Key Points
Question
What is the discriminative accuracy of [18F]flortaucipir positron emission tomography (PET) for differentiating Alzheimer disease dementia from other neurodegenerative disorders?
Finding
In this multicenter cross-sectional study that included 719 participants, the use of [18F]flortaucipir PET had an estimated sensitivity of 89.9% and specificity of 90.6% for Alzheimer disease vs other neurodegenerative diseases, and outperformed established volumetric magnetic resonance imaging measures.
Meaning
Although the [18F]flortaucipir PET scan was able to discriminate Alzheimer disease from other neurodegenerative diseases, further research in clinically more representative populations is needed to understand its potential utility in patient care.
Abstract
Importance
The positron emission tomography (PET) tracer [18F]flortaucipir allows in vivo quantification of paired helical filament tau, a core neuropathological feature of Alzheimer disease (AD), but its diagnostic utility is unclear.
Objective
To examine the discriminative accuracy of [18F]flortaucipir for AD vs non-AD neurodegenerative disorders.
Design, Setting, and Participants
In this cross-sectional study, 719 participants were recruited from 3 dementia centers in South Korea, Sweden, and the United States between June 2014 and November 2017 (160 cognitively normal controls, 126 patients with mild cognitive impairment [MCI], of whom 65.9% were amyloid-β [Aβ] positive [ie, MCI due to AD], 179 patients with AD dementia, and 254 patients with various non-AD neurodegenerative disorders).
Exposures
The index test was the [18F]flortaucipir PET standardized uptake value ratio (SUVR) in 5 predefined regions of interest (ROIs). Cut points for tau positivity were determined using the mean +2 SDs observed in controls and Youden Index for the contrast AD dementia vs controls.
Main Outcomes and Measures
The reference standard was the clinical diagnosis determined at the specialized memory centers. In the primary analysis, the discriminative accuracy (ie, sensitivity and specificity) of [18F]flortaucipir was examined for AD dementia vs all non-AD neurodegenerative disorders. In secondary analyses, the area under the curve (AUC) of [18F]flortaucipir SUVR was compared with 3 established magnetic resonance imaging measures (hippocampal volumes and AD signature and whole-brain cortical thickness), and sensitivity and specificity of [18F]flortaucipir in MCI due to AD vs non-AD neurodegenerative disorders were determined.
Results
Among 719 participants, the overall mean (SD) age was 68.8 (9.2) years and 48.4% were male. The proportions of patients who were amyloid-β positive were 26.3%, 65.9%, 100%, and 23.8% among cognitively normal controls, patients with MCI, patients with AD dementia, and patients with non-AD neurodegenerative disorders, respectively. [18F]flortaucipir uptake in the medial-basal and lateral temporal cortex showed 89.9% (95% CI, 84.6%-93.9%) sensitivity and 90.6% (95% CI, 86.3%-93.9%) specificity using the threshold based on controls (SUVR, 1.34), and 96.8% (95% CI, 92.0%-99.1%) sensitivity and 87.9% (95% CI, 81.9%-92.4%) specificity using the Youden Index–derived cutoff (SUVR, 1.27) for distinguishing AD dementia from all non-AD neurodegenerative disorders. The AUCs for all 5 [18F]flortaucipir ROIs were higher (AUC range, 0.92-0.95) compared with the 3 volumetric MRI measures (AUC range, 0.63-0.75; all ROIs P < .001). Diagnostic performance of the 5 [18F]flortaucipir ROIs were lower in MCI due to AD (AUC range, 0.75-0.84).
Conclusions and Relevance
Among patients with established diagnoses at a memory disorder clinic, [18F]flortaucipir PET was able to discriminate AD from other neurodegenerative diseases. The accuracy and potential utility of this test in patient care require further research in clinically more representative populations.
This diagnostic accuracy study characterizes the ability of [18F]flortaucipir to discriminate Alzheimer from non-Alzheimer neurodegenerative disorders among patients at dementia clinics and research centers.
