Key Points
Question
Can the administration of nitrous oxide reduce or eliminate bothersome tinnitus?
Findings
In this randomized clinical trial of 40 participants, the Tinnitus Functional Index after intervention was lower than before intervention in the placebo arm and in the nitrous oxide arm. The within-participant mean difference in the change in the Tinnitus Functional Index of the placebo arm compared with the nitrous oxide arm was −1.1 points, which was neither clinically or statistically significant.
Meaning
Nitrous oxide is no more effective than placebo for the treatment of tinnitus.
Abstract
Importance
The tinnitus research literature suggests that N-methyl-d-aspartate (NMDA) receptor antagonists may be useful in reducing tinnitus. Nitrous oxide, a member of the NMDA receptor antagonist class, is a widely used general anesthetic and sedative with a proven safety record.
Objective
To investigate whether nitrous oxide can reduce bothersome tinnitus.
Design, Setting, and Participants
Randomized, placebo-controlled crossover trial conducted between October 15, 2016, and June 22, 2017. Participants attended 2 interventional sessions separated by at least 14 days and were randomized to receive either placebo first or nitrous oxide first. Participants were followed up through completion of the second arm of the study. The setting was a clinical research unit at an academic medical center. Adults aged 18 to 65 years with subjective, idiopathic, nonpulsatile bothersome tinnitus of 6 months’ duration or longer were recruited from 2 clinical research databases. Seventy-one individuals were screened, of whom 40 were enrolled. Of those enrolled, 37 participants completed all components of the study.
Interventions
The placebo session consisted of 50% nitrogen and 50% oxygen inhaled for 40 minutes, and the treatment session consisted of 50% nitrous oxide and 50% oxygen inhaled for 40 minutes.
Main Outcomes and Measures
Tinnitus was assessed before and after intervention, with the change in the Tinnitus Functional Index (TFI) as the primary outcome measure. Secondary outcome measures included the Patients’ Global Impression of Change score and the change in the Global Bothersome Scale score.
Results
Among 40 participants in this intent-to-treat randomized clinical trial with 20 participants randomly assigned to each group, the mean (SD) age of participants was 52.9 (11.1) years, with equal numbers of male and female participants. The TFI after intervention was a mean (SD) of 1.8 (8.8) points lower than before intervention in the placebo arm and a mean (SD) of 2.5 (11.0) points lower than before intervention in the nitrous oxide arm. The within-participant mean difference in the change in the TFI of the placebo arm compared with the nitrous oxide arm was −1.1 points (95% CI, −5.6 to 3.4 points). The difference between the placebo and nitrous oxide arms was neither clinically meaningful nor statistically significant.
Conclusions and Relevance
Nitrous oxide was no more effective than placebo for the treatment of subjective, idiopathic tinnitus.
Trial Registration
ClinicalTrials.gov identifier: NCT03365011
This randomized clinical trial investigates whether inhaled nitrous oxide and oxygen vs inhaled nitrogen and oxygen can reduce bothersome tinnitus among adults with subjective, idiopathic, nonpulsatile tinnitus of 6 months’ duration or longer.
Introduction
Subjective, idiopathic, nonpulsatile tinnitus (“tinnitus”) is the perception of sound in the absence of an external acoustic stimulus. Approximately 50 million Americans experience chronic tinnitus; of these, 10 million have bothersome tinnitus.1 Bothersome tinnitus is associated with poorer working memory, slower cognitive processing speeds and reaction times, and deficiencies in selective attention.2
To date, effective treatment for tinnitus is limited to cognitive behavior therapy.3,4 Hearing aids may provide benefit for those patients with tinnitus and hearing loss.5 Sound therapy, or masking, may be of limited benefit.6 Pharmacologic therapy, including antidepressants,7 anxiolytics,8,9 and anticonvulsants,10 are of limited benefit, have significant adverse effects, and are not suitable for long-term use. Dietary supplements,11,12 acupuncture,13 and transcranial magnetic stimulation14 are also not recommended for tinnitus. The identification of an effective and fast-acting intervention would be a significant advantage for the patient with tinnitus.
