore exposed to long drying periods in the open air, which may lead to filth contamination from birds, insects and rodents (Fudholi et al. 2014). Hot-air drying as a commonly used postharvest drying method for agricultural products offers a good alternative to avoid such environmental contaminations during sun-drying. Although drying equipment may be a low-cost investment, process parameters such as drying air temperature and drying time would affect quality of the dried product, so that experimental assessment on such changes is important from a nutritional point of view. Enzymatic and/or non-enzymatic processes may also occur during drying of the fresh plant tissues and would lead to significant changes in the composition of phytochemicals. The main variables that intervene on product’s quality are processing time and air-drying temperature (Chan et al. 1997; Gupta et al. 2011). Little is known about the drying process of edible Chilean seaweeds or about the effect of drying temperature on the dried product´s quality (Tello-Ireland et al. 2011). Studies on the effect of drying on the nutritional properties of brown seaweeds or on the phytochemical constituents such as phenols and flavonoids are not available in literature. Therefore, the aim of the present study was to determine the influence of drying temperatures between 40 and 80 °C on the phytochemical components of the brown seaweed D. antarctica. Total phenolics, total flavonoids, vitamin E, chlorophyll, colour difference, amino acids profile, dietary fibre as well as antioxidant activity as DPPH and ORAC values were determined.
Materials and methods
Raw material and drying process
The brown seaweeds D. antarctica used in this study were harvested from the coast of Coquimbo (IV Region, Chile), in the locality of Puerto Oscuro, Los Vilos, Chile. Seaweeds were selected by visual inspection according to colour and absence of foreign matter. Frond stems (cochayuyo) were separated and cut into cylinders of 2 cm for the drying experiment performed in a convective dryer as described by Uribe et al. (2017), at 40, 50, 60, 70, and 80 ± 0.2 °C under constant air flow of 1.5 ± 0.2 m/s and load density of 1.9 ± 0.1 kg/m2. The fresh seaweed used as control was freeze-dried (Virtis Advantage Plus, Gardiner, NY) at − 80 °C upon arrival at the laboratory. The dried samples were stored at 5 °C, protected from sunlight and vacuum-sealed in low-density polyethylene bags until analysis.
Dietary fibre content
Total dietary fibre (TDF) in D. antarctica was determined using an enzymatic gravimetric method AOAC 993.19 and 991.42 (AOAC 1996) as the sum of insoluble (IDF) and soluble dietary fibre (SDF) using digestion enzymes (Sigma-Aldrich). The seaweeds samples were suspended in phosphate buffer (0.08 M, pH 6.0) and a sequential digestion with thermostable enzymes was performed, starting with α-amylase at 95–100 °C, followed by protease at 60 °C, and finally amyloglucosidase also at 60 °C. Ethanol (95%) preheated at 60 °C was added in a ratio ethanol to suspension of 4–1 to precipitate the soluble fibre that was removed after 1 h using a glass filter. The residue was washed with ethanol and acetone. After drying, the residue was weighed. Half of the samples was analysed for protein (Kjeldahl method, N × 6.25) and the other half was incinerated in a muffle oven at 525 °C for 5 h. Total dietary fibre is the weight of the residue less the weight of the protein and ash. The results were expressed in g/100 g dry matter (dm). All measurements were performed in triplicate.
Vitamin E content
Vitamin E was determined as α-tocopherol in the lipid extracts of the brown seaweeds. Between 20 and 30 g dried seaweeds was first mixed with hexane in a ratio of solid to solvent of 1–3, using an orbital shaker (OS-100, HiLab, Indonesia) at 280 rpm for 60 min, before allowing to macerate for 24 h. The mixture was then centrifuged for 5 min at 5000 rpm and the supernatant was filtered (Whatman filter paper N° 1) and concentrated using a rotary vacuum evaporator (Büchi RE 121, Switzerland). 20 μL were used in the HPLC analysis with a fluorescence detector (Merck-Hitachi F-1050) following a standard AOCS method (AOCS-Ce8-89/1993). Chromatographic column LiChroCART® 250-4 LiChrosorb® Si 60 (5 µm) (Merck, Darmstadt, Germany) was used. The mobile phase consisted of propan-2-ol in hexane (0,5:99,5 v/v) at a flow rate of 1 mL/min. Peaks were detected at excitation and emission wavelengths of 290 and 330 nm respectively. Contents of vitamin E was expressed in mg/100 g dm.
Chlorophyll and total carotenoids
The content of chlorophylls (CHL) was calculated using the spectrophotometric equations of Jeffrey and Humphrey (1975). Briefly, a mass of 0.2 g ground dried brown algae was extracted for 12 h at 4 °C with 25 mL 90% acetone. The mixture was then centrifuged at 5000 rpm for 5 min and the absorbance of the supernatant was measured using 1-cm cell path-length at 630 and 664 nm with a spectrophotometer (Spectronic Instruments, Spectronic 20 Genesys, Rochester, NY, USA). Equations 1 and 2 were used to calculate contents of chlorophyll a and respectively, with symbol Ex denoting the extinction at wavelength x. Results were expressed in μg/g dm.
| 1 |
| 2 |
Total carotenoids were determined following a methodology described by Chan and Matanjun (2017) with some modifications. Ground dried seaweed samples (3.0 g) were extracted for 1 h at room temperature with 75 mL of solvent hexane/acetone/ethanol (2:1:1, v/v). The mixture was filtered, and filtrate made up to 100 mL with extraction solvent. Then, 25 mL distilled water were added and after agitation mixture was left for 30 min to separate in an organic and aqueous layer, protected from light. The absorbance A of the organic layer was measured at 470 nm, and total carotenoids content (TCC) was calculated according to Eq. 3, with v total volume of extract (mL), w sample weight (g) and A1% = 2600 (β-carotene extinction coefficient in hexane). Results were expressed in μg/g dm.
| 3 |
Amino acids profile
Amino acids were determined by HPLC equipped with a UV detector and post-columnn in hydrin derivatization. The samples were first hydrolysed in 6 M HCl for 22 h under nitrogen, buffered to pH 2.2, before injection in the HPLC system. Norleucine was used as quantitative internal standard (Wright et al. 2002). Altogether the following 17 amino acids were analyzed: Alanine (Ala), Arginine (Arg), Aspartic acid (Asp), Cystine (Cys), Glutamic acid (Glc), Glycine (Gly), Histidine (His), Isoleucine (Ile), Leucine (Leu), Lysine (Lys), Methionine (Met), Proline (Pro), Phenylalanine (Phe), Serine (Ser), Threonine (Thr), Tyrosine (Tyr), and Valine (Val).