Introduction
Distinguishing Alzheimer disease (AD) dementia from other neurodegenerative disorders often poses a diagnostic challenge to clinicians due to substantial overlap in symptoms across etiological entities.1 To facilitate the diagnostic process, the National Institute on Aging and the Alzheimer Association (NIA-AA) proposed a revised set of criteria for AD dementia that includes the possibility to use biomarkers of amyloid-β (Aβ) and neurodegeneration to support the clinical diagnosis.2 However, Aβ pathology already starts accumulating 15 to 30 years before symptom onset. The prevalence of Aβ pathology consequently rises steeply with advancing age, which results in high rates of (comorbid) Aβ positivity in non-AD neurodegenerative disorders3 and cognitively normal elderly individuals,4 reducing the specificity of Aβ biomarkers. Furthermore, biomarkers reflecting neurodegeneration (eg, structural magnetic resonance imaging [MRI]) lack specificity and often also sensitivity for AD.5
The advent of [18F]flortaucipir, a positron emission tomography (PET) tracer (currently solely used in investigational settings) with high affinity to the aggregates of tau formed in AD,6 may resolve some of the aforementioned issues because the age-associated increase in tau aggregates in neocortex is less prevalent than that of Aβ in normal populations.7 Initial human [18F]flortaucipir PET studies have shown strong associations between regional tau and cognitive decline8 and neurodegeneration,9 and good discrimination between patients with AD and controls.10,11 However, the accuracy for distinguishing AD from non-AD neurodegenerative disorders is less clear. Some studies have shown subtle to substantial [18F]flortaucipir retention in non-AD tauopathies, including frontotemporal dementia and progressive supranuclear palsy12 or non-tau proteinopathies, such as Parkinson disease or semantic variant primary progressive aphasia,13 while others have not.14
The objectives of this multicenter study were to determine the discriminative accuracy of [18F]flortaucipir PET for AD dementia vs other neurodegenerative disorders, compare [18F]flortaucipir with established MRI markers, and examine the diagnostic performance of [18F]flortaucipir at the prodromal (mild cognitive impairment [MCI]) stage of AD.
Methods
Participants
Informed consent was obtained from all participants and local institutional review boards for human research approved this study. A convenience sample of participants covering a wide range of neurodegenerative diseases was recruited from the Memory Disorder Clinic of Gangnam Severance Hospital (Seoul, South Korea), the Swedish BioFINDER study (http://www.biofinder.se) at Lund University (Lund, Sweden), and the University of California San Francisco (UCSF, United States) Alzheimer Disease Research Center who underwent [18F]flortaucipir PET between June 2014 and November 2017. During this period, most patients visiting these clinics were invited to participate in the study. All underwent a medical history and complete neurological examination, brain MRI, and neuropsychological testing. Patients fulfilled diagnostic criteria (eTable 1 in Supplement 1) for probable AD, mild cognitive impairment (MCI, amnestic, nonamnestic, or mixed phenotype), Parkinson disease with or without cognitive impairment, progressive supranuclear palsy, behavioral variant frontotemporal dementia, dementia with Lewy bodies, corticobasal syndrome, nonfluent variant primary progressive aphasia, semantic variant primary progressive aphasia, or vascular dementia.