Nitrous oxide is an N-methyl-d-aspartate (NMDA) receptor antagonist, a class of drugs shown to have antidepressant effects.6 A previous trial examined the effect of nitrous oxide for treatment-resistant major depressive disorder.15 In that masked, placebo-controlled crossover trial, 20 patients with treatment-resistant depression were randomly assigned to receive 1 hour of 50% nitrous oxide or 50% oxygen (control), followed 1 week later by the other treatment. Depressive symptoms significantly improved at 2 hours and 24 hours after nitrous oxide compared with control. The proof-of-concept trial demonstrated that nitrous oxide had a rapid antidepressant effect. The NMDA receptors are maintained at synapses throughout the auditory pathway, including in the cochlea, dorsal cochlear nucleus, and higher brainstem structures, and have diverse roles in synaptic development, plasticity, and processing of temporal auditory information. Generally, the NMDA receptors promote excitation at synapses throughout the auditory pathway and have diverse roles in synaptic development and auditory information processing. In the setting of chronic damage to the auditory system, overactivation of the NMDA receptors leads to aberrant spontaneous neuronal firing in the cochlea and auditory brainstem structures, which can further perpetuate damage and disease in a feed-forward mechanism.16,17,18,19,20 Studies by Guitton and Dudai18 and by Puel19 showed that the administration of the NMDA receptor antagonists before the administration of salicylate was effective in preventing acute excitotoxic tinnitus, establishing that salicylate induces tinnitus through its action on the NMDA receptors.
Therefore, the NMDA receptors are thought to be implicated in the generation and perpetuation of several auditory diseases, including tinnitus.19,20,21 We hypothesized that the administration of nitrous oxide may provide a rapid reduction in subjective, bothersome tinnitus.
Methods
Study Design and Oversight
This randomized, placebo-controlled crossover trial was approved by the institutional review board at Washington University School of Medicine in St Louis. Each participant attended 2 intervention sessions, one “placebo” and one “treatment.” The 2 intervention sessions were held at least 14 days apart to allow for adequate washout of any treatment effect.15 Participants were followed up through completion of the second arm of the study. Participants enrolled in the study were randomly assigned to receive either placebo that was followed by nitrous oxide or nitrous oxide that was followed by placebo according to a computer-generated randomization sequence. Only the statistician (D.K.) and the anesthesiology team (B.Y. and P.N.) directly involved in the administration of the placebo and nitrous oxide had access to the group assignments. All participants and other study team members (H.Y.H., O.K., and F.T.B.) administering survey assessments remained masked. The intervention sessions were indistinguishable in setting, setup, and monitoring to maintain masking for the participants and study team members. All intervention sessions were performed at the Washington University Clinical Research Unit, a component of the Center for Applied Research Sciences. The full trial protocol can be accessed in Supplement 1.
Participants
Forty participants were recruited from 2 clinical research databases, the Washington University Volunteers for Health Research Participant Registry and the Otolaryngology Research Participant Registry. Eligibility criteria included adults aged 18 to 65 years who had subjective, idiopathic, nonpulsatile bothersome tinnitus of 6 months’ duration or longer and were able to communicate in English and provide written informed consent. Individuals were included only if they self-reported to be “Bothered more than a little but not a lot” or more on a 5-point Global Bothersome Scale (GBS), which ranges from “Not bothered” to “Extremely bothered.” Individuals were excluded if they had a history of bipolar disorder, schizophrenia, or schizoaffective disorder at any time in their life or if they had substance abuse or dependence within the last year. Individuals were also excluded if they demonstrated any of the following: (1) active psychotic symptoms; (2) significant pulmonary disease and/or requirement of supplemental oxygen; (3) contraindication against the use of nitrous oxide, including pneumothorax, bowel obstruction, middle ear occlusion, elevated intracranial pressure, chronic cobalamin and/or folate deficiency treated with folic acid or vitamin B12, pregnancy, or breastfeeding; (4) previous NMDA receptor antagonist treatment (eg, ketamine hydrochloride) within the last 3 months; (5) tinnitus related to cochlear implantation, retrocochlear lesion, Meniere disease, or other known anatomic lesions of the ear or temporal bone; or (6) tinnitus related to any active litigation-related event.
