Abstract
Importance
Endoscopic surgical decompression of the supratrochlear nerve (STN) and supraorbital nerve (SON) is a new treatment for patients with frontal chronic headache who are refractory to standard treatment options.
Objective
To evaluate and compare treatment outcomes of oral medication, botulinum toxin type A (BoNT/A) injections, and endoscopic decompression surgery in frontal secondary headache attributed to STN and supraorbital SON entrapment.
Design, Setting, and Participants
Prospective cohort study of 22 patients from a single institution (Diakonessen Hospital Utrecht) with frontal headache of moderate-to-severe intensity (visual analog scale [VAS] score, 7-10), frontally located, experienced more than 15 days per month, and described as pressure or tension that intensifies with pressure on the area of STN and SON. A screening algorithm was used that included examination, questionnaire, computed tomography of the sinus, injections of local anesthetic, and BoNT/A in the corrugator muscle.
Interventions
Different oral medication therapy for headache encountered in the study cohort, as well as BoNT/A injections (15 IU) into the corrugator muscle. Surgical procedures were performed by a single surgeon using an endoscopic surgical approach to release the supraorbital ridge periosteum and to bluntly dissect the glabellar muscle group.
Main Outcomes and Measures
Headache VAS intensity after oral medication and BoNT/A injections. Additionally, early postoperative follow-up consisted of a daily headache questionnaire that was evaluated after 1 year.
Results
In total, 22 patients (mean [SD] age, 42.0 [15.3] years; 7 men and 15 women) were included in this cohort study. Oral medication therapy reduced the headache intensity significantly (mean [standard error of the mean {SEM}] VAS score, 6.45 [0.20] [95% CI, 0.34-3.02; P < .001] compared with mean [SEM] pretreatment VAS score, 8.13 [0.22]). Botulinum toxin type A decreased the mean (SEM) headache intensity VAS scores significantly as well (pretreatment, 8.1 [0.22] vs posttreatment, 2.9 [0.42]; 95% CI, 3.89-6.56; P < .001). The mean (SEM) pretreatment headache intensity VAS score (8.10 [0.22]) decreased significantly after surgery at 3 months (1.30 [0.55]; 95% CI, 5.48-8.16; P < .001) and 12 months (1.09 [0.50]; 95% CI, 5.71-8.38; P < .001). There was a significant decrease of headache intensity VAS score in the surgical group over the BoNT/A group (mean [SEM] VAS score, 2.90 [0.42]) after 3 months (mean [SEM] VAS score, 1.30 [0.55]; 95% CI, 0.25-2.93; P < .001) and 12 months (mean [SEM] VAS score, 1.09 [0.50]; 95% CI, 0.48-3.16; P < .001) after surgery.
Conclusions and Relevance
Endoscopic decompression surgery had a long-lasting successful outcome in this type of frontal secondary headache. Even though BoNT/A had a positive effect, the effect of surgery was significantly higher.
Level of Evidence
3.
This cohort study compares treatment outcomes of oral medication, botulinum toxin type A injections, and endoscopic decompression surgery in patients with frontal secondary headache attributed to supratrochlear nerve and supraorbital nerve entrapment.
Key Points
Question
Can endoscopic surgical decompression of the supratrochlear nerve and supraorbital nerve alleviate frontal chronic headache?
Findings
In this cohort study of 22 patients with chronic headaches, the headache visual analog scale intensity variables (for medication therapy, botulinum toxin injection, surgery) improved significantly when compared with baseline values. Treatment outcomes of decompression surgery were significantly higher when compared with other treatment options used in this study.
Meaning
Endoscopic decompression surgery can demonstrate a long-lasting successful outcomes in frontal secondary headache attributed to supratrochlear nerve and supraorbital nerve entrapment.
Introduction
Headaches form a heterogenic group of neurological disorders that are classified according to the International Classification of Headache Disorders 3-β (ICHD 3-β) into major groups, types, subtypes, and subforms following explicit diagnostic criteria.1
Guyuron and colleagues2 were some of the first physicians to note that some patients who underwent a brow-lift procedure for aesthetic reasons also experienced an unexpected and beneficial effect on their frontal headache. Their observations were published in a retrospective study2 describing long-term postoperative improvement of headache symptoms in 47 patients who preoperatively fulfilled criteria for migraine. Guyuron and colleagues hypothesized that the cause of frontal headache in these patients could be an entrapment of sensory nerves by the corrugator muscle, through which nerves—specifically, the supratrochlear nerve (STN) and supraorbital nerve (SON)—run in well-defined anatomical trajectories.3,4,5,6,7,8 They later coined the procedure and term “migraine surgery.”
