Key Points
Question
When drilling 3-dimensional (3-D)-printed temporal bones, are harmful volatile organic compounds (VOCs) produced in excess of the safety limits set by the Office of Safety and Health Administration (OSHA)?
Findings
In this occupational safety assessment, air sampling was conducted while a surgeon drilled temporal bones of 3 different commonly used materials; no harmful VOCs were detected in excess of the safety limits.
Meaning
Drilling 3-D–printed models of the 3 tested materials was safe by OHSA standards; continued monitoring and safety testing is needed as 3-D–printed technologies are introduced to our specialty.
This occupational safety assessment evaluates a surgeon’s exposure to volatile organic compounds when drilling 3 commonly used materials in 3-dimensional–printed temporal bone implants using a standard Office of Safety and Health Administration protocol.
Abstract
Importance
Three-dimensional (3-D) printing of temporal bones is becoming more prevalent. However, there has been no measure of the safety of drilling these models to date. It is unknown whether the heat and sheer from the drill may create harmful volatile organic compounds (VOCs).
Objective
To determine the level of exposure to airborne contaminants when conducting high-speed drilling on 3-D–printed models and to explore whether there is a need for exposure control measures.
Design, Setting, and Participants
In this occupational safety assessment carried out in a temporal bone laboratory, 3 individual 3-D–printed temporal bones were made using 3 different materials commonly cited in the literature: polylactic acid (PLA), photoreactive acrylic resin (PAR), and acrylonitrile butadiene styrene (ABS). Each model was drilled for 40 minutes while the surgeon wore a sampling badge. Sampling was conducted for airborne concentrations of VOCs and total particulate (TP). Monitoring for VOCs was conducted using Assay Technology 521-25 organic vapor badge worn at the surgeon’s neckline. Monitoring for TP was conducted using a polyvinyl chloride filter housed inside a cassette and coupled with an SKC AirChek 52 personal air-sampling pump. Samples were collected and analyzed in accordance with NIOSH Method 500.
Main Outcomes and Measures
Presence of VOCs and TP count exposures at Occupational Safety and Health Administration (OSHA) actionable levels.
Results
Results of the VOC sample were less than detection limits except for isopropyl alcohol at 0.24 ppm for PAR. The TP samples were less than the detection limit of 1.4 mg/m3. The results are below all applicable OSHA Action Levels and Permissible Exposure Limits for all contaminants sampled for.
Conclusions and Relevance
Drilling 3-D–printed models made from PLA, ABS, and PAR was safe by OSHA standards. Continued monitoring and safety testing are needed as 3-D–printed technologies are introduced to our specialty.
Introduction
In the past 10 years, great advances in 3-dimensional (3-D)–printing technology have been made. Increasingly, otolaryngologists are investigating new ways to use the technology, from implantable stents to surgical simulation tools.1 One such application that has been explored by several studies in the literature is 3-D printing temporal bones. A number of models have been described and evaluated with generally positive feedback.2,3,4,5,6,7,8,9 What has not been described in the literature, however, is the safety of drilling 3-D–printed temporal bones. When surgical drills are used on the printed models, they will cut the material into pieces, which may be small enough to aerosolize, and the heat from the drill may volatilize components. Much like bone dust, particles from the material can be seen spraying off of the model during drilling. This raises the question of whether there may be harmful chemicals being produced that the surgeon may inhale and whether additional protection is necessary, such as is the case for asbestos and certain chemicals. The objective of the present study was to investigate whether the surgeon may be exposed to airborne contaminants when conducting high-speed drilling on 3-D–printed models and to determine whether there is a need for exposure control measures beyond standard personal protective equipment.
Methods
Institutional review board approval is not required for occupational safety testing at our institution. Three separate 3-D–printed temporal bones were made from 3 commonly described materials for temporal bones in the literature: polylactic acid (PLA), acrylonitrile butadiene styrene (ABS), and photoacyrlic resin (PAR). The PLA model was printed using a commercially available FlashForge Creator Pro fused deposition modeling (FDM) 3-D printer (FlashForge Corp) using PLA filament made by Hatchbox (Figure 1). The ABS model was printed on the same printer using ABS filament made by Hatchbox. The PAR model (Figure 2) was printed using the commercially available Formlabs Form 2 stereolithography (SLA) 3-D printer using the Formlabs Standard White Resin (Formlabs). Safety data sheets produced by Hatchbox10 and Formlabs11 describing the respective materials were reviewed, which describe the compounds in the materials. Using this information, safety specialists from our Office of Environmental Health and Safety generated a list of harmful volatile organic compounds (VOCs) to be sampled during the testing. These compounds were chloroform, ethyl alcohol, hexane, methyl isobutyl, ketone, napthalene, tetrahydrofuran, xylenes, cyclohexanone, ethylbenzene, isopropyl alcohol, methyl methacrylate, perchloroethylene, toluene, ethyl acetate, heptane, methyl ethyl ketone, methylene chloride, styrene, and trcihloroethylene. The Office of Safety and Health Administration (OSHA) 15-minute Exposure limits of these compounds is listed in the Table.