Intervention
Patients attending the placebo session were administered an inhalation of 50% nitrogen and 50% oxygen for 40 minutes, and those attending the active treatment session were administered an inhalation of 50% nitrous oxide and 50% oxygen for 40 minutes. The selection of the 50% nitrous oxide concentration was based on the use in the previous trial for treatment-resistant major depressive disorder.15 Each mixture of gases was administered via a nitrous oxide delivery apparatus approved by the US Food and Drug Administration (FDA). The total gas flow was set at 4 to 6 L/min. Participants were monitored during and after each intervention session according to the American Society of Anesthesiologists standard,22 including continuous 3-lead electrocardiogram, pulse oximetry, and noninvasive blood pressure under the supervision of an anesthesiologist. After the 40-minute intervention, all participants were observed for an additional hour and were then assessed by a study team member for safe discharge. Masking of participants was assessed by asking each participant at the end of the first intervention session to give their best guess as to which intervention (placebo or nitrous oxide) they had just received.
Outcome Measures
The primary outcome measure was the change in the Tinnitus Functional Index (TFI) 1 week after intervention compared with before intervention. The TFI is a 25-question survey assessing tinnitus effect on quality of life. Participants were asked to rate on a scale from 0 (low) to 10 (high) the degree of unpleasantness, cognitive interference, sleep disturbance, auditory difficulties, interference with relaxation, and emotional distress associated with their tinnitus. Subindexes were summed and scaled to an index of 0 to 100. An index less than 25 indicates mild problems due to tinnitus and little need for intervention, while an index between 25 and 50 indicates significant problems due to tinnitus, with possible need for intervention. Secondary outcome measures included the Patients’ Global Impression of Change (PGIC) score and the change in the GBS score. The GBS measured participants’ self-assessment of bothersome tinnitus on the 5-point scale ranging from “Not bothered” to “Extremely bothered.” On the PGIC, participants reported their perception of change due to intervention, if any, with regard to activity limitations, symptoms, emotions, and overall quality of life related to tinnitus. Participants chose from 7 options ranging from “No change or worsened” to “Great deal better, considerable improvement.” The preintervention TFI and GBS data were collected immediately before the participant received the intervention, and the postintervention TFI and GBS data were collected 1 week after each intervention. Participants completed the PGIC 1 week after each intervention. All data were collected using research electronic data capture (REDCap).23
Statistical Analysis
The main outcome variable was the change in the TFI from before to after intervention. The effect size was determined a priori based on the clinically meaningful change of 13 points as defined by Meikle et al24 (the authors of the TFI), assuming the same variability in the TFI change shown in previous tinnitus studies.25,26 We estimated that a sample size of 32 participants would provide us with 81% power to detect an effect size of 0.52 or larger at 2-sided α = .05. Assuming a 20% dropout rate, we planned to enroll 40 participants with tinnitus in this study.
Standard descriptive statistics were used to describe the sample of participants included in the study. Bivariate analysis was performed to compare the distribution of characteristics between participants randomized to receive placebo first and those randomized to receive nitrous oxide first. Given the crossover study design, carryover effect was assessed by comparing the TFI at the start of the first session with the TFI at the start of the second session for the total cohort, the group receiving placebo first, and the group receiving nitrous oxide first.
The change in the TFI from before to after intervention was calculated for each participant and each intervention. The mean change in the TFI was determined for each intervention, and the mean within-participant difference between the placebo and nitrous oxide intervention groups was determined. A repeated-measures mixed linear model using the Kenward-Roger option27 to adjust df for small sample sizes and with participant as a random effect was used to investigate the change in the TFI before and after intervention and to test whether this change between the 2 interventions was significantly different. Participants who experienced a reduction in the TFI of 13 or more points were defined as intervention responders. The number of responders was calculated for each intervention arm and compared between the 2 interventions. Effectiveness of masking was assessed by calculating the percentage of participants who correctly identified which intervention they received during their first session.