In a recent review,7 we expressed our ideas about some different aspects of frontal headache in relation to endoscopic migraine surgery. An important factor in decompression surgery is the release of the supraorbital periosteum attachments through which STN and SON run at the level of the supraorbital ridge. The best candidates for endoscopic decompression surgery are patients with frontal chronic headaches (moderate to severe in intensity; >15 days per month; pressure or tension; intensifies with applied pressure on the area of STN and SON) that are abolished immediately after peripheral injection of local anesthetic in the area of the supraorbital ridge. With endoscopic decompression surgery and release of the periosteum we are not treating migraine, but rather a frontal secondary headache attributed to STN and SON entrapment, that in some cases mimics migraine symptomatology.
We believe that this paradigm change in the treatment of frontally localized headache will lessen some of the criticism that has been raised around migraine surgery by neurologists9 and can facilitate the proper selection of candidates for endoscopic decompression surgery. In this study, describing and evaluating our standard care for these patients, we evaluated and compared treatment outcomes of oral medication, botulinum toxin type A (BoNT/A) injections, and endoscopic decompression surgery in frontal secondary headache attributed to STN and SON entrapment.
Methods
Patient Selection and Screening Algorithm
Patients with headaches were referred to our practice for a second opinion after using different medication therapy. The reasons to seek out alternative treatment options were overall unsatisfactory medical treatment outcome, reluctance to use medication for a longer period of time, and adverse drug effects. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committees, and an oral informed consent for study participation was obtained from the patients (all recruited from Diakonessen Hospital Utrecht).
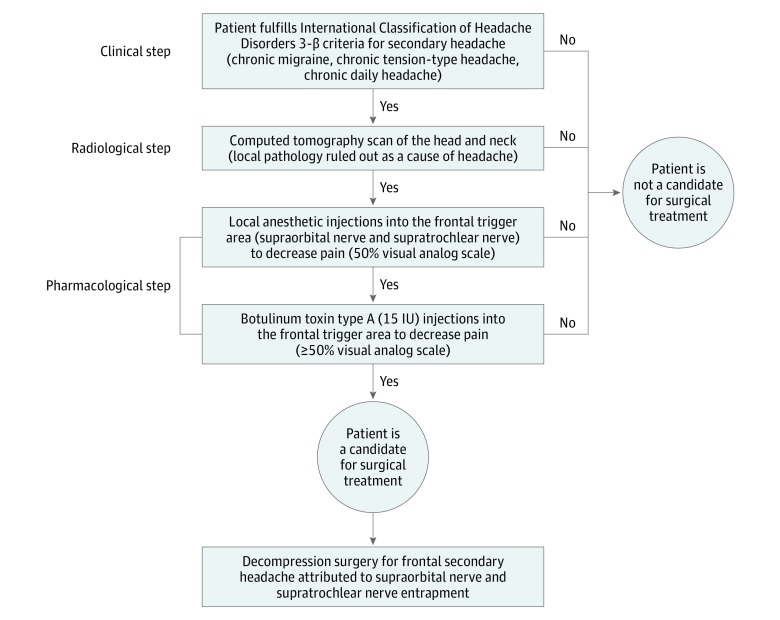
A screening algorithm was used to identify patients with frontal secondary headaches that have the highest chance for a successful surgical decompression.7 The algorithm included clinical, radiological, and pharmacological elements. In total, 22 patients were included in the study after completing each part of the algorithm in a sequence (Figure 1).
Figure 1. Screening Algorithm for Patient Selection.
Clinical Elements
Patients with frontally localized headaches were evaluated by a team member (A.R.) who is an otolaryngological surgeon with a special interest in headache disorders. Initial evaluation included clinical history and physical examination, as well as short questionnaires (scales and scores) for validation of headache symptoms and characteristics (pain duration [months, intensity] on a scale from 0 to 10; location [frontal, temporal, occipital], and accompanying symptoms [nausea, vomiting]).