According to the NIA-AA criteria2,15 and a recently proposed NIA-AA research framework,16 we included only patients with Aβ-positive AD dementia and MCI (ie, MCI due to AD) in the primary analyses. This minimizes the number of patients with clinically misdiagnosed AD dementia1,17 and maximizes the number of patients with MCI at a prodromal stage of AD. Information on Aβ status (determined by PET or cerebrospinal fluid [CSF]; eTable 2 in Supplement 1) was not used to establish the clinical diagnosis, and controls and non-AD neurodegenerative conditions were included regardless of Aβ status (eFigure 1 in Supplement 1). The results of the index test ([18F]flortaucipir PET) were not available to clinicians making the diagnosis. Controls were cognitively unimpaired and had no significant neurological or psychiatric illnesses. They were a mix of research volunteers recruited through advertisements and persons visiting the memory clinic with cognitive complaints but normal performance at neuropsychological testing (ie, “subjective cognitive decline”18).19,20,21
Acquisition and Processing of PET and MRI Data
Images were acquired using previously described scanners and protocols8,19,22 (eTable 3 in Supplement 1). [18F]flortaucipir PET data were locally reconstructed into 4 × 5-minute frames for the 80- to 100-minute interval after injection. Subsequently, PET images were centrally processed at Lund University using previously reported procedures22 by analysts who were blinded to any clinical information including the clinical diagnosis. Briefly, PET images were resampled to obtain the same image size (128 × 128 × 63 matrix) and voxel dimensions (2.0 × 2.0 × 2.0 mm) across centers. These images were motion-corrected using Analysis of Functional NeuroImages’ (AFNI) 3dvolreg, time-averaged, and rigidly coregistered to the skull-stripped MRI scan. Voxelwise standardized uptake value ratio (SUVR) images were created using inferior cerebellar gray matter as the reference region.23 FreeSurfer (version 6.0, https://surfer.nmr.mgh.harvard.edu/) parcellation of the T1-weighted MRI scan was applied to the PET data transformed to participants’ native T1-space to extract mean regional SUVR values for each participant. We performed partial volume correction using the Geometric Transfer Matrix approach24 and report both uncorrected (main report) and corrected (Supplement 1) data. For voxelwise analyses, [18F]flortaucipir images were warped into Montreal Neurological Institute standard space (eTable 3 in Supplement 1).25 MRI data were centrally processed using FreeSurfer (eTable 3 in Supplement 1).22
Regions of Interest
For [18F]flortaucipir PET, we aimed to cover the full spectrum of tau aggregation (from early to later affected areas). We therefore selected 5 FreeSurfer-based volume-weighted bilateral regions of interest (ROI) previously used by several groups to quantify [18F]flortaucipir uptake: entorhinal cortex,11,19 inferior temporal cortex,11 temporal meta-ROI (bilateral entorhinal, amygdala, fusiform, inferior and middle temporal cortices; reflecting Braak stage I to IV),7,19,26 temporoparietal cortex (comprising bilateral inferior, middle and superior temporal cortices, banks of superior temporal sulcus, transverse temporal, supramarginal and inferior parietal cortices),27 and Braak stages V and VI (including widespread neocortical areas).10,19 For head-to-head comparisons with [18F]flortaucipir, we selected 3 established MRI markers: hippocampal volumes, an “AD-signature” cortical thickness composite (bilateral entorhinal cortex, inferior and middle temporal cortices, and fusiform cortex), and whole-brain cortical thickness.7,22,26
Statistical Analyses
For defining accuracy, sensitivity, specificity, and positive and negative likelihood ratios, we used 2 approaches to determine tau positivity. First, we used the mean and SD in the control group and computed thresholds for all ROIs at mean+(2 × SD). Second, we calculated the Youden Index28 (J[c] = Sensitivity[c]+Specificity[c]-1, wherein c represents any given cutoff) in 1 cohort (AD dementia vs controls) and applied the optimal threshold to the other 2 cohorts. Only 4 controls were available from UCSF; thus, only Seoul and BioFINDER control data were used to calculate a Youden Index (eTable 4 in Supplement 1). Diagnostic performance of [18F]flortaucipir was calculated for (1) AD dementia vs all non-AD neurodegenerative disorders combined, (2) AD dementia vs separate non-AD neurodegenerative disorders and controls, and (3) MCI due to AD vs the combined and separate non-AD neurodegenerative disorders and controls.