Results
Participants
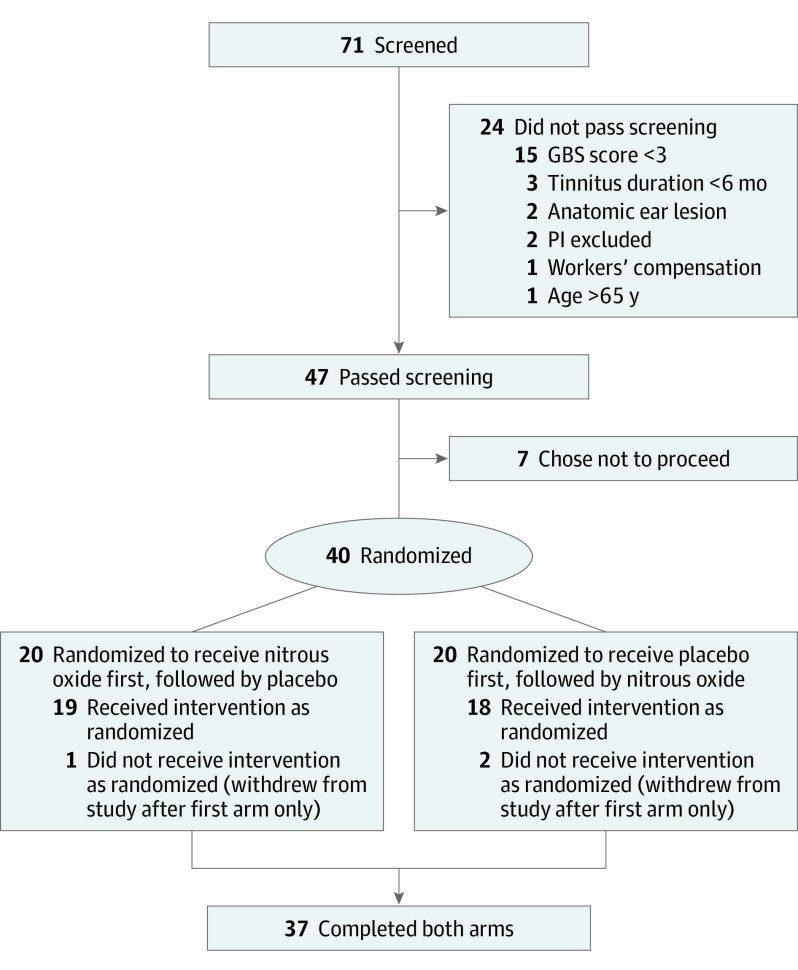
Recruitment of participants and implementation of the trial were conducted between October 15, 2016, and June 22, 2017. A total of 71 individuals were screened for participation in the study. Of these, 24 did not pass screening and were ineligible for participation in the study, and 7 passed screening but chose not to participate. The remaining 40 were enrolled and were evenly randomized into the groups of placebo first or nitrous oxide first. Of those enrolled, 3 participants either withdrew or were lost to follow-up after completion of the first intervention session. Thirty-seven participants completed the entirety of the study, including the intervention sessions and all assessments (Figure 1).
Figure 1. CONSORT Diagram of Study Participation.
CONSORT indicates Consolidated Standards of Reporting Trials; GBS, Global Bothersome Scale; and PI, principal investigator.
The mean (SD) age of the study population was 52.9 (11.1) years. There were equal numbers of male participants (n = 20) and female participants (n = 20). Participants had been experiencing tinnitus for a median duration of 13 years (range, 1-56 years). The median Patient Health Questionnaire19 score was 3 (range, 0-27; the higher the score, the higher the severity of depression: 0-4, none; 5-9, mild; 10-14, moderate; 15-19 moderately severe; and 20-27, severe). The median TFI at baseline was 41 (range, 6-92). On the GBS, 24 participants (60%) reported being “Bothered more than a little but not a lot,” 12 participants (30%) reported being “Bothered a lot,” and 4 participants (10%) reported being “Extremely bothered.” Baseline demographic characteristics, the TFI, and the GBS score are listed in the Table; tinnitus characteristics are listed in the eTable in Supplement 2. There were no significant differences between the group of participants randomized to receive placebo first and the group of participants randomized to receive nitrous oxide first.
Table. Baseline Demographics, TFI, and GBS Response of 40 Participants (20 Receiving Placebo First and 20 Receiving Nitrous Oxide First).
