Key Points
Question
Can Juvéderm Voluma XC hyaluronic acid filler be used safely for treatment of infraorbital hollowing, and are patients satisfied with the results?
Findings
In this retrospective observational study of 202 eyes in 101 patients, only 12 patients (12%) experienced an adverse event after treatment with Juvéderm Voluma XC, and most of these adverse events were temporary and mild in nature. Overall patient satisfaction with their results and decision to treat was 71.1% and 65.6%, respectively; these results are similar to other soft-tissue fillers currently used to treat the infraorbital area.
Meaning
Juvéderm Voluma XC can be used for treatment of infraorbital hollowing. It has a high safety profile and overall patient satisfaction profile, making it an option treating infraorbital hollowing.
Abstract
Importance
Hyaluronic acid filler can be safely used as a soft-tissue filler for correction of infraorbital hollowing. It has a high overall patient satisfaction profile among patients.
Objective
To report safety and patient satisfaction outcomes of Juvéderm Voluma XC for correction of infraorbital hollows.
Design, Setting, and Patients
This was a retrospective observational study performed at a private ambulatory facial plastic and reconstructive surgery practice. Participants were all patients 21 to 85 years old who presented to our practice and underwent Juvéderm Voluma XC treatment for correction of infraorbital hollows as a singular intervention from February 2016 to March 2017.
Interventions
Injection of Juvéderm Voluma XC to the tear trough, nasojugal fold, and/or palpebromalar groove.
Main Outcomes and Measures
Primary outcome measures include the number of recorded short- and long-term adverse events, need for additional treatment, and patient questionnaire FACE-Q scores.
Results
A total of 202 eyes were treated in 101 patients with a mean follow-up of 12 months. Patients were principally female (90 [89%]) with an average age of 54 years (range, 21-85 years). Most patients (99) had Fitzpatrick grade 1 to 4 skin type (98%) and had an infraorbital hollows score of 2 to 4 (89 [88%]). The average initial treatment volume was 1 mL with 18 patients (18%) requiring additional treatment within 3 months. The average time until additional treatment was 35.7 days. Adverse effects include bruising (in 10 [10%], contour irregularities (2 [2%]), swelling (3 [3%]), and Tyndall effect (1 [1%]). Hyaluronidase was required in 3 patients (3%). Forty-one patients completed the FACE-Q Satisfaction With Eyes survey, and 42 patients completed the FACE-Q Satisfaction With Decision survey (41% and 42%). Overall mean (SD) patient satisfaction (based on FACE-Q scores) was 71.1% (27.3) and 65.6% (31.3), respectively.
Conclusions and Relevance
Juvéderm Voluma XC has a high patient satisfaction profile and an acceptable safety profile for the correction of infraorbital hollowing.
Level of Evidence
4.
This observational study reports safety and patient satisfaction outcomes of Juvéderm Voluma XC hyaluronic acid filler for correction of infraorbital hollows.
Introduction
The eyes represent the focal point of the face. Their central position, expressivity, and color contrast draw our attention immediately. They are also one of the first areas of the face to show aging owing to their sensitive, thin periorbital skin.1 Even minor changes in structure and volume are readily apparent, which can alter the perceived emotions and health of a patient.
Traditionally, facial rejuvenation was based on the theory that excess tissue is the cause of facial aging. Thus, surgical excision and “lifting” procedures were the mainstay of treatment. Recently, however, there has been an increased interest in volume loss as the culprit of facial aging. The advent of synthetic fillers, in addition to autologous fat transfer, have provided a safe and efficacious way of treating volume loss.2 The periorbital region, however, presents considerable challenges for volume replacement. Anatomically, this region is challenging for the injector given its thin skin, increased vascularity, and compromised lymphatic drainage system. Improper soft-tissue filling can easily lead way to contour irregularities, asymmetries, and Tyndall effect. For these reasons, periorbital volume rejuvenation should be carried out only by an experienced injector.