We performed sensitivity analyses for the contrast AD dementia vs all non-AD neurodegenerative disorders combined using (1) partial volume-corrected data, (2) cutoffs derived using the Youden Index for the contrast of AD dementia vs all non-AD disorders in one cohort and applied to the others, (3) cutoffs using SUVRs closest to 95% sensitivity and 95% specificity for the contrast AD dementia vs controls in 1 cohort and applied to the 2 other cohorts, and (4) patients with Aβ-negative AD dementia. To identify factors associated with tau negativity in AD dementia and tau positivity in non-AD neurodegenerative disorders, we performed bivariate binary logistic regression models with [18F]flortaucipir status (determined using the mean+[2 × SD] in controls approach) in the temporal meta-ROI as dependent variable and age, sex, apolipoprotein E (APOE) ε4 status, Aβ status (only in non-AD analyses), and Mini-Mental State Examination (MMSE) score as predictors. Additionally, we performed multivariable binary logistic regression models using observed data only and multiple imputations (with 25 multiple imputations and 40 iterations) to account for missing data. Test assumptions for these models were met, as the dependent variable is binary, observations are independent, and there is limited collinearity among independent variables.
Furthermore, we performed receiver operating characteristic analyses to generate the area under the curve (AUC) for both AD dementia and MCI due to AD against non-AD neurodegenerative disorders. We then compared the AUC of the [18F]flortaucipir ROIs with MRI measures, and assessed whether combined PET and MRI improved discriminative accuracy. Differences in AUCs were assessed using bootstrap (n = 1000) procedures. We additionally ran the Voxelstats toolbox29 to compare groups by computing voxelwise [18F]flortaucipir AUC values. To evaluate the added value of [18F]flortaucipir over Aβ status, we performed bootstrapped analyses of the specificities in non-AD neurodegenerative disorders and controls.
The significance level was set at 2-sided P < .05. We used SPSS version 21 (IBM) to determine diagnostic test measures and perform binary logistic regression models, and R version 3.3.2 (R Foundation) for multiple imputations, AUC comparisons, and bootstrapped sensitivity analyses. Caret software (http://www.nitrc.org/projects/caret/) was used for display of voxelwise [18F]flortaucipir SUVRs and AUC analyses.
Results
Participants
The study included 719 participants, including 179 with AD dementia, 254 with non-AD neurodegenerative disorder (Parkinson disease with [n = 70] or without [n = 23] cognitive impairment, progressive supranuclear palsy [n = 40], behavioral variant frontotemporal dementia [n = 33], dementia with Lewy bodies [n = 24], corticobasal syndrome [n = 23], non-fluent variant primary progressive aphasia [n = 17], semantic variant primary progressive aphasia [n = 11], vascular dementia [n = 7], multiple system atrophy [n = 3], chronic traumatic encephalopathy [n = 2], and unspecified primary progressive aphasia [n = 1]), 126 with MCI (83 [66%] with MCI due to AD), and 160 cognitively normal controls (147 research volunteers and 13 participants with subjective cognitive decline). Baseline characteristics are provided in Table 1 and eTables 5 and 6 in Supplement 1.
Table 1. Participant Characteristicsa.