| Variable | All | Placebo First | Nitrous Oxide First |
|---|---|---|---|
| Age, mean (SD), y | 52.9 (11.1) | 53.5 (9.4) | 52.8 (11.1) |
| Sex, No. (%) | |||
| Male | 20 (50) | 7 (35) | 13 (65) |
| Female | 20 (50) | 13 (65) | 7 (35) |
| Education, No. (%) | |||
| High school or GED equivalent | 6 (15) | 1 (5) | 5 (25) |
| Associate degree or some college | 8 (20) | 5 (25) | 3 (15) |
| Bachelor degree | 11 (28) | 8 (40) | 3 (15) |
| Master’s degree or equivalent | 14 (35) | 6 (30) | 8 (40) |
| Doctorate | 1 (3) | 0 | 1 (5) |
| Race/ethnicity, No. (%) | |||
| White | 31 (78) | 16 (80) | 15 (75) |
| Black | 3 (8) | 1 (5) | 2 (10) |
| Other | 5 (13) | 3 (15) | 2 (10) |
| Did not wish to respond | 1 (3) | 0 | 1 (5) |
| Household and family, No. (%) | |||
| Single | 8 (20) | 5 (25) | 3 (15) |
| Married | 22 (55) | 11 (55) | 11 (55) |
| Divorced/separated/widowed | 10 (25) | 4 (20) | 6 (30) |
| Employment, No. (%) | |||
| Full-time | 27 (68) | 15 (75) | 12 (60) |
| Part-time | 2 (5) | 1 (5) | 1 (5) |
| Retired | 7 (18) | 2 (10) | 5 (25) |
| Disabled | 1 (3) | 0 | 1 (5) |
| Unemployed | 1 (3) | 1 (5) | 0 |
| Student | 1 (3) | 0 | 1 (5) |
| Missing | 1 (3) | 0 | 0 |
| Smoking, No. (%) | |||
| Never | 24 (60) | 13 (65) | 11 (55) |
| Previous | 15 (38) | 6 (30) | 9 (45) |
| Current | 1 (3) | 1 (5) | 0 |
| PHQ score, median (range) | 3 (0-23) | 3 (0-20) | 4 (0-23) |
| TFI, median (range) | 41 (6-92) | 42 (6-82) | 40 (21-92) |
| GBS response, No. (%) | |||
| Not bothered | 0 | 0 | 0 |
| Bothered a little but not much | 0 | 0 | 0 |
| Bothered more than a little but not a lot | 24 (60) | 12 (60) | 12 (60) |
| Bothered a lot | 12 (30) | 7 (35) | 5 (25) |
| Extremely bothered | 4 (10) | 1 (5) | 3 (15) |
Abbreviations: GBS, Global Bothersome Scale; GED, general equivalency diploma; PHQ, Patient Health Questionnaire; TFI, Tinnitus Functional Index.
Main Outcome Variable
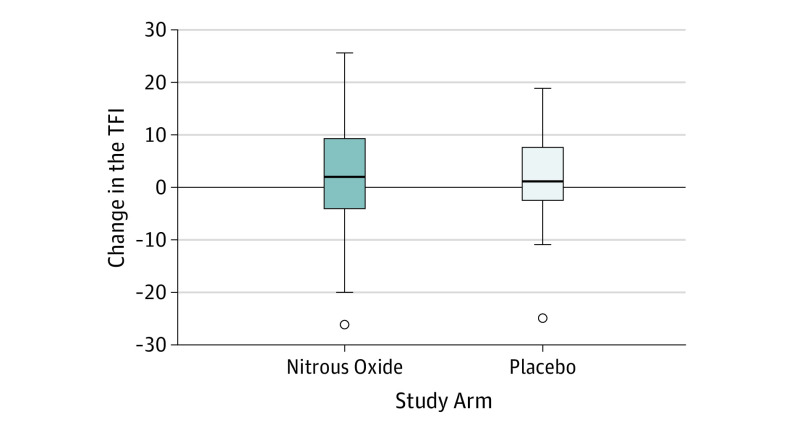
There was no significant difference in the TFI at the beginning of the second session compared with the beginning of the first session for the total cohort independent of the order of interventions (mean difference, 1.2 points; 95% CI, −4.5 to 2.1 points), nor was there a significant difference between the group receiving placebo first (mean difference, −2.9 points; 95% CI, −8.5 to 2.7 points) and the group receiving nitrous oxide first (mean difference, 0.5 points; 95% CI, −3.6 to 4.6 points). The TFI after intervention was a mean (SD) of 1.8 (8.8) points lower than before intervention in the placebo arm and a mean (SD) of 2.5 (11.0) points lower than before intervention in the nitrous oxide arm (Figure 2). Mixed model analysis revealed that the change in the TFI from before to after intervention did not differ between the groups. The within-participant mean difference in the change in the TFI among participants receiving placebo compared with nitrous oxide was −1.1 points (95% CI, −5.6 to 3.4 points). The effect size of −1.1 points and even the upper bound of the 95% CI were less than the desired clinically significant change of a 13-point reduction in the TFI. Four participants in the placebo arm and 5 participants in the nitrous oxide arm achieved a reduction in the TFI of 13 points or greater.