As of 2017, there were no US Food and Drug Administration (FDA)-approved soft-tissue fillers for the periorbital complex. Their current use is considered an off-label use.3 Many, including the lead authors (M.B.H., S.R., and E.D.B.), have reported success treating periorbital volume loss with synthetic fillers without significant adverse effects.4,5,6,7 Currently in our practice, we use Juvéderm Voluma XC (VYC-20L; Allergan Inc), a volumizing hyaluronic acid (HA) filler approved by the FDA for cheek augmentation to correct age-related volume loss in the midface in adults older than 21 years, off-label for the treatment of infraorbital hollows.
There has been an increase in literature examining volume rejuvenation of the periorbital region recently.8,9,10,11,12 Most of this literature, as well as injector preference, revolves around fillers such as Belotero or Restylane, owing to various perceived advantages.8,9,10,11,12 To our knowledge, there is no literature currently examining volume rejuvenation of the periorbital region specific to Juvéderm Voluma XC. This study sought to fill that gap in the literature. Juvéderm Voluma XC exhibits greater stability and a longer in vivo duration relative to other Juvéderm products, making it ideal for volumizing the infraorbital region through layered injection techniques.3,4
Methods
The medical records of 101 patients who underwent cosmetic injection of Juvéderm Voluma XC for correction of infraorbital hollowing as a singular intervention from February 2016 to March 2017 were reviewed. This culminated in a total of 202 injections. All procedures were performed at the Buckingham Center for Facial Plastic Surgery, a private facial plastic and reconstructive surgery practice, under the supervision of the senior author (E.D.B.). All injections were elective in nature and performed by a certified facial plastic and reconstructive surgeon or certified nurse injector. In our practice, 27-gauge (G) 1.5-inch DermaSculpt Microcannulas are used for all fillers in the infraorbital region. These cannulas are blunt, flexible, and atraumatic—excellent for use in this delicate area. First, an infraorbital nerve block consisting of lidocaine, 1%, is performed using a 27-G, 1.5-inch needle through a gingivobuccal approach. A puncture site on the anterior cheek is then made with a 26-G, 0.5-inch needle. Finally, the microcannula is inserted through the puncture site, and the filler is injected in a layered fashion in the supraperiosteal or submuscular plane until the desired correction is achieved.
Study participants include men and women ages 21 to 85 years and from all racial/ethnic backgrounds. Patients with congenital or acquired deformities of the face, lipodystrophy, or poor nutritional status were excluded. No participants were coerced or influenced as part of the study, and participation was entirely voluntary. Institutional review board approval was obtained from IntegReview, and no external funding or remuneration was received as part of the study. Patients were not compensated for their participation.
Prior to any cosmetic intervention at our practice, all patients are asked to sign a photography and procedural consent form. Preprocedural photographs are taken with a Canon EOS Rebel T2i Digital SLR camera in a professional studio with standard oblique frontal flashes, controlled settings, positioning, and standardized views. For all patients, infraorbital hollowing was evaluated using the Allergan Infraorbital Hollows Scale.13 The requirement for written informed consent to access their medical record for demographic information, treatment data and complications was waived by IntegReview because all data analysis was accomplished without any identifying information. Primary end points of the study were short- and long-term adverse effects (prolonged swelling, bruising, contour irregularities, intravascular injection, and related sequelae), and posttreatment patient satisfaction questionnaire scores. FACE-Q Satisfaction With Eyes and FACE-Q Satisfaction With Decision surveys, 2 validated patient-centric questionnaires with high validity and reliability,14,15 were sent to all patients after treatment regarding their experience via e-mail through an online survey web link. The initial survey was sent in March 2017 with a reminder in April 2017 for those who had not responded. All responses were kept anonymous, making determination of exact response time not feasible. Response time could range from 1 month to 1 year. Univariate analysis was performed to analyze the data.
Results
Patients
Demographic data are presented in Table 1. Most of our patients (58 [57%]) were middle-aged women 45 to 65 years old. Most patients were of Fitzpatrick skin types 1 to 4 (99 [98%]) with a preintervention Infraorbital Hollows score of 2 to 4 (89 [88%]). Two patients had previous lower blepharoplasty with concurrent autologous fat transfer to the midface. Average follow-up time for all patients was 12 months (range, 4-17 months).