Colour parameters
Surface colour of D. antarctica samples was measured using a colorimeter (HunterLab, MiniScan™ XE Plus, Reston, VA, USA) and CIE chromatic coordinates, L*, a* and b* at standard illuminant D65 and 10º observer angle. The measurements were performed in five replicates. Total colour difference ΔE = [(L* − L0)2 + (a* − a0)2 + (b* − b0)2]½ was calculated with Lo, ao and bo as control values of fresh seaweeds (Tello-Ireland et al. 2011). Hue h* and chroma ΔC = (Δa2 + Δb2)½ were also determined (McGuire 1992).
Total phenolic (TPC), total flavonoids (TFC) content and antioxidant activity
The extraction of the brown seaweed was performed according to a method described by Vasco et al. (2008) but slightly modified. Dried samples of brown seaweeds (2 g) were extracted at room temperature with 50 mL aqueous acetone (70% v/v) under continuous stirring for 24 h at 200 rpm using an orbital shaker (OS-20, Boeco, Hamburg, Germany). After extraction, mixture was filtered using Whatman filter paper Nº 1 and filtrate dried at 37 °C on a rotary vacuum evaporator (Büchi R-210, Switzerland). The extracts were reconstituted in 25 mL volumetric flasks with distilled water and newly filtered before use. Extractions were performed in triplicate and the extracts were used for analysis of TPC, TFC and antioxidant activity.
TPC was determined using Folin-Ciocalteu (FC) reagent according to Vasco et al. (2008) with some modifications. Briefly, 0.5 mL aliquot of seaweed extracts was transferred to a Falcon centrifuge tube and mixed with 0.5 mL of FC reagent; after 5 min 2 mL Na2CO3 solution (200 mg/mL) were added and mixed by vortexing for 20 s. The reaction proceeded for 15 min at ambient temperature protected from sunlight. 10 mL of ultra-pure water were then added, and precipitate formed was removed by centrifugation (5804 R, Eppendorf, Hamburg, Germany) at 5000 g for 5 min. Finally, the absorbance was measured at 725 nm (SpectronicTM 20 Genesys, NY, USA) and compared to a gallic acid calibration curve. Results were expressed as mg gallic acid equivalent (GAE)/100 g dm. All reagents were purchased from Merck (Merck KGaA, Darmstadt, Germany). All measurements were done in triplicate.
TFC was measured by a colorimetric assay developed by Dini et al. (2010). Briefly, 0.5 mL aliquot of extract of brown seaweeds was added to a volumetric flask containing 2 mL double distilled water (ddH2O). At time zero, 0.15 mL NaNO2 aqueous solution (5 g/100 mL) was added to the flask. After 5 min, 0.15 mL AlCl3 aqueous solution (10 g/100 mL) was added. After another 6 min, 1 mL 1 M NaOH was added and the mixture in the reaction flask was diluted to volume with the addition of 1.2 mL of ddH2O and thoroughly mixed. Absorbance of the mixture was determined at 415 nm versus prepared water blank using a spectrophotometer (SpectronicTM 20 Genesys, NY, USA). Total flavonoid content was expressed as mg catechin equivalent (CE)/100 g dm.
Free radical scavenging activity of the samples was determined using the 1,1-diphenyl-2-picryl hydrazyl (DPPH) method (Brand-Williams et al. 1995) with some modifications. Different dilutions of the extract were prepared in triplicate. A solution of DPPH radical was prepared using 2 mg dissolved in100 mL methanol 80% (v/v). For the analysis 3.9 mL of the DPPH solution was vortex-mixed for 30 s with 0.1 mL of seaweed extracts prepared at different concentrations and the reaction mixture was left to stand in the dark at room temperature for 20 min. The absorbance was measured at 517 nm (Spectronic® 20 GenesysTM, Illinois, USA) and compared to a methanol blank. Calibration was performed within a linear range between 0.1 and 1.0 mM Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) and results were expressed as μmoles of Trolox equivalent (TE)/100 g dm.
The ORAC assay was performed as described by Zulueta et al. (2009). Briefly, the 2,2’-azobis(2-amidinopropane) dihydrochloride (AAPH) was dissolved in 10 mL of 75 mM phosphate buffer (pH 7.4) to a final concentration of 153 mM. A fluorescein stock solution (4 × 10−3 mM) was made in 75 mM phosphate buffer (pH 4) and stored; the stock solution was diluted 1:1000 with buffer phosphate. 150 μL of working sodium fluorescein solution were added. In addition, blank wells standards received 25 μL of 75 mM phosphate buffer (pH 7.4), while standards received 25 μL of Trolox dilution and samples received 25 μL of the seaweeds extracts (diluted 1:100). A fluorescence spectrophotometer was used at 485 nm excitation and 535 nm emission. Reaction was initiated by the addition of 25 μL of AAPH reagent. ORAC value was calculated according to Huang et al. (2002). Final ORAC values were calculated using the regression equation between Trolox concentration and the net AUC and expressed as Trolox Equivalent Antioxidant Capacity (TEAC) in μmoles of Trolox equivalent (TE) per 100 g dm.
Statistical analysis
The effect of air-drying temperature on each quality parameter was estimated using Statgraphics® Plus v. 5.1 (Statistical Graphics Corp., Herndon, VA, USA). An analysis of variance (ANOVA) was performed and differences among media were analysed using the least significant difference (LSD) test at significance level α = 0.05 and confidence interval of 95% (p < 0.05). The multiple range test (MRT) included in the statistical program was used to demonstrate the existence of homogeneous groups within each of the parameters.