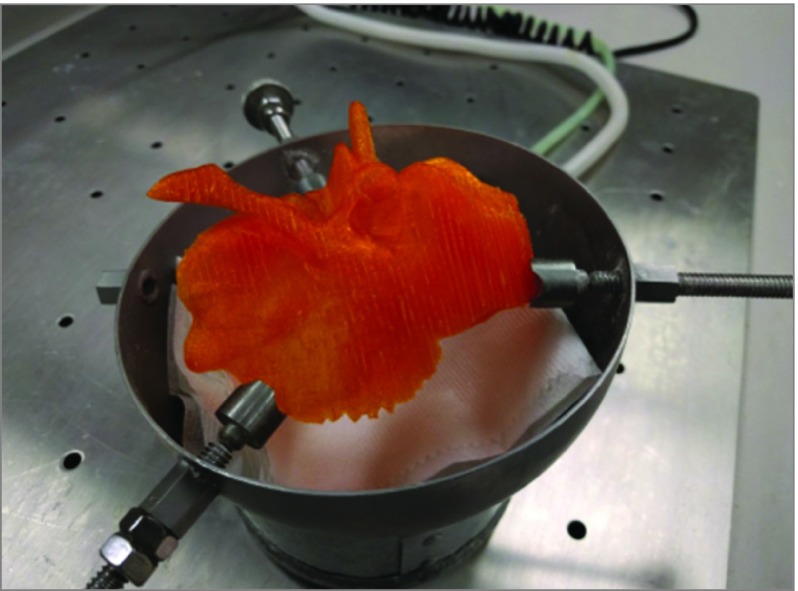
Figure 1. Temporal Bone Printed Using Polylactic Acid (PLA).
Pictured is a temporal bone made from PLA positioned in the temporal bone holder. Polylactic acid is a thermoplastic material that is melted and then rehardens during the printing process with a filament deposition printer. Acrylonitrile butadiene styrene is also thermoplastic and printed using a filament deposition printer. The underlying plate with holes is the lid to the dissection station and is not part of a downdraft ventilation system.
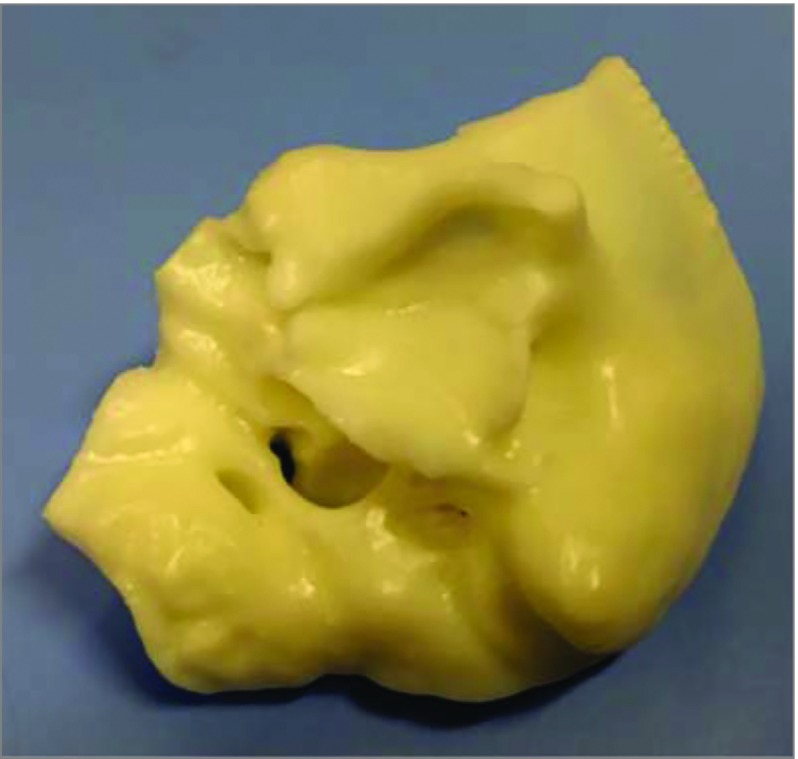
Figure 2. Temporal Bone Printed Using Photoreactive Acrylic Resin.