Table 1. Demographic Information for 101 Study Patients.
| Characteristic | Patients, No. |
|---|---|
| Age, mean (range), y | 54 (32-75) |
| Sex, No. (%) | |
| Male | 11 (11) |
| Female | 90 (89) |
| Fitzpatrick skin type | |
| 1 | 14 |
| 2 | 54 |
| 3 | 18 |
| 4 | 13 |
| 5 | 1 |
| 6 | 1 |
| Infraorbital hollows grade | |
| 1 | 9 |
| 2 | 30 |
| 3 | 34 |
| 4 | 25 |
| 5 | 3 |
| Follow-up, mo | 12 |
Treatment
The total mean injection volume was 1.0 mL of HA gel. The sites of injection were customized to each patient as determined by their anatomy and degree of volume loss. In our practice, we typically do not inject more than 1.0 mL of HA gel at a given sitting when treating the infraorbital groove as a means to prevent excessive swelling. There is an expectation that a certain number of patients may need additional treatment in the future, and this is explained to the patients in advance. Exact quantitative distribution of injection was limited, but, in general, a total of 0.5 mL was used for each side and disbursed evenly throughout the orbital rim and zygomaticomalar depression with some going toward the septal confluence. All injections were placed in the supraperiosteal or submuscular plane, just deep to the orbicularis oculi. The number of patients requiring a touch-up procedure and when is listed in Table 2. A touch-up was defined as additional treatment to the infraorbital area within 3 months of the initial treatment. The mean touch-up volume was 0.9 mL in total (range, 0.5-1.0 mL). Most patients presenting for a touch-up required an additional 1 mL of Voluma, with only 3 patients requiring an additional 0.5 mL. Two patients required more than 1 touch-up. Five of 11 men (45%) required a touch-up procedure compared with 13 women (14%). Twenty-four patients pursued further treatment after 3 months.
Table 2. Treatment and Adverse Events.
| Treatment Information | No. (%) |
|---|---|
| Initial treatment volume, mL | 1 |
| Patients requiring touch-up procedures | 18 (18) |
| Time to touch-up, d | 35.7 |
| Adverse events | 12 (12) |
| Bruising | 10 (10) |
| Contour irregularities | 2 (2) |
| Tyndall effect | 1 (1) |
| Edema | 3 (3) |
| Patients requiring hyaluronidase | 3 (3) |
Safety
A total of 12 patients (12%) had adverse events related to the injection of Juvéderm Voluma XC. Of those 12 patients, 3 had more than 1 adverse event (25%). A summary of adverse events is seen in Table 2. Often some degree of bruising, swelling, or contour irregularity was evident immediately after injection. Recorded events included prolonged episodes noticed by the physician, those requiring intervention, or those where the patient found the events noticeable or objectionable.
Despite the thin skin of the periorbital region, only 1 patient had Tyndall effect, the bluish hue visible within the skin caused by too superficial placement of HA filler. Three patients developed diffuse doughy edema of the infraorbital area that seemed to spread to adjacent facial subunits or caused lymphedema. Most of these adverse events were temporary, with only 3 patients requiring hyaluronidase to reverse the injection. The typical dose was 0.05 mL per side of a 150-U/mL concentration solution.
Satisfaction
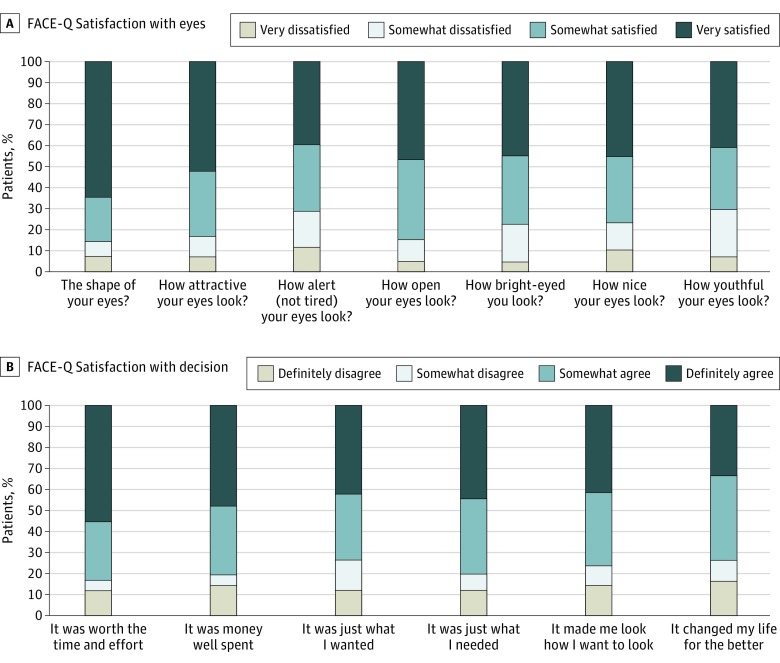
All patients were sent a patient-centric questionnaire after their treatment which included the FACE-Q Satisfaction With Eyes and FACE-Q Satisfaction With Decision surveys. Forty-one patients completed the FACE-Q Satisfaction With Eyes and 42 patients completed the FACE-Q Satisfaction With Decision surveys, 41% and 42%, respectively. The results from these surveys are charted in Figure 1. FACE-Q scores were calculated for each response (range, 0-100), with higher scores indicating greater satisfaction with appearance or treatment decision.