The patients had frontal pain that was moderate to severe in intensity (visual analog scale [VAS] score of 7-10), frontally located (>15 days per month), and described as pressure or tension that intensified with applied pressure on the area of the STN and SON (eTable 1 in the Supplement). When adhering to the ICHD 3-β, participants headache fulfilled the criteria for frontal secondary headache that, in some cases, mimicked chronic migraines, chronic tension-type headaches, and headaches from medication overuse.1
At the beginning of the study, the participants had already been taking different pain medications on a daily basis, and the effect was evaluated by using a headache intensity VAS. The daily dosage of the drugs used varied: 4 doses of paracetamol 1000 mg; 3 doses of ibuprofen 500 mg; 3 doses of tramadol 50 mg; 2 or 3 doses of pregabalin 150 mg to 300 mg; 1 dose of amitriptyline 10 mg to 25 mg; 6 doses of oxycodone 5 mg; 2 doses of oxycontin 10 mg; 1 dose of naratriptan 5 mg; 1 dose of topiramate 100 mg; 3 doses of sumatriptan 50 mg; 6 doses of 1 tablet of Excedrin (Novartis) for 3 to 4 days (1 tablet = acetylsalicylic acid 250 mg, paracetamol 250 mg, caffeine 65 mg). Patients were asked to stop using medication before receiving injections of local anesthetic and BoNT/A, and later, nerve decompression surgery.
Radiological Elements
Computed tomographic scan of the sinus was performed to exclude secondary pathology (frontal sinusitis, malignancy, etc), and only patients without secondary pathology were included in the study.
Pharmacological Elements
Pain reduction after injection of local anesthetic, and later BoNT/A, in the corrugator muscle was regarded as evidence of a peripheral origin of headache, and importantly, a positive sign that surgery could have a successful outcome.7 In line with that, a local anesthetic lidocaine 2%, adrenaline 1:80 000 (Xylocaine; APP Pharmaceuticals LLC) was injected in the corrugator muscle around the exit points of the STN and SON at the supraorbital rim (eFigure 1A in the Supplement). Patients with pain reduction (at least half of their initial VAS score) that started immediately after lidocaine injection and that lasted up to several hours after injection were included for further study.
In these patients, BoNT/A dissolved in sodium chloride 0.9% solution (Botox; Allergan) was injected (25-gauge needle) into the corrugator muscle on both sides (5 injection points per side; each injection 3 IU) adding up to approximately 15 IU per corrugator muscle (eFigure 1B in the Supplement). Patients were asked to fill out a daily headache questionnaire (diary documenting) to evaluate postinjection headache progress (effect and duration). Patients included in the further study reported pain reduction of at least half of the initial VAS score that lasted for several weeks.
Endoscopic Decompression Surgery in the Frontal Area
All surgical procedures were performed by a single surgeon (P.J.F.M.L.) using general anesthesia. The endoscopic surgical approach was used to completely release the STN and SON by cutting the periosteum at the level of the supraorbital ridge and by bluntly dissecting the entire glabellar muscle group. Schematic presentation of the procedure with the surgical set used for endoscopic approach are shown in Figure 2.
Figure 2. Surgical Approach and Instruments.
The endoscopic decompression surgical approach was used to completely release the supratrochlear nerve and supraorbital nerve by cutting the periosteum at the level of the supraorbital ridge and by bluntly dissecting the entire glabellar muscle group. Artwork credit: Boris Filipović, MD, PhD.
Prior to the incision, the skin was marked and the forehead injected with lidocaine to minimize the bleeding in the operating field. A pretrichial W-shaped, 12-mm skin incision was made in the midline with a 15-blade scalpel all the way to the periosteum that was incised and elevated with the Obwegeser periosteum elevator (eFigure 2 in the Supplement).