The pictured temporal bone is printed using a stereolithography printer that uses a laser to cure each layer.
Table. Office of Safety and Health Administration (OSHA) Exposure Limits for Volatile Organic Compoundsa .
| Compoundb | Parts per Million | |
|---|---|---|
| PELc | STELd | |
| 1,1,1-Trichloroethane | 350 | 450 |
| 1-Butanol | 100 | None |
| 4-Phenyl cyclohexane | None | None |
| Acetone | 1000 | 750 |
| Benzene | 1 | 5 |
| Butyl acetate | 200 | 200 |
| Chloroform | 10 | CL: 50 |
| Cyclohexanone | 50 | None |
| Ethyl | ||
| Acetate | 400 | None |
| Alcohol | 1000 | None |
| Ethylbenzene | 100 | 125 |
| Heptane | 500 | None |
| Hexane | 500 | None |
| Isopropyl alcohol | 400 | 500 |
| Methyl | ||
| Ethyl ketone | 200 | 300 |
| Isobutyl ketone | 100 | 75 |
| Methacrylate | 100 | None |
| Methylene chloride | 25 | 125 |
| Napthalene | 10 | 15 |
| Perchloroethylene | 100 | 200 |
| Styrene | 100 | 200 |
| Tetrahydrofuran | 200 | 250 |
| Toluene | 200 | 300 |
Abbreviations: PAR, photoreactive acrylic resin; PEL, permissible exposure limit; STEL, short-term exposure limit.
Exposure limits set by the OSHA for the volatiles measured in this study.
With few exceptions, volatile organic compounds not reported in this table were not detected. Exceptions are as follows: for total particulate count, PEL was 15 mg/m3; PAR, <1.4 mg/m3; polylactic acid, <1.1 mg/m3; and acrylonitrile butadiene styrene, <0.97 mg/m3. For isopropyl alcohol, PAR was 0.24 parts per million.
Maximum amount or concentration of a chemical to which a worker may be exposed.
Acceptable average exposure over 15 minutes.
In addition to VOC testing, total particulate (TP) count was performed to assess how much particulate material of any size 5 μm or greater was detectable at the neckline. Monitoring of VOCs was conducted using Assay Technology 521-25 organic vapor badge worn at the surgeon’s neckline. Monitoring of TP was conducted using a 37-mm 5-μm polyvinyl chloride filter housed inside a cassette and coupled with an SKC AirChek 52 personal air-sampling pump operating at a flow rate of approximately 2.0 L per minute. Prior to starting any drilling, the safety officers sampled the air in the temporal bone laboratory as a control. Surgeons wore a standard operating room gown, mask, and nonsterile nylon gloves to protect them from possible harmful exposure. The filter/pump combination was calibrated prior to and after sampling and the average flow rate was calculated.
Drilling of each bone was then separately conducted using an Anspach EMAX 2 surgical drill with Acumen cutting and diamond burrs. The drill was operated at a speed of 80 000 rotations per minute. A suction irrigator was used to closely simulate surgical conditions, as is standard when drilling in the temporal bone laboratory. There were no ventilation hoods, down-draft or negative pressure ventilation systems in the temporal bone laboratory. Throughout the drilling, VOC and TP count sampling was performed. We chose this amount of time because this is, in our experience, about the length of time that is used to drill these models and we wanted to ensure adequate sampling time to minimize sampling error. To maximize the “bone dust” created, we focused on drilling the material continuously during the 40 minutes, rather than precise anatomic drilling, with the majority of the material drilled away by the end of sampling. During the drilling, the surgeon wore the sampling badge and particulate sampler at the neckline. We chose a sampling time of 40 uninterrupted minutes, which was well over the mandated OSHA testing time of 15 minutes, to better mimic actual standard time spent in a laboratory drilling. The Office of Safety and Health Administration only requires 1 sample for safety testing. Therefore, drilling multiple temporal bones of the same compound to evaluate for reproducibility of sampling is not encouraged nor compensated for when undergoing formal OSHA safety testing as undertaken in this pilot study.