Results and discussion
Dietary fibre
In general, total dietary fibre (TDF) includes the structural (cellulose, hemicellulose and pectic substances) and non-structural (gum, mucilage) components of vegetable cell walls (AACC 2001) and can be classified as water soluble (SDF) and water insoluble (IDF). In Table 1 the contents of dietary fibre in cochayuyo can be seen. In the fresh product IDF predominated with 43.3 ± 0.5 g/100 g dm, which corresponded to 76% of TDF, showing a high level of dietary fibre, characteristic for seaweeds (Lahaye 1991). According to Murata and Nakazoe (2001) seaweeds Undaria and Fucus contain respectively 58% and 50% of TDF, while Porphyra and Saccharina have only 30% and 29% TDF respectively. In Fucus and laminaria IDF contents are 40% and 27%, respectively. SDF values fluctuates between 15% and 22% in Undaria pinnatifida (wakame), Chondrus and Porphyra (Fleury and Lahaye 1991). Therefore, the brown seaweed D. antarctica can be classified among seaweeds with high content of dietary fibre known for their anti-tumour and anti-herpetitic bioactivity as well as for their capacity to prevent obesity, large intestine cancer and diabetes and having antiviral activities (Løvstad-Holdt and Kraan 2011; Murata and Nakazoe 2001). Drying temperature between 40 and 80 °C (Table 1) showed in general no significant influence on dietary fibre content, except for an observed significant drop in SDF at 70 °C, which could be occurring within a critical temperature range where the soluble dietary fibres could be more vulnerable to chemical destruction due to prolonged drying at a relatively high temperature. Drying time to achieve equilibrium moisture content at 70 °C was around 220 min that was nearer to the corresponding drying time at 60 °C (240 min) than to that at 80 °C that was around 170 min (Uribe et al. 2017).
Table 1.
Dietary fibre in fresh and dehydrated brown seaweed D. antarctica
| Temperature (°C) | Dietary fibre (g /100 g dm) | ||
|---|---|---|---|
| IDF | SDF | TDF | |
| Control | 43.4 ± 0.5a | 13.7 ± 1.2a | 57.1 ± 0.7a |
| 40 | 45.9 ± 0.2ab | 11.5 ± 0.0a | 57.4 ± 0.2a |
| 50 | 46.3 ± 1.2ab | 11.8 ± 1.2a | 58.1 ± 0.0a |
| 60 | 46.3 ± 2.3b | 11.7 ± 1.2a | 58.0 ± 1.1a |
| 70 | 45.9 ± 0.8ab | 8.50 ± 1.2b | 54.4 ± 2.0b |
| 80 | 45.7 ± 0.8ab | 13.2 ± 0.2a | 58.9 ± 1.0a |
Different letters in the same column indicate significant difference (p < 0.05)
Chlorophyll, carotenoids and α-tocopherol
The pigments (chlorophyll and carotenoids) and vitamin E (α-tocopherol) content is reported in Table 2. Chlorophyll a (CHLa) predominated over (CHLc) and total carotenoids content (TCC). The control sample contained 698.6 ± 5.3 mg/kg dm of CHLa, 108.3 ± 2.6 mg/kg dm of CHLc and 34.1 ± 1.7 mg/kg dm of TCC, which is comparable to content of CHLa (677 mg/kg dry weight) in Petalonia fascia (O. F. Muller) Kuntze, an edible brown alga from Araña beach, Málaga, southern Spain (Flores-Moya and Fernández 1998). However, P. fascia has higher contents of CHLc (308 mg/kg dm) and carotenoids (580 mg/kg dm). Vitamin E, determined as α-tocopherol, was found in an amount of 84.0 ± 0.5 mg/kg dm in brown seaweed D. antarctica and has been reported to occur in the lipid fraction of the fresh seaweed (Ortiz et al. 2006). During drying at all temperatures between 40 and 80 °C, a significant degradation of the chlorophyll pigments was observed with a minimum level of 59% of the initial chlorophyll content occurring at 60 °C, while the least degradation occurred at 50 °C with an average retention of 87%. It was also observed in a previous work (Uribe et al. 2017) that the final dried products at these two temperatures had no-significantly different water contents (9%) with respect to fresh weight, but moisture diffusivities were 1.42 × 10−9 m2/s at 60 °C being higher than that at 50 °C (1.04 × 10−9 m2/s). Consequently, a moisture ratio of around 0.1 was achieved in 120 and 240 min at 60 and 50 °C respectively, indicating for a similar moisture loss preponderance of drying temperature over drying time. At 70 and 80 °C chlorophyll losses were observed to be lower than at 40 °C. In this case the moisture ratio of 0.1 was reached within 60 min at 70 and 80 °C, while at 40 °C drying would last more than 250 min, which could be the limit to prolonged exposure.
Table 2.
Chlorophyll, carotenoids and vitamin E in fresh and dehydrated brown seaweeds D. antarctica
| Temperature (°C) | Pigments (mg/kg dm) | Tocopherol (mg/kg dm) | ||
|---|---|---|---|---|
| Chlorophyll a | Chlorophyll c1 + c2 | Carotenoids | α-Tocopherol (Vit. E) | |
| Control | 698.6 ± 5.3a | 108.3 ± 2.6a | 34.1 ± 1.70ab | 84.0 ± 0.5ab |
| 40 | 438.0 ± 2.0b | 58.0 ± 2.2b | 23.1 ± 1.95c | 100.3 ± 17.2b |
| 50 | 606.3 ± 7.7c | 93.5 ± 4.4c | 35.9 ± 1.27a | 65.8 ± 8.0a |
| 60 | 412.0 ± 20.3d | 62.7 ± 3.2b | 32.9 ± 1.27b | 78.1 ± 11.4ab |
| 70 | 499.0 ± 21.4e | 76.7 ± 1.5d | 35.4 ± 1.83ab | 83.4 ± 1.8ab |
| 80 | 483.9 ± 5.7e | 72.3 ± 1.5d | 28.9 ± 1.14d | 89.0 ± 4.4b |
Different letters in the same column indicate significant difference (p < 0.05)
With respect to carotenoids between 50 and 70 °C there were no significant differences to initial content, indicating a good stability during drying under such temperature conditions. Significant losses were observed at 40 °C (32%) and 80 °C (15%), with a lower loss at 80 °C, similar to degradation behaviour of chlorophyll at 80 °C, where a prolonged exposure > 250 min may cause more damage that the higher temperature, due to a predominating oxidation process of the highly unsaturated carotenoids (Christaki et al. 2012). As to α-tocopherol no significant loss was observed during drying at any of the assayed temperature, which could be due to the occurrence of that component in the lipid fraction that shielded from a thermal destruction (Van Hoed et al. 2009).