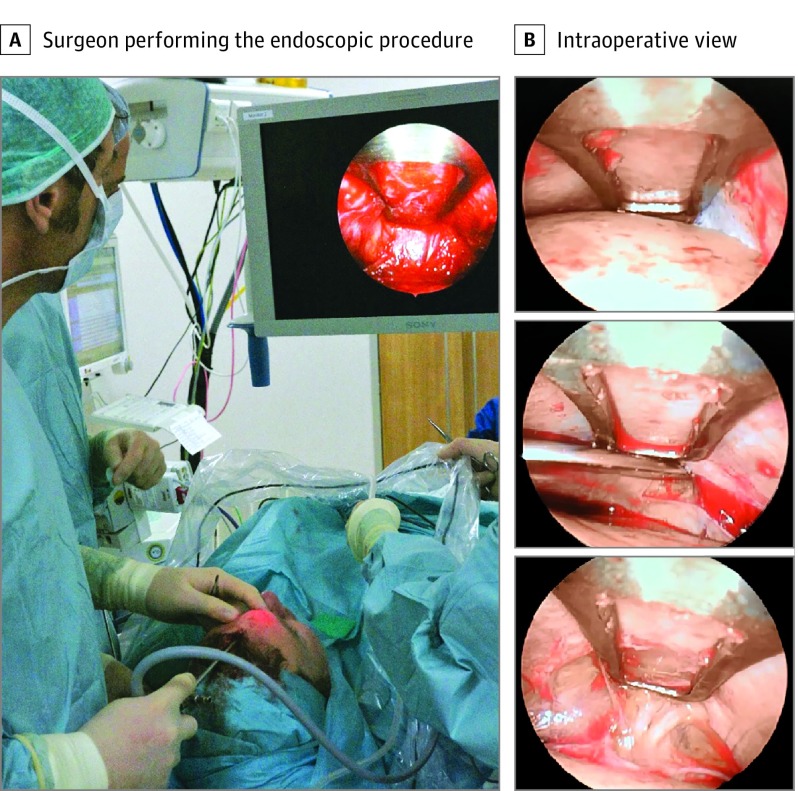
Under direct control of the endoscope, the Obwegeser periosteum elevator was used to further elevate skin, muscle, and the periosteum flap in a subperiosteal plane as the dissection was carried caudally in the medial line until the supraorbital rims were visualized, at which level the periosteum was incised. We did not remove any of the glabellar muscles. Instead, we used the elevator for blunt dissection of the muscles through the periosteal incision, until both STN and SON and accompanying vessels were well visualized. Surgery was finished with hemostasis and stapling of the skin incision. A circular bandage was placed around the forehead for 1 day. Parts of the endoscopic procedure are shown in Figure 3, as well as in the Video.
Figure 3. Clinical Photographs of the Endoscopic Decompression Procedure.
A, A clinical photograph shows the surgical field on the screen and the patient’s head position during surgery. B, The top panel shows elevation of the frontal muscle in the subperiosteal plane; the middle panel, incision of the periosteum with blunt dissection of the glabellar muscles; and the bottom panel, decompression of the supraorbital and supratrochlear nerves.
Video. The Endoscopic Decompression Procedure.
Postoperative Follow-up
Our patients were examined in the early (7 days) and late (3 and 12 months) postoperative periods to assess possible adverse effects of the surgery. They were asked to fill out a daily headache diary (4 points only) for the first 12 postoperative months to evaluate treatment outcomes. The collected 12-month diary data were entered into a headache database containing each participant’s anonymous diary. After the last study patient had finished the 12-month postoperative period, all patients were contacted by telephone (S.H.) and asked again to complete the headache questionnaire. At that moment the longest postoperative follow up was 107 months.
Statistical Analysis
The treatment outcomes of medication therapy, BoNT/A injection, and decompression surgery on headache intensity were presented as mean (standard error of the mean [SEM]) and analyzed by ANOVA (analysis of variance) test, followed by the Bonferroni post hoc test for between-group differences; P values less than .05 were considered to be significant. Differences between male and female study participants regarding the treatment outcomes were analyzed by paired t tests; P values less than .05 were considered significant. Statistical analysis was performed using SPSS version 22 (IBM Corp).
Results
Effect of Medication Therapy on Frontal Secondary Headache
In this cohort, the daily treatment with different drugs (paracetamol, ibuprofen tramadol, pregabalin, amitriptyline, oxycodone, oxycontin, naratriptan, topiramate, sumatriptan, Excedrin) reduced the frontal headache intensity significantly. The mean (SEM) VAS score with medication was 6.45 (0.20) (95% CI, 0.34-3.02; P < .001) compared with the pretreatment mean (SEM) VAS score, which was 8.13 (0.22) (eTable 2 in the Supplement) (Figure 4).
Figure 4. Treatment Effects on Reduction of Headache Intensity.
The plot points represent the mean VAS score, and the error bars represent the standard error of the mean. VAS indicates visual analog scale.
aComparison of pretreatment with medication (95% CI, 0.34-3.02).
bComparison of medication with botulism toxic type A (BoNT/A) treatment (95% CI, 2.21-4.88), results of surgery at 3 months (95% CI, 3.80-6.47), and results of surgery at 12 months (95% CI, 4.03-6.70).
cComparison of pretreatment with BoNT/A treatment (95% CI, 3.89-6.56), results of surgery at 3 months (95% CI, 5.48-8.16), and results of surgery at 12 months (95% CI, 5.71-8.38).
dComparison of BoNT/A treatment with results of surgery at 3 months (95% CI, 0.25-2.93) and results of surgery at 12 months (95% CI, 0.48-3.16).