Once sampling was performed for each model, the filters were sent to laboratories accredited by the American Industrial Hygiene Association. The VOC samples were analyzed by Assay Technology using gas chromatography in accordance with OSHA Method 7. The particulate samples were analyzed using gravimetric analysis in accordance with the National Institute of Occupational Safety and Health (NIOSH) Method 0500.12
The Office of Safety and Health Administration has established a personal exposure limit of 15 mg/m3 of air for employee exposure to airborne TP based on an 8-hour time-weighted average exposure.13 The Office of Safety and Health Administration has also established an action level, defined as a level at which an intervention must occur, of 5 µg/m3 based on an 8-hour exposure and 15 1.1 µg/m3 based on a 15-minute exposure. The VOC exposure limits for the measured compounds vary from 10 to 1000 ppm depending on the compound.14
Results
For PLA, the results of the VOC testing were less than the analytical detection limits for all analytes. The TP count was less than the analytical detection limit of 1.1 µg/m3.
For ABS, the results of the VOC testing were less than the analytical detection limits for all analytes. The TP count was less than the analytical detection limit of 1.1 µg/m3. Of note, although no harmful VOCs were detected, the smell of ABS while drilling was putrid.
For PAR, the results of the VOC testing were less than the analytical detection limits for all analytes except isopropyl alcohol, detected at 0.24 ppm. This is well under the OSHA personal exposure limit of 400 ppm. The TP count was 1.4 µg/m3.
The results were below all applicable OSHA Action Levels and Permissible Exposure Limits for all contaminants sampled for.
Discussion
As 3-D printing technology continues to be explored in our specialty, we have a responsibility to our trainees, our patients, and ourselves to consider the safety of using the materials as intended. To our knowledge, there are no other studies looking at the safety of drilling 3-D–printed models using a standard surgical drill.
In this study, we performed safety testing using standard OSHA protocol to evaluate the safety of drilling 3 commonly cited materials, PLA, ABS, and PAR.
When looking into the data available for these materials, we turned first to the data safety sheets (SDS), which manufactures are required to publish. In each case, general precautions were emphasized with little specific information on dosing or parameters. Polylactic acid is a man-made polymer made from lactic acid, a naturally organic acid produced from fermenting sugars. It is used to make biodegradable materials, bioabsorbable sutures, and bone screws.13 For PLA, users are instructed to “avoid eye contact and inhalation of dusts. Use adequate safety equipment, eg, protective clothing, eye protection glasses, heat protection gloves. In case of dust formation wear mask with particle filter. Avoid breathing dust and vapors. Dust can cause irritation of eyes, respiratory organs and skin,” in the safety data sheet. It notes that the melting temperature is 155°C and decomposition products include carbon monoxide, carbon dioxide, and hydrocarbons.
Acrylonitrile butadiene styrene is a terpolymer made by polymerizing styrene and acrylonitrile in the presence of polybutadiene. Several studies have found an association between styrene exposure and the development of cancer.15 Styrene is classified as a possible human carcinogen by the International Agency for Research on Cancer (IARC classification group 2B). The amount of styrene in ABS material for 3-D printing can vary, but for Hatchbox brand it is no more than 0.1% weight concentration. For ABS, the safety data sheet recommends a local exhaust ventilation system to “effectively remove and prevent buildup of any dusts or fumes that may be generated during handling or thermal processing.” The decomposition temperature is 250°C.
Photoreactive acrylic resin is made from a mixture of methacrylic acid esters and photoinitiator.11 For PAR, prior to printing the material, it is in liquid form, and the data sheet describes it in this phase. It is classified as a category 1 irritant and may be “irritating to eyes, respiratory system and skin,” according to the safety data sheet. The flash point temperature is 100°C and decomposition products include carbon oxides, nitrogen, and hydrocarbon fragments.
In addition to the safety data sheets, there have been studies looking at exposures with 3-D printing the materials. A study performed by Azimi et al16 looked at emissions of particles and VOCs when actively printing the materials, which involves heating the materials. They quantified emissions of ultrafine particles and VOCs from 5 filament extrusion desktop 3-D printers and 9 different filaments in a closed testing chamber that sampled for at least 2 hours. Included in this study was PLA and ABS. There was a significant difference comparing the various materials. Overall, the PLA material printed on the FlashForge was among the safest. It was a low emitter of VOCs (<40 µg per minute). The only VOCs detected were lactide and 12-Crown-4 of the many VOCs tested. Lactide is the cyclic diester of lactic acid and when polymerized forms PLA. Neither of these have evidence for being carcinogenic.13,17 There was some difference in emitted particles even between different printers with the same PLA material, with a small amount of styrene noted with Lutzbot printing of PLA.