Amino acids profile
The amino acids profiles of brown seaweed D. antarctica after drying is reported in Table 3. Amino acids (AAs) have important roles in nutrition and whole-body homeostasis. They are not only building blocks for proteins, they also have multiple regulatory functions in cells and are crucial nutrients for growth, development, and health of animals and humans (Wu 2010). In the brown seaweed D. antarctica all traditionally known as essential AAs (leucine, isoleucine, lysine, methionine, phenylalanine, threonine and valine) have been determined. The so-called functional AAs (arginine, cysteine, glutamine, leucine and proline) known to regulate key metabolic pathways, necessary for maintenance, growth, reproduction and immunity (Wu 2010), were also found. Only tryptophan was not determined. The essential AAs in the control seaweed D. antarctica was found in an amount of 578 mg/g protein, which accounted for 43% of total AAs in the seaweed’s protein. After drying at any temperature between 40 and 80 °C, this percentage did not significantly change, remaining between 42 and 44%. Therefore, D. antarctica can contribute an adequate level of total essential AAs (309 mg/g protein), according to FAO/WHO/UNU (1985) protein requirement pattern, implying a high biological protein value of the essential AAs. Among the AAs found in D. antarctica, glycine followed by glutamic acid and leucine predominated at a level > 150 mg/g protein. After drying an amount > 100 mg/g protein was maintained. A slight but significant decrease in all AAs was observed at drying temperatures of 50 and 60 °C, although the level of essential AAs did not fall below 395 mg/g protein, which was the lowest value determined at 60 °C. Therefore, D. antarctica still retained it high biological protein value after drying. A thermal treatment may improve, reduce or maintain essential AAs content depending on drying conditions or the amino acid itself (Deng et al. 2014). In this study a general decrease in AAs content was observed, except for alanine during drying at 70 °C and lysine at 40, 50 and 70 °C where a significant increase (p < 0.05) was observed (Table 3), which might be due to the progress of proteolysis during the drying process (Zhao et al. 2017).
Table 3.
Amino acids profile of brown seaweed D. antarctica before and after dehydration at different drying temperatures
| Amino acids (g /100 g protein) | Control | 40 °C | 50 °C | 60 °C | 70 °C | 80 °C |
|---|---|---|---|---|---|---|
| Alanine | 9.57 ± 0.10a | 9.68 ± 0.31a | 9.07 ± 0.39a | 8.14 ± 0.28b | 12.06 ± 0.70c | 9.42 ± 0.34a |
| Arginine | 4.83 ± 0.53a | 4.22 ± 0.17b | 3.62 ± 0.27c | 3.29 ± 0.18c | 4.91 ± 0.32a | 4.46 ± 0.30ab |
| Aspartic acid | 4.17 ± 0.47a | 1.78 ± 0.12b | 2.08 ± 0.07b | 1.96 ± 0.09b | 2.89 ± 0.26c | 2.93 ± 0.04c |
| Cystine | 0.78 ± 0.09a | 0.65 ± 0.04b | 0.51 ± 0.05c | 0.48 ± 0.01c | 0.49 ± 0.05c | 0.52 ± 0.09c |
| Glutamic acid | 17.87 ± 1.27a | 13.06 ± 0.32b | 12.84 ± 0.73b | 12.23 ± 1.26b | 14.86 ± 0.03c | 16.40 ± 1.02ac |
| Glycine | 18.36 ± 0.47a | 13.22 ± 0.06b | 12.19 ± 0.16c | 13.31 ± 0.37b | 15.78 ± 0.39d | 16.32 ± 0.50d |
| Histidine | 2.26 ± 0.04a | 1.63 ± 0.07b | 1.46 ± 0.03c | 1.48 ± 0.01c | 2.10 ± 0.06d | 1.84 ± 0.02e |
| Isoleucine | 8.05 ± 0.42a | 5.91 ± 0.28b | 5.27 ± 0.08c | 5.42 ± 0.41bc | 7.29 ± 0.37d | 6.75 ± 0.37d |
| Leucine | 15.88 ± 0.89a | 11.37 ± 0.19b | 10.22 ± 0.39c | 10.33 ± 0.69c | 14.59 ± 0.38d | 13.10 ± 0.67e |
| Lysine | 4.22 ± 0.30a | 6.56 ± 0.05b | 6.17 ± 0.05bd | 3.47 ± 0.15c | 5.75 ± 0.68d | 4.23 ± 0.24a |
| Methionine | 3.89 ± 0.18a | 2.60 ± 0.16bd | 2.29 ± 0.12b | 2.27 ± 0.21b | 3.50 ± 0.17c | 2.80 ± 0.27d |
| Proline | 7.95 ± 0.66a | 5.18 ± 0.06bc | 4.94 ± 0.21b | 5.91 ± 0.47c | 7.50 ± 0.71ad | 6.86 ± 0.61d |
| Phenylalanine | 9.97 ± 0.42a | 7.20 ± 0.13b | 6.60 ± 0.19c | 6.57 ± 0.47c | 9.21 ± 0.08d | 8.10 ± 0.40e |
| Serine | 5.38 ± 0.45a | 3.92 ± 0.10bc | 3.73 ± 0.13b | 4.21 ± 0.15c | 5.71 ± 0.13a | 5.34 ± 0.23a |
| Threonine | 5.84 ± 0.44a | 4.40 ± 0.09b | 4.21 ± 0.25b | 4.53 ± 0.10b | 5.83 ± 0.31ac | 5.40 ± 0.02c |
| Tyrosine | 4.45 ± 0.15a | 3.34 ± 0.11b | 2.99 ± 0.07b | 2.84 ± 0.20c | 3.89 ± 0.14d | 3.51 ± 0.12b |
| Valine | 9.97 ± 0.32a | 6.38 ± 0.56b | 6.66 ± 0.13b | 6.92 ± 0.41b | 9.13 ± 0.15c | 8.73 ± 0.08c |
Different letters in the same row indicate significant differences (p < 0.05)