Effect of BoNT/A Injection on Frontal Secondary Headache
Treatment with BoNT/A bilateral injections into the corrugator muscle in total dose of 15 IU/mL per side (5 injection points per side, each of 0.1 mL) statistically decreased the frontal headache intensity mean (SEM) VAS values significantly (pretreatment, 8.10 [0.22] vs posttreatment, 2.90 [0.42]; 95% CI, 3.89-6.56; P < .001 (Figure 4). The effect of BoNT/A on headache intensity was observed 3 days after peripheral injection, which is in line with the previously reported time period required for the drug to reach its maximum activity.10 The mean BoNT/A effect duration was 7.2 weeks.
Effect of Endoscopic Decompression Surgery on Frontal Secondary Headache
The mean (SEM) VAS headache intensity value measured before treatment was 8.10 (0.22) and decreased significantly at both 3 months (mean [SEM] VAS score, 1.30 [0.55]; 95% CI, 5.48-8.16; P < .001) and 12 months (mean [SEM] VAS score, 1.09 [0.50]; 95% CI, 5.71-8.38; P < .001) after surgery (Figure 4). There was no statistical difference in the surgical treatment outcome at 3 and 12 months after surgery between male and female study participants (results not shown).
A comparison of outcomes between the treatment groups demonstrated significant decrease of headache VAS intensity after both surgery at 3 months (95% CI, 3.80-6.47; P < .001) and 12 months (95% CI, 4.03-6.70; P < .001) and BoNT/A injections (95% CI, 2.21-4.88; P < .001) compared with medication therapy (Figure 4). Additionally, the study shows a significant decrease of headache intensity VAS score in the surgical group over the BoNT/A group (mean [SEM] VAS score, 2.90 [0.42]) after 3 months (mean [SEM] VAS score, 1.30 [0.55]; 95% CI, 0.25-2.93; P < .001) and 12 months (mean [SEM] VAS score, 1.09 [0.50]; 95% CI, 0.48-3.16; P < .001) after surgery. There was no significant difference in headache intensity VAS between the periods of 3 and 12 months after surgery (Figure 4).
Additionally, improvements in headache symptoms remained stable after surgery even at the longest study follow up of 107 months, while the mean (SEM) study end follow-up was 29.5 (5.8) months. The accompanying headache symptoms (photophobia, phonophobia, nausea, vomiting) were completely abolished in patients who underwent a successful surgical procedure, except in 1 case (No. 17) who continued to experience photophobia (Table).
Table. Long-term Effect of Endoscopic Decompression Surgery on Frontal Secondary Headache Intensity and Accompanying Symptoms.
| Patient No. | Pretreatment | 3 mo | 12 mo | End Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Headache, Years | VAS Score | Accompanying Symptoms | VAS Score | Accompanying Symptoms | VAS Score | Accompanying Symptoms | Period, Weeks | VAS Score | Accompanying Symptoms | |
| 1 | 10 | 9 | No | 0 | No | 0 | No | 107 | 0 | No |
| 2 | 8 | 8 | No | 0 | No | 0 | No | 95 | 0 | No |
| 3 | 13 | 7 | No | 6 | No | 6 | No | 59 | 6 | No |
| 4 | 15 | 6 | No | 0 | No | 0 | No | 48 | 0 | No |
| 5 | 1 | 8 | No | 0 | No | 2 | No | 48 | 2 | No |
| 6 | 12 | 10 | No | 0 | No | 0 | No | 32 | 0 | No |
| 7 | 7 | 9 | N, V, Photo, Phono | 0 | No | 0 | No | 31 | 0 | No |
| 8 | 1,5 | 8 | N, Photo, Phono | 0 | No | 0 | No | 29 | 0 | No |
| 9 | 3 | 8 | No | 0 | No | 0 | No | 29 | 0 | No |
| 10 | 1,5 | 8 | No | 0 | No | 0 | No | 28 | 0 | No |
| 11 | 5 | 9 | N, Photo | 0 | No | 0 | No | 25 | 0 | No |
| 12 | 13 | 10 | Photo, Phono | 9 | Photo, Phono | 9 | Photo, Phono | 23 | 9 | Photo, Phono |
| 13 | 30 | 8 | N, Photo, Phono | 2 | No | 0 | No | 21 | 0 | No |
| 14 | 5 | 8 | No | 3 | No | 1 | No | 19 | 1 | No |
| 15 | 20 | 8 | Photo | 0 | No | 0 | No | 19 | 0 | No |
| 16 | 3 | 8 | Photo, Phono | 0 | No | 0 | No | 17 | 0 | No |
| 17 | 8 | 8 | Photo | 7 | Photo | 4 | Photo | 16 | 4 | Photo |
| 18 | 2 | 9 | N, Photo | 0 | No | 0 | No | 15 | 0 | No |
| 19 | 3 | 7 | N, Photo, Phono | 2 | No | 2 | No | 15 | 2 | No |
| 20 | 2,5 | 9 | N, Photo, Phono | 0 | No | 0 | No | 14 | 0 | No |
| 21 | 1 | 6 | N | 0 | No | 0 | No | 13 | 0 | No |
| 22 | 15 | 8 | Photo | 0 | No | 0 | No | 12 | 0 | No |
Abbreviations: N, nausea; Phono, phonophobia; Photo, photophobia; V, vomiting; VAS, visual analog scale.