Styrene, a possible human carcinogen as classified by the International Agency for Research on Cancer, is a known component of ABS in small quantities. In the present study, we evaluated for but did not detect styrene with any material. All ABS filaments in the study by Azimi et al16 emitted large amounts of styrene. They calculated what the concentrations of styrene would be based on their data if 1 desktop 3-D printer were operating continuously in a well-mixed 45-m3 furnished and conditioned office space. The predicted styrene concentration in this scenario, which would be 150 µg/m3, would be 20 times higher than the average concentration in commercial and residential properties in the United States. Although printing the materials is not equivalent to drilling the materials, it does again support that not all materials are created equally; some may produce harmful chemicals and safety testing is important.
In the present study, we found that for PLA, no VOCs were detected and the TP count was under the detection limit of 1.1 µg/m3. For ABS, no VOCs were detected and the TP count was under the detection limit of 1.1 µg/m3. The operating surgeon did however note a putrid odor. It is unclear what compound this represents, but likely a volatile compound that is present in a quantity under the detection limit (1.1 µg/m3). Human olfaction can be as sensitive as 0.77 parts per trillion.18
For PAR, there was 1 contaminant detected, isopropyl alcohol at 0.24 ppm, well under the OHSA limit, and the TP count was also under the OHSA limit at 1.4 µg/m3. Isopropyl alcohol is a compound commonly found in rubbing alcohol, hand sanitizers, and cleaning products. According to the National Institutes of Health toxicology sheet, which describes the inhalation limit of 400 ppm, effects if ingested or inhaled at toxic doses include drowsiness, ataxia, stupor, coma and respiratory depression, irritation of mucous membranes and eyes, gastritis, gastric hemorrhage, vomiting, pancreatitis, cold clammy skin, hypothermia, miosis, tachycardia, slow and noisy respiration.19 It has been used in emergency departments to increase nausea relief.20,21 There is inadequate evidence to classify it as a carcinogen in humans or animals.19 Isopropyl alcohol is used in the post processing of SLA printed models. In this study, models were drilled within 1 hour of postprocessing in isopropyl alcohol to maximize detection. Allowing models to fully dry (1-2 days) would likely minimize the level of residual isopropyl alcohol in the model.
All of our results are below all applicable OSHA Action Levels and Permissible Exposure Limits for all contaminants sampled for. We are relieved to find that this pilot study did not identify the release of carcinogenic or irrigating VOCs while drilling and using suction/irrigation at the levels set by OHSA. If suction/irrigation had not been used, the materials may have heated up to a greater extent and potentially released a significant amount of VOCs. Our temporal bone laboratory is not equipped with a ventilation hood or negative pressure ventilation system, and thus our results are most applicable to centers in which an active ventilation system is not installed. However, we would recommend having a good ventilation system in place in the temporal bone laboratory if using 3-D–printed materials, particularly if ABS is going to be used given the known presence of styrene, the foul odor when drilling, and the recommendations set forth by the safety data sheets. Although OSHA guidelines are set for safety based on currently available data, there may be health effects of drilling that have yet to be discovered or VOCs that have not been measured.
Limitations
This study is limited to 3 of the many materials available for 3-D printing and only 1 of the many uses being explored in otolaryngology. Moreover, these same materials when made by other manufactures may differ slightly in composition and additives. We encourage other institutions to perform safety testing and share their data. Many institutions including our own have environmental safety departments and safety officers that can be invaluable with designing and carrying out safety testing, as well as implementing any changes that need to occur based on the results.
Conclusions
Drilling 3-D–printed models made from PLA, ABS, and PAR was safe by Occupational Safety and Health Administration standards. Continued monitoring and safety testing is needed as 3-D printing technologies are introduced to our specialty.