| Characteristic | Mean (SD) | |||
|---|---|---|---|---|
| Cognitively Normal (n = 160) | Mild Cognitive Impairment (n = 126) | Alzheimer Disease Dementia (n = 179) | Non-Alzheimer Disease (n = 254) | |
| Age, mean (SD) [range], y | 69.1 (9.5) [41-90] | 68.7 (10.4) [32-89] | 68.8 (9.6) [44-91] | 68.7 (8.0) [37-89] |
| Male, % | 40.6 | 50.8 | 40.8 | 57.5 |
| Duration of education, y | 12.3 (4.1) | 13.1 (4.7) | 13.3 (5.1) | 13.0 (5.3) |
| MMSE scoreb | 28.5 (1.6) | 26.1 (2.8) | 20.2 (5.5) | 23.6 (6.0) |
| CDR scale | ||||
| Globalc | 0 (0.1) | 0.5 (0.2) | 1.0 (0.6) | 0.6 (0.6) |
| Sum of boxesd | 0.1 (0.3) | 1.8 (1.0) | 5.4 (3.0) | 3.3 (3.4) |
| Amyloid-β positivity, No./total No. (%) | 42/160 (26.3) | 83/126 (65.9) | 179/179 (100) | 50/210 (23.8) |
| APOE ε4 positivity, No./total No. (%) | 49/154 (31.8) | 46/108 (42.6) | 88/156 (56.4) | 43/143 (30.1) |
| Time between PET and diagnosis, d | 108 (156) | 29 (59) | 48 (76) | 47 (142) |
| No. of patients by center | ||||
| Seoul | 90 | 64 | 55 | 89 |
| BioFINDER | 66 | 29 | 52 | 73 |
| UCSF | 4 | 33 | 72 | 92 |
| [18F]flortaucipir, SUVR | ||||
| Entorhinal cortex | 1.17 (0.15) | 1.46 (0.37) | 1.73 (0.31) | 1.18 (0.20) |
| Inferior temporal cortex | 1.17 (0.10) | 1.46 (0.43) | 2.09 (0.56) | 1.23 (0.21) |
| Temporal meta-ROI | 1.16 (0.09) | 1.42 (0.36) | 1.95 (0.47) | 1.20 (0.19) |
| Temporoparietal cortex | 1.11 (0.09) | 1.33 (0.34) | 1.89 (0.53) | 1.15 (0.18) |
| Braak stage V and VI | 1.14 (0.09) | 1.29 (0.32) | 1.71 (0.42) | 1.14 (0.16) |
Abbreviations: APOE, apolipoprotein E; CDR, Clinical Dementia Rating; MMSE, Mini-Mental State Examination; PET, positron emission tomography; ROI, regions of interest; SUVR, standardized uptake value ratio; UCSF, University of California, San Francisco.
eTables 5 and 6 in Supplement 1 provide more detailed information on the non-Alzheimer disease and mild cognitive impairment due to Alzheimer disease group, and the distribution across the 3 centers, respectively.
Range: 0 to 30, lower scores indicate worse global cognition.
Range: 0 to 3, higher scores indicate more advanced disease severity.
Range: 0 to 18, higher scores indicate more advanced disease severity.
Age (F = 1.39, P = .98) and years of education (F = 1.39, P = .25) did not differ significantly by group. There were more males in the non-AD neurodegenerative disorder group compared with the AD dementia (57.5% vs 40.8%, P = .001) and control (57.5% vs 40.6%, P = .001) groups. MMSE (F = 94.78; all post-hoc pairwise comparisons: P < .001) and Clinical Dementia Rating (CDR) (F = 123.66; all post-hoc pairwise comparisons: P < .01) scores were more impaired in AD dementia (mean [SD]: MMSE, 20.2 [5.5]); CDR, 1.0 [0.6]) than in non-AD neurodegenerative disorders (MMSE: 23.6 [6.0]; CDR, 0.6 [0.6]), followed by MCI (MMSE, 26.1 [2.8]; CDR, 0.5 [0.2]) and controls (MMSE, 28.5 [1.6]; CDR, 0.0 [0.1]). Rates of Aβ positivity (AD: 100% [by design], non-AD: 23.8%, MCI: 65.9%, controls: 26.3%) and APOE ε4 positivity (AD: 56.4%, non-AD: 30.1%, MCI: 42.6%, controls: 31.8%) were in line with prevalence estimates from the literature.3,4,30 For all [18F]flortaucipir ROIs, mean SUVR was higher in the AD dementia group compared with all other groups (F range: 184.81-268.25; all post-hoc pairwise comparisons: P < .001), higher in MCI due to AD compared with non-AD neurodegenerative disorder and control groups (all post-hoc pairwise comparisons: P < .001), and did not differ significantly between non-AD neurodegenerative disorders and controls (P range, .12-.91). In 6 participants with autopsy data, the clinical diagnoses of AD dementia (n = 1) or a non-AD neurodegenerative disorder (n = 5) were neuropathologically confirmed.