Figure 2. Box and Whisker Plot.
Shown is the change in the Tinnitus Functional Index (TFI) (before intervention minus after intervention) for each study arm. Error bars indicate 95% CIs.
Secondary Outcome Variables
The PGIC response was recorded for each participant 1 week after each session. Comparing the placebo and nitrous oxide arms, similar numbers of participants reported the change in their tinnitus as “No change or worsened” (24 in the placebo arm and 20 in the nitrous oxide arm) at one extreme and as “Better, definite improvement” (1 in the placebo arm and 1 in the nitrous oxide arm) at the other extreme. No participants reported the highest level of change (“Great deal better, considerable improvement”). A summary of the PGIC responses is shown in Figure 3.
Figure 3. PGIC Response Summary for Each Study Arm.
PGIC indicates Patients’ Global Impression of Change.
Most participants did not have any change in the GBS score after intervention compared with before intervention in both the placebo arm (28 participants) and the nitrous oxide arm (34 participants). Within each arm, the remaining responses were almost evenly split between an increase in score indicating worsening of bothersome tinnitus by 1 point (5 in the placebo arm and 2 in the nitrous oxide arm) and a decrease in score indicating improvement of bothersome tinnitus by 1 point (5 in the placebo arm and 2 in the nitrous oxide arm). In addition, 1 participant in the placebo arm experienced an increase of 2 points in the GBS score.
Of the 20 participants who were assigned to the group receiving placebo first, 17 correctly guessed they had received placebo during the first session. Similarly, of the 20 participants who were assigned to the group receiving nitrous oxide first, 18 participants correctly guessed they had received nitrous oxide treatment during the first session. Overall, 88% (35 of 40) of participants guessed correctly, which is significantly higher than what we would expect by chance alone if the masking was effective.
Safety
In total, 3 adverse events occurred in 3 different participants. All adverse events were temporary and fully resolved. The 3 adverse events were nausea, light-headedness, and panic attack, and all occurred during the administration of nitrous oxide. No serious adverse events were reported.
Discussion
In this intent-to-treat randomized clinical trial, we demonstrated that nitrous oxide is no more effective than placebo for the treatment of tinnitus. Because of the crossover study design, each participant received both placebo and nitrous oxide at different points during the study, allowing participants to serve as their own controls. In both the placebo and nitrous oxide arms, the observed effects on tinnitus and bothersome tinnitus can be interpreted as small as measured by the change in the TFI from before to after intervention. The change in the TFI was not statistically significantly different between the 2 intervention arms, and the upper bound of the 95% CI did not exceed the value needed to indicate a clinically meaningful effect.
Masking of participants was assessed by determining whether the percentage of participants who correctly guessed the intervention they had received was significantly greater than 50%. In this study, a large percentage of participants were able to identify the intervention. Although extensive measures were taken to preserve masking for the study team members administering surveys, masking was not achieved for most participants. Nitrous oxide is a colorless and odorless anesthetic that serves as a sedative at lower doses, as used in this study. It is likely that the dissociative effects of nitrous oxide itself were recognizable to participants even at routinely used doses. Despite ineffective masking, there was no effect of the nitrous oxide intervention on the results of the study, which strengthens the validity of the findings as a true negative finding. Furthermore, the fact that an identical dose of nitrous oxide has rapid antidepressant effects in patients with treatment-resistant major depression,15 but not in tinnitus as found herein, suggests that the underlying neurobiology between depression and tinnitus is different.
Another consideration applicable to any study assessing tinnitus is the fact that directing attention to a patient’s tinnitus in the absence of an evidence-based approach, such as cognitive behavior therapy, may in fact increase his or her perception of tinnitus severity or bother.28 Counter to this effect, the placebo effect in tinnitus investigations has been documented to be as large as 40% of patients reporting a change in tinnitus.29 The placebo effect was small in this study, which may be explained by the inadequate participant masking.