Adverse Effects
The 3 most common adverse effects of medication therapy in the study were nausea, dizziness, and constipation. The BoNT/A injections did not produce any adverse effects. The only observed adverse effects of decompression surgery were transient paresthesia from the hair line to the orbital rims in 2 patients (3-month duration for both) and temporary hair loss above the incision in 1 patient (12-month duration).
Discussion
Recently, a new headache subclass was identified, and a term was proposed in accordance with the ICHD 3-β: frontal secondary headache attributed to supraorbital and supratrochlear nerves entrapment.7 The exact incidence and prevalence of this type of headache is elusive. Although the headache seems to be caused by nerve entrapment (it partially fulfills diagnostic criteria for cranial neuralgia), it was classified by our team of investigators in a previous review7 as secondary headache because its pain symptomatology (described as pressure or tension) is completely different compared with the classical cranial neuralgias symptoms (shooting or jabbing pain that may feel like an electric shock) and because the pain is sometimes accompanied by symptoms of photophobia and nausea, often mimicking a primary headache (chronic migraine or chronic tension type headache).1 Different anatomical structures (muscle [corrugator muscle], periosteum [fascia bands], bone [supraorbital foramina, notch]) at the level of the supraorbital ridge have been identified as the potential source of STN and SON entrapment, either separately or combined.11 Subsequently, it was shown that the entrapment of peripheral trigeminal nerve branches induces intracranial sensitization of dural nociceptors, which is one of the main generators of pain and hallmarks in primary headaches.12
Different treatment modalities (pharmacological, BoNT/A injection, decompression surgery) are used in clinical practice for this type of frontal secondary headache; however, to our knowledge, their outcomes have never been investigated and compared in a single study.
Nowadays, pharmacological treatment is the first line in headache therapy with different classes of abortive (triptans, nonsteroidal anti-inflammatory drugs, paracetamol, etc) and preventive (anticonvulsants, calcium channel blockers, antidepressants, etc) drugs available.13,14,15 Even though, these drugs have different mechanism of action, they all aim to decrease sensitization of the trigeminal sensory system, which leads to temporary elimination of headache symptoms (pain, nausea, etc), while the cause of headache still remains untreated. Furthermore, for the drugs to be clinically effective, therapy needs to be individually modified, meaning that each patient will strive different combinations of drugs for some period of time (usually months to years).15,16
Pericranially injected BoNT/A has been approved for the treatment of chronic migraine as preventive medication.17 Its effectiveness in decreasing pain symptoms was confirmed by a meta-analysis study including 27 placebo-controlled trials (with 5313 patients).6 The positive effect of BoNT/A was demonstrated in several types of headache, including tension-type headache with pericranial tenderness and trigeminal neuralgia, migraine, and secondary headache attributed to STN and SON entrapment.18,19 Also, in our study, BoNT/A injections into the corrugator muscle were effective (Figure 4).20
Botulinum toxin type A mechanisms of action in headache disorders, including migraine, is still elusive.10 Yet, BoNT/A mechanisms of action in frontal secondary headache attributed to STN and SON entrapment can potentially be ascribed to peripheral chemodenervation of an overactive corrugator muscle, resulting in nerve muscular de-entrapment. However, we previously hypothesized that this type of headache might rather be caused by nonmuscular nerve entrapments at the supraorbital ridge (periosteum attachments and/or bony foramina) and that the mechanism of action of BoNT/A in this region would be therefore more likely be axonal transport to dural afferents where it prevents dural neurogenic inflammation by suppressing transmission of calcitonin gene-related peptides.8,12
In the last decade, several studies have reported that decompression surgery can be used as a safe treatment modality for frontal secondary headache attributed to STN and SON nerve entrapment.21,22,23,24,25 In these studies, treatment outcome was measured by the percentage of patients who experienced late postoperative reduction of headache symptoms of 50% or more on a VAS (0-10). Surgical success rates from the earliest publication to the latest published study have improved from 68.3%21 to 93.3%.25
In our cohort, the headache intensity decreased significantly at both 3 months and 12 months after endoscopic decompression surgery, and this effect was maintained in all patients who underwent a successful surgical procedure, even at the longest follow-up of 107 months (P < .001) (Figure 4). Additionally, the surgery was also effective in abolishing the accompanying primary headache symptoms (photophobia, phonophobia, nausea, vomiting). If we analyze surgical treatment outcomes based on the criteria used in previously published studies (reduction of headache symptoms of 50% or more on a VAS 0-10), we can conclude that surgery in our study was effective in 90.9% of the cases (20 of 22 patients). Furthermore, the complete elimination of headache symptoms was observed in 72.7% of the cases (16 of 22 patients), while all patients who underwent a successful surgical procedure (90.9%) ceased using pain medication on a daily basis. In our opinion, the high and long-lasting effect of decompression surgery resides in the fact that surgically we are able to permanently remove 3 possible causes of nerve entrapment: (1) corrugator muscle with blunt dissection; (2) periosteum with release of fascial bands anterior to the supraorbital ridge; and (3) bony foramina with release of the nerve from the bony canal (if necessary).