There were no missing data for the index test ([18F]flortaucipir), the reference standard (diagnosis), age, or sex. Aβ status was available in 675 of 719 participants (94%), APOE ε4 status in 561 (78%), education in 682 (95%), MMSE in 660 (92%), and CDR in 696 (97%).
AD Dementia vs Non-AD Neurodegenerative Disorders
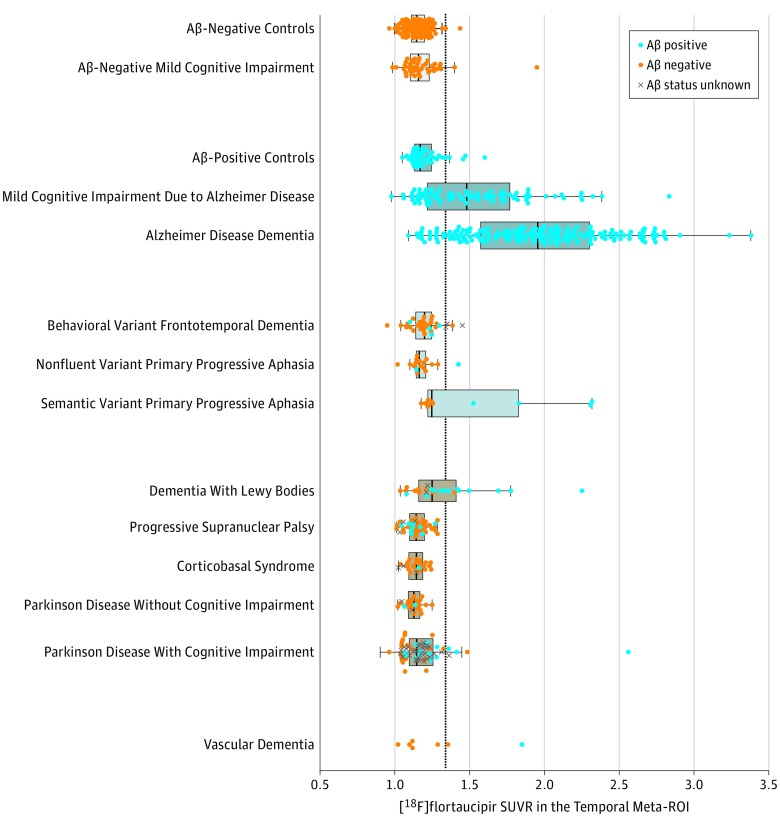
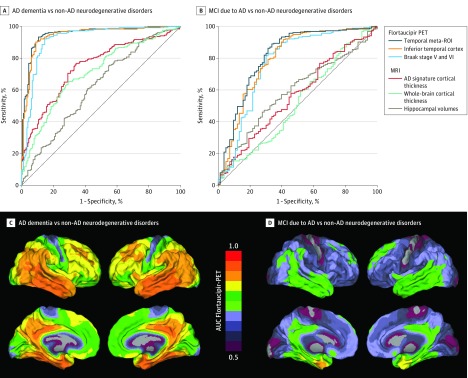
Figure 1 shows the mean temporal meta-ROI and Figure 2 shows the whole-brain voxelwise [18F]flortaucipir uptake for the various groups. eFigure 2 in Supplement 1 shows SUVR values in the other 4 ROIs. Using both cut-point approaches and across ROIs, [18F]flortaucipir showed high accuracy for discriminating AD dementia from the combined group of non-AD neurodegenerative disorders (Table 2). The temporal meta-ROI, for example, yielded 90.3% (95% CI, 87.1%-92.9%) accuracy, 89.9% (95% CI, 84.6%-93.9%) sensitivity, and 90.6% (95% CI, 86.3%-93.9%) specificity using the threshold derived in controls (SUVR, 1.34). Further, accuracy was 91.7% (95% CI, 87.9%-94.6%); sensitivity, 96.8% (95% CI, 92.0%-99.1%), and specificity, 87.9% (95% CI, 81.9%-92.4%) when using the Youden Index–derived cutoff in the Seoul cohort (SUVR, 1.27) applied to BioFINDER and UCSF cohorts, and accuracy was 87.7% (95% CI, 83.5%-91.1%); sensitivity, 92.1% (95% CI, 86.0%-96.2%); and specificity, 84.5% (95% CI, 78.4%-89.5%) using the Youden Index–derived cutoff in the BioFINDER cohort (SUVR, 1.27) applied to Seoul and UCSF cohorts. Voxelwise analyses indicated that temporoparietal regions, including inferior and middle temporal cortices, medial temporal lobe structures, and posterior cingulate yielded the highest AUC (range, 0.85-0.95) for separating AD dementia from non-AD neurodegenerative conditions (Figure 3C).