Figure 1. FACE-Q Satisfaction Results for 101 Patients.
For each question in the FACE-Q Satisfaction With Eyes Questionnaire, most patients answered “definitely satisfied” or “somewhat satisfied,” indicating they were happy with their treatment outcome (70%-85%). The mean (SD) overall calculated satisfaction score using the FACE-Q conversion table was 71.1% (27.3%). Looking at the FACE-Q Satisfaction With Decision survey, similar results were seen. Most patients answered “definitely agree” or “somewhat agree” for all questions, indicating satisfaction with their decision to undergo treatment (73%-85%). The overall satisfaction score for the decision to undergo treatment was 65.6% (31.3%).
Discussion
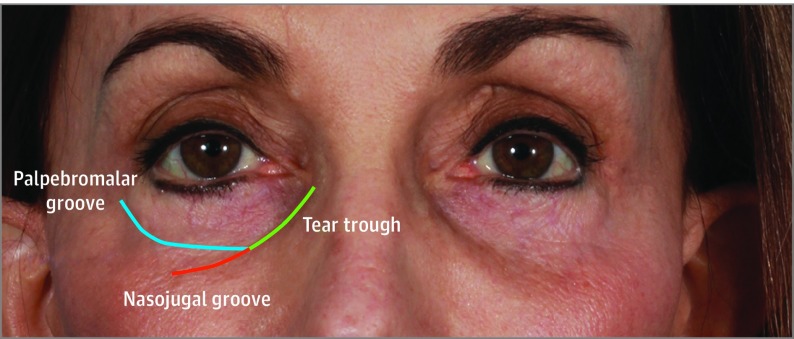
The infraorbital hollow refers to the U-shaped or curvilinear depression under the eyes and includes the tear trough, nasojugal fold, and palpebromalar groove (Figure 2). This area presents challenging anatomy for using injectable fillers, with little room for error. Overcorrection and/or the use of unsuitable fillers can lead way to complications or undesirable results. These irregularities are difficult to conceal owing to the thin skin overlying bone in this region. Thus, many physicians tend to undertreat or err on the side of undercorrection when it comes to periorbital rejuvenation. While this approach may avoid contour irregularities, edema, and bruising, it may lead to less than optimal results or a false sense of inadequacy with soft-tissue filler to this region. The ideal filler for the infraorbital area should be nonimmunogenic, biocompatible, and stable, providing good cosmetic results with a long duration of action.
Figure 2. Anatomy of Infraorbital Hollowing.
In 2016, the American Society of Plastic Surgery published data showing that soft-tissue filler injections have increased by 298% since the year 2000, with a 2% increase from the year 2015. Hyaluronic acid fillers specifically constituted over 2 million procedures in 2016, a 3% increase from 2015 (American Society of Plastic Surgeons Annual Procedural Statistics, 201616). Hyaluronic acid consists of repeating polysaccharide polymer chains with internal cross links of agents that bind the polymers together. The characteristics of the filler can be altered by varying the amount and type of cross-linking material used.