Despite its prevalence, tinnitus and its pathophysiologic causes are still not well understood. The NMDA receptors are maintained at synapses throughout the auditory pathway, including in the cochlea, dorsal cochlear nucleus, and higher brainstem structures, and have diverse roles in synaptic development, plasticity, and processing of temporal auditory information.20,30 While the NMDA receptors are likely implicated in both the generation and perpetuation of tinnitus, as well as in models of auditory plasticity, they do not represent the entire picture. Guitton and Dudai18 induced tinnitus via acute noise insult in a rat model; subsequently, at varying time points after insult, they administered an NMDA receptor antagonist, ifenprodil, to abate the development of tinnitus induced by long-term noise.
At 2 weeks after insult, they assessed whether tinnitus had developed. They concluded that tinnitus undergoes a consolidation period of several days, meaning there is a particular time window during which tinnitus could be avoided through the use of the NMDA receptor antagonists acting on cochlear receptors. No animals in the study arm that received ifenprodil in the first 4 days after insult developed tinnitus; in contrast, a significant proportion of animals in the study arm that received ifenprodil 8 days after exposure developed tinnitus. Furthermore, the authors make a comparison between tinnitus and memory insofar as the 2 processes share the property of consolidation, characterized as a time window of susceptibility to blockers of experience-dependent plasticity. Therefore, it is conceivable that application of the NMDA receptor antagonists, such as nitrous oxide, is more effective in an immediate postexposure setting in response to noise insult or high doses of salicylates.
Bothersome tinnitus is a medical condition with the potential to be debilitating. Coupled with the fact that to date the FDA has not approved any pharmacologic treatments for tinnitus and other treatment options are limited, we see that a substantial tinnitus problem exists for 50 million people in the United States, or 15% of the population. This study serves as another call in highlighting the need for further research into both the pathophysiologic causes of tinnitus and the development of effective treatment.
Limitations
The limitations of the study include a small sample size that prevented exploration of differential treatment effects in unique clinical subgroups. In addition, a large number of participants were likely unmasked to treatment assignment, and this could have affected their assessment of treatment outcome. We believe that unmasking only served to strengthen the negative findings of the study.
Conclusions
Our randomized clinical trial demonstrated that nitrous oxide, a member of the NMDA receptor antagonist class, was no more effective than placebo for the treatment of subjective, idiopathic, nonpulsatile tinnitus. To date, no FDA-approved pharmacologic treatments exist for tinnitus, which remains a prevalent and debilitating condition. Further efforts are needed to understand the pathology involved in long-standing tinnitus and to develop effective pharmaceutical agents.
Trial Protocol
eTable. Tinnitus Baseline Characteristics
References
- 1.Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-718. doi: 10.1016/j.amjmed.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 2.Pierce KJ, Kallogjeri D, Piccirillo JF, Garcia KS, Nicklaus JE, Burton H. Effects of severe bothersome tinnitus on cognitive function measured with standardized tests. J Clin Exp Neuropsychol. 2012;34(2):126-134. doi: 10.1080/13803395.2011.623120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;(9):CD005233. [DOI] [PubMed] [Google Scholar]
- 4.Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(2)(suppl):S1-S40. doi: 10.1177/0194599814545325 [DOI] [PubMed] [Google Scholar]
- 5.Hoare DJ, Edmondson-Jones M, Sereda M, Akeroyd MA, Hall D. Amplification with hearing aids for patients with tinnitus and co-existing hearing loss. Cochrane Database Syst Rev. 2014;(1):CD010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2012;11:CD006371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldo P, Doree C, Molin P, McFerran D, Cecco S. Antidepressants for patients with tinnitus. Cochrane Database Syst Rev. 2012;(9):CD003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson RM, Brummett R, Schleuning A. Use of alprazolam for relief of tinnitus. A double-blind study. Arch Otolaryngol Head Neck Surg. 1993;119(8):842-845. doi: 10.1001/archotol.1993.01880200042006 [DOI] [PubMed] [Google Scholar]