Figure 1. Mean [18F]flortaucipir Uptake Across Diagnostic Groups in the Temporal Meta-Region of Interest (ROI).
The figure shows the volume-weighted average of the temporal meta-ROI, which includes the bilateral entorhinal, amygdala, fusiform, and inferior and middle temporal cortices. The dots indicate individuals within the diagnostic groups. Box and whisker plots are only shown for groups with at least 10 participants. The box ranges from the first to the third quartile, the vertical line represents the median of the diagnostic group, and the whiskers indicate the range from the minimum to quartile 1 and from quartile 3 to the maximum, excluding outliers. Outliers were defined as standardized uptake value ratios (SUVRs) less than quartile 1 or greater than quartile 3 by more than 1.5 times the interquartile range, and were shown as separate plotted points. The dotted line represents the cutoff (SUVR: 1.34, defined using the mean +2 × SD in all controls). Aβ indicates amyloid-β.
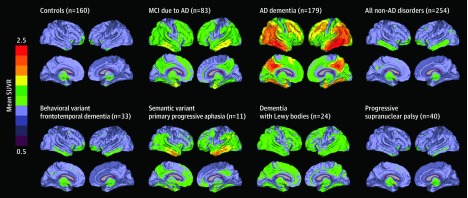
Figure 2. Mean Whole-Brain [18F]flortaucipir Uptake Across Groups.
Mean [18F]flortaucipir standardized uptake values ratios (SUVRs) across all participants within different diagnostic groups. AD indicates Alzheimer disease; MCI, mild cognitive impairment.
Table 2. Diagnostic Performance of [18F]flortaucipir PET in Distinguishing AD Dementia From Non-AD Neurodegenerative Diseasea.
| ROI (Threshold) | AUC (95% CI) | % (95% CI) | Positive Likelihood Ratio (95% CI) | Negative Likelihood Ratio (95% CI) | ||
|---|---|---|---|---|---|---|
| Accuracy | Sensitivity | Specificity | ||||
| Threshold Approach: Mean +2 × SD in All Controls (n = 160) | ||||||
| BioFINDER + UCSF + Seoul | n = 433 | n = 433 | n = 179 | n = 254 | n = 433 | (n = 433) |
| Entorhinal cortex (SUVR: 1.39) | 0.94 (0.91-0.96) | 87.8 (84.3-90.7) | 80.5 (73.9-86.0) | 92.9 (89.0-95.8) | 11.4 (7.2-17.8) | 0.21 (0.16-0.28) |
| Inferior temporal cortex (SUVR: 1.31) | 0.94 (0.92-0.97) | 90.1 (86.9-92.7) | 89.9 (84.6-93.9) | 90.2 (85.8-93.5) | 9.1 (6.3-13.3) | 0.11 (0.07-0.17) |
| Temporal meta-ROI (SUVR: 1.34)b | 0.95 (0.93-0.97) | 90.3 (87.1-92.9) | 89.9 (84.6-93.9) | 90.6 (86.3-93.9) | 9.5 (6.5-14.0) | 0.11 (0.07-0.17) |
| Temporoparietal cortex (SUVR: 1.26) | 0.93 (0.91-0.96) | 90.8 (87.6-93.3) | 86.0 (80.1-90.8) | 94.1 (90.5-96.7) | 14.6 (8.9-23.9) | 0.15 (0.10-0.21) |