References
- 1.Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi: 10.1177/0194599816678372 [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Reyes SA. Creation of a 3D printed temporal bone model from clinical CT data. Am J Otolaryngol. 2015;36(5):619-624. doi: 10.1016/j.amjoto.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 3.Da Cruz MJ, Francis HW. Face and content validation of a novel three-dimensional printed temporal bone for surgical skills development. J Laryngol Otol. 2015;129(suppl 3):S23-S29. doi: 10.1017/S0022215115001346 [DOI] [PubMed] [Google Scholar]
- 4.Hochman JB, Kraut J, Kazmerik K, Unger BJ. Generation of a 3D printed temporal bone model with internal fidelity and validation of the mechanical construct. Otolaryngol Head Neck Surg. 2014;150(3):448-454. doi: 10.1177/0194599813518008 [DOI] [PubMed] [Google Scholar]
- 5.Longfield EA, Brickman TM, Jeyakumar A. 3D printed pediatric temporal bone: a novel training model. Otol Neurotol. 2015;36(5):793-795. doi: 10.1097/MAO.0000000000000750 [DOI] [PubMed] [Google Scholar]
- 6.Mowry SE, Jammal H, Myer C IV, Solares CA, Weinberger P. A novel temporal bone simulation model using 3D printing techniques. Otol Neurotol. 2015;36(9):1562-1565. doi: 10.1097/MAO.0000000000000848 [DOI] [PubMed] [Google Scholar]
- 7.Roosli C, Sim JH, Möckel H, Mokosch M, Probst R. An artificial temporal bone as a training tool for cochlear implantation. Otol Neurotol. 2013;34(6):1048-1051. doi: 10.1097/MAO.0b013e31828f4907 [DOI] [PubMed] [Google Scholar]
- 8.Rose AS, Kimbell JS, Webster CE, Harrysson OL, Formeister EJ, Buchman CA. Multi-material 3D models for temporal bone surgical simulation. Ann Otol Rhinol Laryngol. 2015;124(7):528-536. doi: 10.1177/0003489415570937 [DOI] [PubMed] [Google Scholar]
- 9.Rose AS, Webster CE, Harrysson OL, Formeister EJ, Rawal RB, Iseli CE. Pre-operative simulation of pediatric mastoid surgery with 3D-printed temporal bone models. Int J Pediatr Otorhinolaryngol. 2015;79(5):740-744. doi: 10.1016/j.ijporl.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 10.Hatchbox PLA 3D Printer Filament [safety data sheet]. Pomona, CA: Hatchbox; 2016.
- 11.Photoreactive Resin for Form 1, Form 1+, Form 2 [safety data sheet]. Somerville, MA: Formlabs; 2016.
- 12.National Institute for Occupational Safety and Health (NIOSH) Particulates Not Otherwise Regulated, Total 0500. The NIOSH Manual of Analytical Methods, 4th ed. 1994. https://www.cdc.gov/niosh/docs/2003-154/method-cas0.html. Accessed July 10, 2018.
- 13.Occupational Safety and Health Administration Permissible Exposure Limits–Annotated Tables. Washington, DC: US Department of Labor. https://www.osha.gov/dsg/annotated-pels/. Accessed July 10, 2018.
- 14.Occupational Safety and Health Administration Limits for Air Contaminants. Washington, DC: US Department of Labor; 2018. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=9992. Accessed July 10, 2018.
- 15.Huff J, Infante PF. Styrene exposure and risk of cancer. Mutagenesis. 2011;26(5):583-584. doi: 10.1093/mutage/ger033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azimi P, Zhao D, Pouzet C, Crain NE, Stephens B. Emissions of ultrafine particles and volatile organic compounds from commercially available desktop three-dimensional printers with multiple filaments. Environ Sci Technol. 2016;50(3):1260-1268. doi: 10.1021/acs.est.5b04983 [DOI] [PubMed] [Google Scholar]
- 17.12-Crown-4 [safety data sheet]. St. Louis, MO: Sigma-Aldrich; 2014.
- 18.Sela L, Sobel N. Human olfaction: a constant state of change-blindness. Exp Brain Res. 2010;205(1):13-29. doi: 10.1007/s00221-010-2348-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US National Library of Medicine Isopropanol. Toxicology Data Network; 1991. https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+116. Accessed July 10, 2018.
- 20.Beadle KL, Helbling AR, Love SL, April MD, Hunter CJ. Isopropyl alcohol nasal inhalation for nausea in the emergency department: a randomized controlled trial. Ann Emerg Med. 2016;68(1):1-9e1. [DOI] [PubMed] [Google Scholar]
- 21.Merritt BA, Okyere CP, Jasinski DM. Isopropyl alcohol inhalation: alternative treatment of postoperative nausea and vomiting. Nurs Res. 2002;51(2):125-128. doi: 10.1097/00006199-200203000-00009 [DOI] [PubMed] [Google Scholar]