- 9.Jalali MM, Kousha A, Naghavi SE, Soleimani R, Banan R. The effects of alprazolam on tinnitus: a cross-over randomized clinical trial. Med Sci Monit. 2009;15(11):PI55-PI60. [PubMed] [Google Scholar]
- 10.Hoekstra CE, Rynja SP, van Zanten GA, Rovers MM. Anticonvulsants for tinnitus. Cochrane Database Syst Rev. 2011;(7):CD007960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Person OC, Puga ME, da Silva EM, Torloni MR. Zinc supplementation for tinnitus. Cochrane Database Syst Rev. 2016;11:CD009832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;(3):CD003852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, White AR, Ernst E. Efficacy of acupuncture as a treatment for tinnitus: a systematic review. Arch Otolaryngol Head Neck Surg. 2000;126(4):489-492. doi: 10.1001/archotol.126.4.489 [DOI] [PubMed] [Google Scholar]
- 14.Meng Z, Liu S, Zheng Y, Phillips JS. Repetitive transcranial magnetic stimulation for tinnitus. Cochrane Database Syst Rev. 2011;CD007946(10):CD007946. [DOI] [PubMed] [Google Scholar]
- 15.Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18. doi: 10.1016/j.biopsych.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 16.Sahley TL, Nodar RH, Musiek FE. Endogenous dynorphins: possible role in peripheral tinnitus. Int Tinnitus J. 1999;5(2):76-91. [PubMed] [Google Scholar]
- 17.Nicolas-Puel C, Faulconbridge RL, Guitton M, Puel JL, Mondain M, Uziel A. Characteristics of tinnitus and etiology of associated hearing loss: a study of 123 patients. Int Tinnitus J. 2002;8(1):37-44. [PubMed] [Google Scholar]
- 18.Guitton MJ, Dudai Y. Blockade of cochlear NMDA receptors prevents long-term tinnitus during a brief consolidation window after acoustic trauma. Neural Plast. 2007;2007:80904. doi: 10.1155/2007/80904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puel JL. Cochlear NMDA receptor blockade prevents salicylate-induced tinnitus. B-ENT. 2007;3(suppl 7):19-22. [PubMed] [Google Scholar]
- 20.Sanchez JT, Ghelani S, Otto-Meyer S. From development to disease: diverse functions of NMDA-type glutamate receptors in the lower auditory pathway. Neuroscience. 2015;285:248-259. doi: 10.1016/j.neuroscience.2014.11.027 [DOI] [PubMed] [Google Scholar]
- 21.Guitton MJ. Tinnitus: pathology of synaptic plasticity at the cellular and system levels. Front Syst Neurosci. 2012;6:12. doi: 10.3389/fnsys.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Society of Anesthesiologists . Standards for basic anesthetic monitoring. https://www.asahq.org/~/media/Sites/ASAHQ/Files/Public/Resources/standards-guidelines/standards-for-basic-anesthetic-monitoring.pdf. Published 2015. Accessed June 18, 2018.
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meikle MB, Henry JA, Griest SE, et al. The Tinnitus Functional Index: development of a new clinical measure for chronic, intrusive tinnitus [published correction appears in Ear Hear. 2012;33(3):443]. Ear Hear. 2012;33(2):153-176. doi: 10.1097/AUD.0b013e31822f67c0 [DOI] [PubMed] [Google Scholar]
- 25.Krings JG, Wineland A, Kallogjeri D, et al. A novel treatment for tinnitus and tinnitus-related cognitive difficulties using computer-based cognitive training and D-cycloserine. JAMA Otolaryngol Head Neck Surg. 2015;141(1):18-26. doi: 10.1001/jamaoto.2014.2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roland LT, Lenze EJ, Hardin FM, et al. Effects of mindfulness based stress reduction therapy on subjective bother and neural connectivity in chronic tinnitus. Otolaryngol Head Neck Surg. 2015;152(5):919-926. doi: 10.1177/0194599815571556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983-997. doi: 10.2307/2533558 [DOI] [PubMed] [Google Scholar]
- 28.Andersson G, Westin V. Understanding tinnitus distress: introducing the concepts of moderators and mediators. Int J Audiol. 2008;47(suppl 2):S106-S111. doi: 10.1080/14992020802301670 [DOI] [PubMed] [Google Scholar]
- 29.Duckert LG, Rees TS. Placebo effect in tinnitus management. Otolaryngol Head Neck Surg. 1984;92(6):697-699. doi: 10.1177/019459988409200618 [DOI] [PubMed] [Google Scholar]
- 30.Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206(1-2):200-226. doi: 10.1016/j.heares.2005.02.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Tinnitus Baseline Characteristics