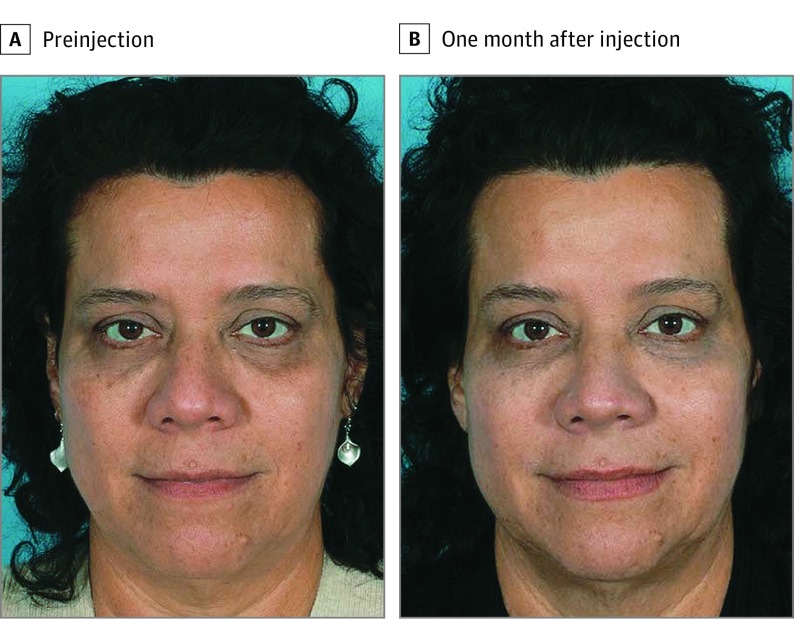
Over the past 1.5 years, our practice has been using Juvéderm Voluma XC, a volumizing HA filler approved by the FDA for correction of volume loss in the midface in adults older than 21 years, to correct infraorbital hollows in patients desiring an effective and long-lasting intervention (Figure 3). Juvéderm Voluma XC is a sterile, biodegradable, viscoelastic gel implant consisting of a 20-mg/mL mixture of low– and high–molecular-weight HA, efficiently cross-linked with Vycross technology, in combination with lidocaine, 0.3%. It is this cross-linking technology that makes Voluma more durable, resistant to degradation, and longer lasting. Studies have shown that the effects of Juvéderm Voluma XC can last for up to 2 years, making it one of the longest-lasting fillers on the market.3 In general, Juvéderm differs from other HA fillers in that it is a homogeneous gel rather than a particulate-based product. Juvéderm Voluma XC specifically exhibits greater firmness (higher G′), higher viscosity and a longer in vivo duration relative to other Juvéderm products. It has a relatively low G′ and viscosity, however, when compared with other fillers, such as Restylane, Perlane, and Radiesse. The overall low G′ and viscosity of Juvéderm Voluma XC give it a softer feel, which is especially ideal for the lower eyelids, where palpability and visibility are more of a concern, but the softer feel does not come at the expense of increased propensity of the filler to spread, a characteristic associated with those fillers with the lowest G′ and viscosity. In our opinion, this makes Juvéderm Voluma XC ideal for volumizing the infraorbital region through layered injection techniques.
Figure 3. Before and After Juvéderm Voluma XC Treatment.
The patient had 0.5 mL of Voluma injected in each lower eyelid.
An often-heard criticism of the use of other Juvéderm products (Ultra and Ultra Plus) for the correction of infraorbital hollowing is that they are too hydrophilic, which can cause excessive swelling under the eyes, or that it is more likely to cause a Tyndall effect. This has not been our experience with Juvéderm Voluma XC. Overall, the study treatment was well tolerated, with most complications and adverse events being temporary in nature. Furthermore, the number of complications and adverse events, including swelling and palpability, were similar to those in studies involving other soft-tissue fillers in the periorbital region.5,6,8,11 Although in general rare occurrences, there were no accounts of intravascular injections, injection site necrosis, blindness, or hypersensitivity reactions in our study. This may be a result of the limited sample size in the study, however, rather than a true absence of occurrences. Also, the use of 27-G DermaSculpt Microcannulas for all injections, instead of needles, may contribute to the paucity of complications. Additional long-term studies are needed to clarify further.