Chromatic coordinates and surface colour
The chromatic coordinates and surface colour of fresh and dried D. antarctica are shown in Table 1. The L-value (brightness [100 = white, 0 = black]) of the samples increased due to drying. The L-value achieved at 40 °C is more than twice the L-value of the control sample. As drying temperature was increased, the L-values showed a continuous gradual increase from 54.33 ± 0.05 at 40 °C to 61.32 ± 0.21 at 80 °C, obeying a linear function (R2 = 0.9859) that may be given as: L-value = (47.484 + 0.1776·T), being T the air temperature in °C. The increase in brightness is typical for dried products of D. antarctica that acquire a plane glossy surface. The a-value that represents in the CIE L* a* b* system the greenness-redness axis (+ a* red direction, − a* green direction), showed a tendency to reddish colour at 40 °C with an increase in a-value to 2.26 ± 0.04 departing from 0.23 ± 0.03 for the control sample. However, at drying temperatures between 50 and 80 °C there was a shift to the green direction from − 1.41 ± 0.06 at 50 °C to − 0.33 ± 0.02 at 80 °C. The b-value that represents in the CIE L* a* b* system the yellowness-blueness axis (blue [negative] and yellow [positive]), showed at 40 °C a strong increase in the yellow direction to a value of 28.04 ± 0.07 (Table 4), but afterwards dropped to a value around 20 at temperatures between 50 and 80 °C. The combination of red and yellow at 40 °C showed tendency to colour saturation resulting in a dark brownish tone, which was confirmed by the ΔC-value or (Δa2 + Δb2)½ of 28.13 ± 0.07, related to chroma (McGuire 1992), being also highest at 40 °C (Table 4). Between 50 and 80 °C the combination of green and yellow predominated, which conferred to the seaweed a lighter brownish tone. The hue of the dried seaweed contributed mainly to chroma saturation, showing drastically opposite effect at 40 °C compared to the temperature range between 50 and 80 °C. ΔE (= ΔL2 + Δa2 + Δb2)½ (McGuire 1992), which is the colorimetric difference between the sample and the white standard reflectance plate, increased with drying temperature and was congruent with chroma saturation. In whatever direction, the colour change was similar at an average value around 40 (Table 4).
Table 4.
Chromatic coordinates and surface colour of fresh and dehydrated brown seaweed D. antarctica
| Temperature (°C) | L* | a* | b* | ∆E | h* | C* |
|---|---|---|---|---|---|---|
| Control | 21.52 ± 0.06a | 0.23 ± 0.03a | 1.50 ± 0.11a | – | 1.42 ± 0.03a | 1.52 ± 0.11a |
| 40 | 54.33 ± 0.05b | 2.26 ± 0.04b | 28.04 ± 0.07b | 42.25 ± 0.13a | 1.49 ± 0.00b | 28.13 ± 0.07b |
| 50 | 56.35 ± 0.15c | − 1.41 ± 0.06c | 19.69 ± 0.05c | 39.33 ± 0.18b | − 1.50 ± 0.00c | 19.74 ± 0.05c |
| 60 | 58.59 ± 0.44d | − 1.31 ± 0.03d | 20.25 ± 0.36d | 41.57 ± 0.59c | − 1.51 ± 0.00c | 20.30 ± 0.36d |
| 70 | 60.13 ± 0.29e | − 0.52 ± 0.02e | 22.90 ± 0.21e | 44.15 ± 0.40d | − 1.55 ± 0.00d | 22.91 ± 0.21e |
| 80 | 61.32 ± 0.21f | − 0.33 ± 0.02f | 20.99 ± 0.22f | 44.32 ± 0.34d | − 1.56 ± 0.00d | 20.99 ± 0.22f |
Different letters in the same column indicate significant differences (p < 0.05) for each parameter
Total phenolics and total flavonoids contents
Total phenolic content (TPC) of 660 ± 34 mg GAE/100 g dm was determined in the fresh brown seaweed, which was lower than TPC reported for the edible Irish brown seaweed, Himanthalia elongata at a level of 1.55 ± 0.026 g GAE/100 g dry seaweed (Gupta et al. 2011), but higher than the phenolic content exhibited in the aqueous fraction of Sargassum marginatum and Turbinaria conoides of 24.61 and 49.16 mg GAE/g of seaweed extract (or 0.29 and 0.86 mg GAE/g of seaweed on dry weight basis), respectively (Chandini et al. 2008). There are also other reports where the phenolic content of the brown algae from the Aegean Sea have been shown to vary between 0.4 ± 0.2 and 189.6 ± 8.6 mg GAE/g extract from 15 g of the freeze-dried seaweeds Colpomenia sinuosa, Dictyota dichotoma, Dictyota dichotoma var. implexa, Petalonia fascia and Scytosiphon lomentaria (Demirel et al. 2009). The phenol content of seaweed would strongly depend on sunlight and climate, such that analogous seaweeds species may contain higher phenol levels in samples taken from a warmer climate (Flodin et al. 1999).