When we compare the 3 different treatment modalities for this type of frontal headache in our study, we conclude that, after proper patient selection, surgery has better and longer-lasting treatment outcomes. It was shown that invasive treatments can have a strong placebo effect as high as 35%,26,27 but this is known to wear out postoperatively. In our study, the effect of surgery was maintained in all patients who underwent a successful surgical procedure, even after a follow-up of 107 months (Table). We think that surgery is more favorable as a treatment modality because it is treating the cause of the headache (nerve entrapment) rather than just the symptoms, and we consider the release of the supraorbital periosteum attachments the most important factor in the decompression procedure.
Limitations
The main study limitation is that the number of study participants, at 22, is relatively low; another limitation is the fact that this is not a multicenter study.
Conclusions
Based on our results, we think that after proper patient selection there is a group of patients with frontal headaches who can benefit from surgery and receive a solution for their problem that is cost-effective and final. Endoscopic decompression surgery of STN and SON is not intended to treat migraine, but rather treats a form of extracranial elicited secondary headache attributed to STN and SON entrapment, that in some cases mimics migraine symptomatology.
eTable 1. Characteristics of patients included in the study
eFigure 1. Pharmacological screening of patients
eFigure 2. Decompression surgery for frontal secondary headache; steps before introduction of the endoscope
eTable 2. Effect of different medication therapy on frontal secondary headache attributed to supratrochlear and supraorbital nerve entrapment
References
- 1.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. [DOI] [PubMed] [Google Scholar]
- 2.Guyuron B, Varghai A, Michelow BJ, Thomas T, Davis J. Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg. 2000;106(2):429-434. [DOI] [PubMed] [Google Scholar]
- 3.Janis JE, Ghavami A, Lemmon JA, Leedy JE, Guyuron B. Anatomy of the corrugator supercilii muscle: part I. Corrugator topography. Plast Reconstr Surg. 2007;120(6):1647-1653. [DOI] [PubMed] [Google Scholar]
- 4.Janis JE, Ghavami A, Lemmon JA, Leedy JE, Guyuron B. The anatomy of the corrugator supercilii muscle: part II. Supraorbital nerve branching patterns. Plast Reconstr Surg. 2008;121(1):233-240. [DOI] [PubMed] [Google Scholar]
- 5.Janis JE, Hatef DA, Hagan R, et al. Anatomy of the supratrochlear nerve: implications for the surgical treatment of migraine headaches. Plast Reconstr Surg. 2013;131(4):743-750. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JL, Kuriyama A, Hayashino Y. Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: a meta-analysis. JAMA. 2012;307(16):1736-1745. [DOI] [PubMed] [Google Scholar]
- 7.Filipović B, de Ru JA, van de Langenberg R, Borggreven PA, Lacković Z, Lohuis PJFM. Decompression endoscopic surgery for frontal secondary headache attributed to supraorbital and supratrochlear nerve entrapment: a comprehensive review. Eur Arch Otorhinolaryngol. 2017;274(5):2093-2106. [DOI] [PubMed] [Google Scholar]
- 8.Lacković Z, Filipović B, Matak I, Helyes Z. Activity of botulinum toxin type A in cranial dura: implications for treatment of migraine and other headaches. Br J Pharmacol. 2016;173(2):279-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew PG. A critical evaluation of migraine trigger site deactivation surgery. Headache. 2014;54(1):142-152. [DOI] [PubMed] [Google Scholar]
- 10.Matak I, Lacković Z. Botulinum toxin A, brain and pain. Prog Neurobiol. 2014;119-120:39-59. [DOI] [PubMed] [Google Scholar]