By design, total injection volume with Juvéderm Voluma XC was primarily 0.5 mL per side, with only 17% of patients receiving some filler to areas other than the infraorbital groove. Eighteen percent of patients required an additional touch-up procedure within 3 months, with men requiring more than women. On average, 0.9 mL of additional Voluma was required. The reason for this is unclear and may be due to the limited sample size of men in the study, anatomical differences, or initial degree of infraorbital hollowing on presentation. There was no difference in age between those patients requiring touch-ups and those who did not, indicating that age alone was not a good predictor of need for additional treatment. Three months was selected to define a touch-up procedure because it was expected that all filler from the initial injection would still be present and any short-term complication would have resolved.
As the implementation of evidence-based medicine grows in everyday practice, there is increasing pressure to adopt validated survey instruments to demonstrate patient-reported outcomes.17 FACE-Q Quality of Life and Perception questionnaires have been adopted as a validated measure of health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment.18 Previous studies investigating the use of soft-tissue fillers for volume rejuvenation of the infraorbital region have indicated a high level of patient satisfaction (50%-99%).9,19,20,21,22,23,24 Unfortunately, none of these studies used FACE-Q validated patient satisfaction surveys. They were mainly based on patients’ perception or subjective phone interviews. Our study is unique in this regard.
Overall, this study showed a high level of patient satisfaction, similar to findings of those previous studies9,19,20,21,22,23,24 in which FACE-Q surveys were not used. Most patients (71%) were very satisfied with their treatment and reported an improved aesthetic appearance of their eyes. Most agreed their decision to pursue treatment was beneficial and worth the time and effort (Figure 1). Response rates of 40.6% and 41.6% pose some uncertainty. It is not our impression that those patients who did not respond to our survey were unhappy or had a poor result, but they represent a potential source of bias. Conversely, they may have all been satisfied with their result and chose not to fill out the survey.
Limitations
Potential limitations to this study include differences in follow-up among patients and the use of subjective data points. The homogeneity and sampling of our patient population also brings a potential for selection bias. While this minimizes the variables among our population, it makes it difficult to extrapolate conclusions to a larger audience. The retrospective nature of this study also brings inherent recall bias when using patient surveys for data collection. This is especially true when identifying adverse events that tend to occur in the acute phase. Respondents who were further from their treatment may not recall those events as accurately as someone with a shorter response time. Further long-term, prospective studies are necessary to control for such variables.
Conclusions
The periorbital region can be a challenging area for nonsurgical rejuvenation. Proper training, knowledge of the anatomy, injection technique, and choice of material are crucial for success. In experienced hands, Juvéderm Voluma XC can be used as an alternative to other soft-tissue fillers for treatment of infraorbital hollowing. Its overall low G′, which correlates to low elasticity and firmness, minimizes visibility in a delicate area, such as the periorbital region. However, Voluma’s higher G′, compared with other Juvéderm products, allows it to maintain its shape and makes it more resistant to spreading then those fillers on the lowest end of the G′ spectrum. It not only has an immediate early filling effect, but also long-lasting results owing to its HA cross-linking, making it an ideal filler choice. Furthermore, its high patient satisfaction profile and a similar safety profile among other soft-tissue fillers make it an excellent adjunct in the plastic surgeon’s armamentarium.