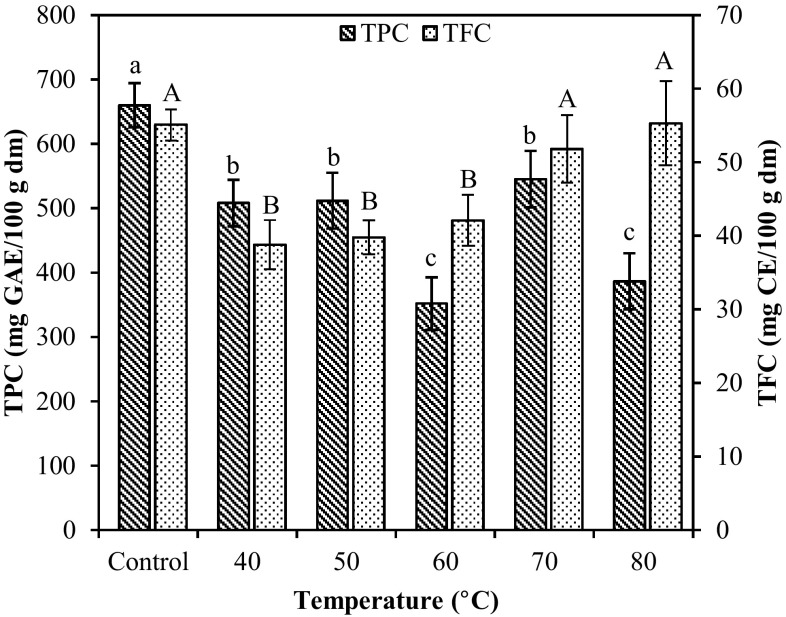
As can be seen in Fig. 1 a decrease in TPC of 23% and 22% occurred at drying temperatures of 40 and 50 °C respectively, which was less than the 29% decrease in TPC reported by Gupta et al. (2011) for the edible H. elongata dried at 40 °C for 24 h. At 60 °C a total loss of 47% of TPC was observed, which was not significantly different (p < 0.05) to the decrease occurred at 80 °C (41%), while at 70 °C the reduction in TPC (17%) was not significantly different to the decrease observed at 40 and 50 °C. The loss occurred at 60 °C may be attributed to intense and prolonged thermal treatment usually responsible for loss of natural antioxidants, due to relative instability of most of these compounds (Lim and Murtijaya 2007) or to the binding of polyphenols with proteins, as well as alterations in the chemical structure of polyphenols (Martín-Cabrejas et al. 2009). The deviation from this decreasing tendency that occurred at 70 °C was unexpected. However, formation of phenolic compounds at high temperatures has been reported and has been ascribed to availability of precursors of phenolic molecules arising from non-enzymatic interconversion between phenolic molecules (Martín-Cabrejas et al. 2009). Therefore, between 60 and 70 °C simultaneous degradation and formation of phenolics might be occurring and since drying time was shorter at 70 °C, content of phenolics could be maintained at a higher level. This observation was also supported by the fact that after drying at 80 °C for shorter drying time, loss of phenolics was slightly less than at 60 °C, even though this difference might not be significant at p < 0.05 (Fig. 1).
Fig. 1.
Total phenolics and total flavonoids in brown seaweeds D. antarctica dried at different temperatures. Different lower-case or upper-case letters on same-shaded columns indicate significant difference (p < 0.05)
Flavonoids are also plant phenolics, belonging to a large group that accounts for more than 50% of the eight thousand naturally occurring phenolic compounds. The potential benefits of flavonoids for the human health are supported by epidemiological and in vitro evidences of antioxidant, cardioprotective, anticarcinogenic activities, and against other non-transmissible chronic diseases (Celli et al. 2011). In Fig. 1 the variation in TFC for the five different drying temperatures are shown. The TFC in the fresh seaweed D. antarctica used as control was 55.09 ± 2.12 mg CE/100 g dm. Drying led to a significant reduction in TFC at 40 °C, but the reduction continuously declined as temperature increased; at 80 °C loss of flavonoids was not significant (p < 0.05). For the edible H. elongata a percentage reduction of 49% and 30% in TFC was reported for drying at 25 and 40 °C (Gupta et al. 2011). The total flavonoids in the brown seaweed showed higher resistance than total phenolics (Fig. 1) probably due to a difference in structural solidity; D. antarctica may contained predominantly glycosylated flavonoids that are more heat stable.
Antioxidant activity
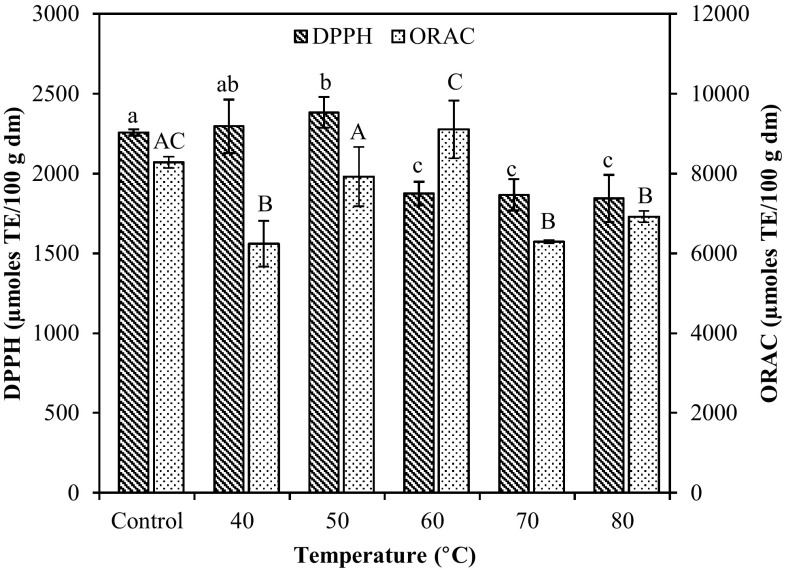
The antioxidant capacity of dried D. antarctica was determined by means of DPPH and Oxygen Radical Absorbance Capacity (ORAC) methodology. The DPPH radical scavenging activity is commonly used to evaluate antioxidant activity of phenolic compounds and is based on the reaction of antioxidants with this stable radical. The ORAC assay is used as an in vitro method for measuring antioxidant activity of seaweed extracts by evaluating the scavenge ability to certain peroxyl radicals that induce oxidation in the presence of fluorescein (Kindleysides et al. 2012). According to Zulueta et al. (2009), the ORAC method combines total inhibition time and degree of the free-radical damage by the antioxidant into a single quantity, ensuring that at the end of the process all antioxidants present in the sample would have reacted with the radicals generated. Fresh D. antarctica presented DPPH value of 2257 ± 20 μmol TE/100 g dm and ORAC value of 8278 ± 140 μmol TE/100 g dm. Drying resulted in significantly higher decrease (p < 0.05) in the antioxidant activity at temperatures over 70 °C. The reduction in DPPH free radical scavenging activity was not significant (p < 0.05) at 40 and 50 °C, while for the ORAC value compared to the fresh samples did not show significant difference at 50 and 60 °C. As reported for H. elongata higher decrease in antioxidant activity occurred as drying temperature was increased from 25 to 50 °C (Gupta et al. 2011). In general, the antioxidant capacity may be related to the amount of TPC and flavonoids, since these compounds act as scavengers of the free radicals produced during oxidation reactions. According to the experimental results as shown in Fig. 2, phenolics and flavonoids could be considered as an important source of bioactive compounds with relatively high antioxidant activity.