- 11.Hagan RR, Fallucco MA, Janis JE. Supraorbital rim syndrome: definition, surgical treatment, and outcomes for frontal headache. Plast Reconstr Surg Glob Open. 2016;4(7):e795. doi: 10.1097/GOX.0000000000000802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipović B, Matak I, Lacković Z. Dural neurogenic inflammation induced by neuropathic pain is specific to cranial region. J Neural Transm (Vienna). 2014;121(5):555-563. [DOI] [PubMed] [Google Scholar]
- 13.Yancey JR, Sheridan R, Koren KG. Chronic daily headache: diagnosis and management. Am Fam Physician. 2014;89(8):642-648. [PubMed] [Google Scholar]
- 14.Olesen J, Peer Tfelt-Hansen K, Welch A, Goadsby PJ, Ramadan NM. The Headaches. 3rd ed Philadelphia, PA; Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 15.Villalón CM, Centurión D, Valdivia LF, de Vries P, Saxena PR. Migraine: pathophysiology, pharmacology, treatment and future trends. Curr Vasc Pharmacol. 2003;1(1):71-84. [DOI] [PubMed] [Google Scholar]
- 16.Omranifard M, Abdali H, Ardakani MR, Talebianfar M. A comparison of outcome of medical and surgical treatment of migraine headache: In 1 year follow-up. Adv Biomed Res. 2016;5(5):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diener HC, Dodick DW, Aurora SK, et al. PREEMPT 2 Chronic Migraine Study Group. Onabotulinumtoxin A for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804-814. [DOI] [PubMed] [Google Scholar]
- 18.Gady J, Ferneini EM. Botulinum toxin A and headache treatment. Conn Med. 2013;77(3):165-166. [PubMed] [Google Scholar]
- 19.Morra ME, Elgebaly A, Elmaraezy A, et al. Therapeutic efficacy and safety of Botulinum Toxin A Therapy in Trigeminal Neuralgia: a systematic review and meta-analysis of randomized controlled trials. J Headache Pain. 2016;17(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Ru JA, Buwalda J. Botulinum toxin A injection into corrugator muscle for frontally localised chronic daily headache or chronic tension-type headache. J Laryngol Otol. 2009;123(4):412-417. [DOI] [PubMed] [Google Scholar]
- 21.Dirnberger F, Becker K. Surgical treatment of migraine headaches by corrugator muscle resection. Plast Reconstr Surg. 2004;114(3):652-657. [DOI] [PubMed] [Google Scholar]
- 22.Bearden WH, Anderson RL. Corrugator superciliaris muscle excision for tension and migraine headaches. Ophthal Plast Reconstr Surg. 2005;21(6):418-422. [DOI] [PubMed] [Google Scholar]
- 23.de Ru JA, Schellekens PP, Lohuis PJ. Corrugator supercilii transection for headache emanating from the frontal region: a clinical evaluation of ten patients. J Neural Transm (Vienna). 2011;118(11):1571-1574. [DOI] [PubMed] [Google Scholar]
- 24.Gfrerer L, Maman DY, Tessler O, Austen WG Jr. Nonendoscopic deactivation of nerve triggers in migraine headache patients: surgical technique and outcomes. Plast Reconstr Surg. 2014;134(4):771-778. [DOI] [PubMed] [Google Scholar]
- 25.Edoardo R, Giorgia C. Frontal endoscopic myotomies for chronic headache. J Craniofac Surg. 2015;26(3):e201-e203. doi: 10.1097/SCS.0000000000001353 [DOI] [PubMed] [Google Scholar]
- 26.Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8):786-792. [DOI] [PubMed] [Google Scholar]
- 27.Freeman TB, Vawter DE, Leaverton PE, et al. Use of placebo surgery in controlled trials of a cellular-based therapy for Parkinson’s disease. N Engl J Med. 1999;341(13):988-992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of patients included in the study
eFigure 1. Pharmacological screening of patients
eFigure 2. Decompression surgery for frontal secondary headache; steps before introduction of the endoscope
eTable 2. Effect of different medication therapy on frontal secondary headache attributed to supratrochlear and supraorbital nerve entrapment