References
- 1.Bravo BS, Rocha CR, Bastos JT, Silva PM. Comprehensive treatment of periorbital region with hyaluronic acid. J Clin Aesthet Dermatol. 2015;8(6):30-35. [PMC free article] [PubMed] [Google Scholar]
- 2.Finn JC, Cox S. Fillers in the periorbital complex. Facial Plast Surg Clin North Am. 2007;15(1):123-132, viii. [DOI] [PubMed] [Google Scholar]
- 3.Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39(11):1602-1612. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan Allemann I, Baumann L. Hyaluronic acid gel (Juvéderm) preparations in the treatment of facial wrinkles and folds. Clin Interv Aging. 2008;3(4):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Few J, Cox SE, Paradkar-Mitragotri D, Murphy DK. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35(5):589-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaser DA, Kenkel JM, Paradkar-Mitragotri D, Murphy DK, Romagnano L, Drinkwater A. Duration of effect by injection volume and facial subregion for a volumizing hyaluronic acid filler in treating midface volume deficit. Dermatol Surg. 2015;41(8):942-949. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RA, Fiaschetti D. Filling the periorbital hollows with hyaluronic acid gel: initial experience with 244 injections. Ophthal Plast Reconstr Surg. 2006;22(5):335-341. [DOI] [PubMed] [Google Scholar]
- 9.Morley AM, Malhotra R. Use of hyaluronic acid filler for tear-trough rejuvenation as an alternative to lower eyelid surgery. Ophthal Plast Reconstr Surg. 2011;27(2):69-73. [DOI] [PubMed] [Google Scholar]
- 10.Sharad J. Dermal fillers for treatment of tear trough deformity: a review of anatomy, treatment techniques and their outcomes. J Cutan Aesthet Surg. 2012;5(4):229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinsapir KD, Steinsapir SM. dermal fillers for the treatment of tear trough deformity: a review of anatomy, treatment techniques, and their outcomes. Ophthal Plast Reconstr Surg. 2006;22(5):344-348.16985416 [Google Scholar]
- 12.Huber-Vorländer J, Kürten M. Correction of tear trough deformity with a cohesive polydensified matrix hyaluronic acid: a case series. Clin Cosmet Investig Dermatol. 2015;8:307-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donofrio L, Carruthers J, Hardas B, et al. Development and validation of a photonumeric scale for evaluation of infraorbital hollows. Dermatol Surg. 2016;42(suppl 1):S251-S258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klassen AF, Cano SJ, Grotting JC, et al. FACE-Q eye module for measuring patient-reported outcomes following cosmetic eye treatments. JAMA Facial Plast Surg. 2017;19(1):7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klassen AF, Cano SJ, Schwitzer JA, Scott AM, Pusic AL. FACE-Q scales for health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment: development and validation. Plast Reconstr Surg. 2015;135(2):375-386. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Plastic Surgeons Annual Procedural Statistics. 2016. https://www.plasticsurgery.org/news/plastic-surgery-statistics?sub=2016+Plastic+Surgery+Statistics. Accessed March 5, 2018.
- 17.Barone M, Cogliandro A, La Monaca G, Tambone V, Persichetti P. Cognitive investigation study of patients admitted for cosmetic surgery: information, expectations, and consent for treatment. Arch Plast Surg. 2015;42(1):46-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barone M, Cogliandro A, Cagli B, Persichetti P. FACE-Q scales for health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment: development and validation. Plast Reconstr Surg. 2015;136(2):272e-273e. [DOI] [PubMed] [Google Scholar]
- 19.Wollina U. Improvement of tear trough by monophasic hyaluronic acid and calcium hydroxylapatite. J Clin Aesthet Dermatol. 2014;7(10):38-43. [PMC free article] [PubMed] [Google Scholar]
- 20.Schierle CF, Casas LA. Nonsurgical rejuvenation of the aging face with injectable poly-L-lactic acid for restoration of soft tissue volume. Aesthet Surg J. 2011;31(1):95-109. [DOI] [PubMed] [Google Scholar]
- 21.Viana GA, Osaki MH, Cariello AJ, Damasceno RW. Treatment of tear trough deformity with hyaluronic acid gel filler [in Portuguese]. Arq Bras Oftalmol. 2011;74(1):44-47. [DOI] [PubMed] [Google Scholar]
- 22.Tung R, Ruiz de Luzuriaga AM, Park K, Sato M, Dubina M, Alam M. Brighter eyes: combined upper cheek and tear trough augmentation: a systematic approach utilizing two complementary hyaluronic acid fillers. J Drugs Dermatol. 2012;11(9):1094-1097. [PubMed] [Google Scholar]
- 23.Lim HK, Suh DH, Lee SJ, Shin MK. Rejuvenation effects of hyaluronic acid injection on nasojugal groove: prospective randomized split face clinical controlled study. J Cosmet Laser Ther. 2014;16(1):32-36. [DOI] [PubMed] [Google Scholar]
- 24.Berguiga M, Galatoire O. Tear trough rejuvenation: a safety evaluation of the treatment by a semi-cross-linked hyaluronic acid filler. Orbit. 2017;36(1):22-26. [DOI] [PubMed] [Google Scholar]