Fig. 2.
Antioxidant activity in dried brown seaweeds D. antarctica dried at different temperatures. Different lower-case or upper-case letters on same-shaded columns indicate significant difference (p < 0.05)
Conclusion
The brown seaweed D. antarctica can be classified among seaweeds with high content of insoluble dietary fibre that is not significantly affected during drying between 40 and 80 °C. The chlorophyll pigments showed significant degradation at drying temperature over 60 °C, while the carotenoids were more stable, and degradation became significant only at drying temperatures over 80 °C. As to α-tocopherol no significant loss was observed during drying within the assayed temperature range, probably due to shielding from thermal destruction by the lipid fraction. The essential amino acids in the fresh seaweed D. antarctica, did not significantly change during drying, showing glycine, glutamic acid and leucine to predominate with a level above 150 mg/g protein. A slight but significant decrease in total amino acids occurred at drying temperatures between 50 and 60 °C. Nonetheless, dried D. antarctica still retained a high biological protein value, according to requirement pattern of FAO/WHO/UNU, with an adequate level of total essential amino acids (309 mg/g protein). At 40 °C a colour shift to darker brown colour occurred, while a lighter brown tone of the dried seaweed may be obtained between 50 and 80 °C. The level of TPC decreased significantly at 60 °C with a total loss of 47% of the total phenolics, while the loss in TFC continuously declined as drying temperature increased and at 80 °C the loss of flavonoids was not significant. D. antarctica proved to be an important source of bioactive compounds with relatively high antioxidant activity, which may satisfy the requirements for development of functional foods.
Acknowledgments
The authors gratefully acknowledge financial support of the Research Department of Universidad de la Serena (DIULS Project No PR16332) and CONICYT-Chile, Fondecyt Project No 1160597 for publication of this research.
Contributor Information
Elsa Uribe, Email: muribe@userena.cl.
Antonio Vega-Gálvez, Email: avegag@userena.cl.
Natalia Vargas, Email: nataliavvg@live.cl.
Alexis Pasten, Email: alexisfapc@gmail.com.
Katia Rodríguez, Email: krodrigu@userena.cl.
Kong Shun Ah-Hen, Phone: 56 63221619, Email: kshun@uach.cl.
References
- American Association of Cereal Chemists (AACC) Dietary Fiber Technical Committee The definition of dietary fiber. Cereal Food World. 2001;46:112–126. [Google Scholar]
- AOAC . Official methods of analysis of the Association of Official Analytical Chemists. 16. Virginia: AOAC International; 1996. [Google Scholar]
- Astorga-España MS, Mansilla A. Sub-Antarctic macroalgae: opportunities for gastronomic tourism and local fisheries in the Region of Magallanes and Chilean Antarctic Territory. J Appl Phycol. 2014;26:973–978. doi: 10.1007/s10811-013-0141-1. [DOI] [Google Scholar]
- Brand-Williams M, Curvelier ME, Berset C. Use of free radical method to evaluate antioxidant capacity. LWT-Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Celli G, Pereira-Netto A, Beta T. Comparative analysis of total phenolic content, antioxidant activity, and flavonoids profile of fruits from two varieties of Brazilian cherry (Eugenia uniflora L.) throughout the fruit developmental stages. Food Res Int. 2011;44:2442–2451. doi: 10.1016/j.foodres.2010.12.036. [DOI] [Google Scholar]
- Chan PT, Matanjun P. Chemical composition and physicochemical properties of tropical red seaweed, Gracilaria changii. Food Chem. 2017;221:302–310. doi: 10.1016/j.foodchem.2016.10.066. [DOI] [PubMed] [Google Scholar]
- Chan JCC, Cheung PCK, Ang PO. Comparative studies on the effect of three drying methods on the nutritional composition of Seaweed Sargassum hemiphyllum (Turn.) C. Ag. J Agric Food Chem. 1997;45:3056–3059. doi: 10.1021/jf9701749. [DOI] [Google Scholar]
- Chandini SK, Ganesan P, Bhaskar N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008;107:707–713. doi: 10.1016/j.foodchem.2007.08.081. [DOI] [Google Scholar]
- Christaki E, Bonos E, Giannenas I, Florou-Paneri P. Functional properties of carotenoids originating from algae. J Sci Food Agric. 2012;93:5–11. doi: 10.1002/jsfa.5902. [DOI] [PubMed] [Google Scholar]
- Demirel Z, Yilmaz-Koz FF, Karabay-Yavasoglu UN, Ozdemir G, Sukatar A. Antimicrobial and antioxidant activity of brown algae from the Aegean Sea. J Serb Chem Soc. 2009;74:619–628. doi: 10.2298/JSC0906619D. [DOI] [Google Scholar]
- Deng Y, Wang Y, Yue J, Liu Z, Zheng Y, Qian B, Zhong Y, Zhao Y. Thermal behavior, microstructure and protein quality of squid fillets dried by far-infrared assisted heat pump drying. Food Control. 2014;36:102–110. doi: 10.1016/j.foodcont.2013.08.006. [DOI] [Google Scholar]
- Dini I, Tenore GC, Dini A. Antioxidant compound contents and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. LWT-Food Sci Technol. 2010;43:447–451. doi: 10.1016/j.lwt.2009.09.010. [DOI] [Google Scholar]
- FAO/WHO/UNU (1985) Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation. WHO technical report series no. 724. Geneva [PubMed]
- Fleury N, Lahaye M. Chemical and physicochemical characterization of fibers from Laminaria digitata (Kombu Breton)—a physiological approach. J Sci Food Agric. 1991;55:389–400. doi: 10.1002/jsfa.2740550307. [DOI] [Google Scholar]
- Flodin C, Helidoniotis F, Whitfield FB. Seasonal variation in bromophenol content and bromoperoxidase activity in Ulva lactuca. Phytochemistry. 1999;51:135–138. doi: 10.1016/S0031-9422(98)00668-2. [DOI] [Google Scholar]
- Flores-Moya A, Fernández JA. Photosynthetic use of light and inorganic carbon in air and water by the intertidal alga Petalonia fascia (Scytosiphonales, Phaeophyta) Phycol Res. 1998;46:125–130. doi: 10.1111/j.1440-1835.1998.tb00104.x. [DOI] [Google Scholar]
- Fudholi A, Sopian K, Othman MY, Ruslan MH. Energy and exergy analyses of solar drying system of red seaweed. Energy Build. 2014;68:121–129. doi: 10.1016/j.enbuild.2013.07.072. [DOI] [Google Scholar]
- Gupta S, Rajauria G, Abu-Ghannam N. Study of the microbial diversity and antimicrobial properties of Irish edible brown seaweeds. Int J Food Sci Technol. 2010;45:482–489. doi: 10.1111/j.1365-2621.2009.02149.x. [DOI] [Google Scholar]
- Gupta S, Cox S, Abu-Ghannam N. Effect of different drying temperatures on the moisture and phytochemical constituents of edible Irish brown seaweed. LWT-Food Sci Technol. 2011;44:1266–1272. doi: 10.1016/j.lwt.2010.12.022. [DOI] [Google Scholar]
- Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RI. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- Jeffrey SW, Humphrey GF. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biocbem Physiol Pflanzen. 1975;167:191–194. doi: 10.1016/S0015-3796(17)30778-3. [DOI] [Google Scholar]
- Kindleysides S, Quek S-Y, Miller MR. Inhibition of fish oil oxidation and the radical scavenging activity of New Zealand seaweed extracts. Food Chem. 2012;133:1624–1631. doi: 10.1016/j.foodchem.2012.02.068. [DOI] [Google Scholar]
- Lahaye M. Marine-algae as sources of fibers—determination of soluble and insoluble dietary fiber contents in some sea vegetables. J Sci Food Agric. 1991;54:587–594. doi: 10.1002/jsfa.2740540410. [DOI] [Google Scholar]
- Lim YY, Murtijaya J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci Technol. 2007;40:1664–1669. doi: 10.1016/j.lwt.2006.12.013. [DOI] [Google Scholar]
- Liu L, Heinrich M, Myers S, Dworjanyn SA. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol. 2012;142:591–619. doi: 10.1016/j.jep.2012.05.046. [DOI] [PubMed] [Google Scholar]
- Løvstad-Holdt S, Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011;23:543–597. doi: 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- Mansilla A, Ávila M, Yokoya NS. Current knowledge on biotechnological interesting seaweeds from the Magellan Region, Chile. Braz J Pharmacogn. 2012;22:760–767. doi: 10.1590/S0102-695X2012005000074. [DOI] [Google Scholar]
- Martín-Cabrejas MA, Aguilera Y, Pedrosa M, Cuadrado C, Hernández T, Díaz S, et al. The impact of dehydration process on antinutrients and protein digestibility of some legume flours. Food Chem. 2009;114:1063–1068. doi: 10.1016/j.foodchem.2008.10.070. [DOI] [Google Scholar]
- McGuire RG. Reporting of objective color measurements. Hortic Sci. 1992;27:1254–1255. [Google Scholar]
- Murata M, Nakazoe J. Production and use of marine algae in Japan. JARQ-Jpn Agric Res Q. 2001;35:281–290. doi: 10.6090/jarq.35.281. [DOI] [Google Scholar]
- Ortiz J, Romero N, Robert P, Araya J, López-Hernández J, Bozzo C, et al. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006;99:98–104. doi: 10.1016/j.foodchem.2005.07.027. [DOI] [Google Scholar]
- Tello-Ireland C, Lemus-Mondaca R, Vega-Gálvez A, López J, Di Scala K. Influence of hot-air temperature on drying kinetics, functional properties, color, phycobiliproteins, antioxidant capacity and agar yield of alga Gracilaria chilensis. LWT-Food Sci Technol. 2011;44:2112–2118. doi: 10.1016/j.lwt.2011.06.008. [DOI] [Google Scholar]
- Uribe E, Vega-Gálvez A, Vásquez V, Lemus-Mondaca R, Callejas L, Pastén A. Hot-air drying characteristics and energetic requirement of the edible brown seaweed Durvillaea antarctica. J Food Process Preserv. 2017;00:e13313. doi: 10.1111/jfpp.13313. [DOI] [Google Scholar]
- Van Hoed V, de Clercq N, Echim C, Adnsjelkovic M, Leber E, De Wettinck K, et al. Berry seeds: a source of speciality oils with high content of bioactives and nutritional value. J Food Lipids. 2009;16:33–49. doi: 10.1111/j.1745-4522.2009.01130.x. [DOI] [Google Scholar]
- Vasco C, Ruales J, Kamal-Eldin A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008;111:816–823. doi: 10.1016/j.foodchem.2008.04.054. [DOI] [Google Scholar]
- Wong K, Cheung PC. Influence of drying treatment on three Sargassum species 2. Protein extractability, in vitro protein digestibility and amino acid profile of protein concentrates. J Appl Phycol. 2001;13:51–58. doi: 10.1023/A:1008188830177. [DOI] [Google Scholar]
- Wright K, Pike O, Fairbanks D, Huber C. Composition of Atriplex hortensis, sweet and bitter Chenopodium quinoa seeds. J Food Sci. 2002;67:1380–1385. doi: 10.1111/j.1365-2621.2002.tb10294.x. [DOI] [Google Scholar]
- Wu G. Functional amino acids in growth, reproduction, and health. Adv Nutr. 2010;1:31–37. doi: 10.3945/an.110.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Jiang Y, Zheng B, Zhuang W, Zheng Y, Tian Y. Influence of microwave vacuum drying on glass transition temperature, gelatinization temperature, physical and chemical qualities of lotus seeds. Food Chem. 2017;228:167–176. doi: 10.1016/j.foodchem.2017.01.141. [DOI] [PubMed] [Google Scholar]
- Zulueta A, Esteve MJ, Frígola A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009;114:310–331. doi: 10.1016/j.foodchem.2008.09.033. [DOI] [Google Scholar]