| Braak stage V and VI (SUVR: 1.28)c | 0.92 (0.89-0.95) | 88.5 (85.1-91.3) | 79.9 (73.3-85.5) | 94.5 (90.9-97.0) | 14.5 (8.7-24.2) | 0.21 (0.16-0.29) |
| Threshold Approach: Youden Index Derived in Seoul Cohort (55 AD Dementia vs 90 Controls) | ||||||
| BioFINDER + UCSF | n = 289 | n = 289 | n = 124 | n = 165 | n = 289 | n = 289 |
| Entorhinal cortex (SUVR: 1.41) | 0.95 (0.93-0.98) | 89.6 (85.5-92.9) | 82.3 (74.4-88.5) | 95.2 (90.7-97.9) | 17.0 (8.6-33.5) | 0.19 (0.13-0.27) |
| Inferior temporal cortex (SUVR: 1.29) | 0.98 (0.96-0.99) | 88.6 (84.3-92.0) | 96.8 (92.0-99.1) | 82.4 (75.7-87.9) | 5.5 (4.0-7.7) | 0.04 (0.01-0.10) |
| Temporal meta-ROI (SUVR: 1.27)b | 0.98 (0.96-1.0) | 91.7 (87.9-94.6) | 96.8 (92.0-99.1) | 87.9 (81.9-92.4) | 8.0 (5.3-12.1) | 0.04 (0.01-0.10) |
| Temporoparietal cortex (SUVR: 1.27) | 0.97 (0.95-0.99) | 93.4 (89.9-96.0) | 90.3 (83.7-94.9) | 95.8 (91.5-98.3) | 21.3 (10.3-44.1) | 0.10 (0.06-0.17) |
| Braak stage V and VI (SUVR: 1.21)c | 0.96 (0.93-0.99) | 89.3 (85.1-92.6) | 93.6 (87.7-97.2) | 86.1 (79.8-91.0) | 6.7 (4.6-9.8) | 0.07 (0.04-0.15) |
| Threshold Approach: Youden Index Derived in BioFINDER Cohort (52 AD Dementia vs 66 Controls) | ||||||
| Seoul + UCSF | n = 308 | n = 308 | n = 127 | n = 181 | n = 308 | n = 308 |
| Entorhinal cortex (SUVR: 1.26) | 0.94 (0.91-0.97) | 82.1 (77.4-86.3) | 95.3 (90.0-98.3) | 72.9 (65.8-79.3) | 3.5 (2.8-4.5) | 0.06 (0.03-0.14) |
| Inferior temporal cortex (SUVR: 1.35) | 0.94 (0.91-0.97) | 89.6 (85.7-92.8) | 89.0 (82.2-93.8) | 90.1 (84.7-94.0) | 9.0 (5.8-13.9) | 0.12 (0.07-0.20) |
| Temporal meta-ROI (SUVR: 1.27)b | 0.94 (0.92-0.97) | 87.7 (83.5-91.1) | 92.1 (86.0-96.2) | 84.5 (78.4-89.5) | 6.0 (4.2-8.4) | 0.09 (0.05-0.17) |
| Temporoparietal cortex (SUVR: 1.21) | 0.93 (0.89-0.96) | 86.7 (82.4-90.3) | 89.8 (83.1-94.4) | 84.5 (78.4-89.5) | 5.8 (4.1-8.2) | 0.12 (0.07-0.20) |
| Braak stage V and VI (SUVR: 1.27)c | 0.91 (0.87-0.95) | 88.6 (84.6-92.0) | 79.5 (71.5-86.2) | 95.0 (90.8-97.7) | 16.0 (8.4-30.4) | 0.22 (0.15-0.30) |
Abbreviations: AD, Alzheimer disease; AUC, area under the curve; PET, positron emission tomography; ROI, region of interest; SUVR, standardized uptake value ratio; UCSF, University of California, San Francisco.
Diagnostic test measures were obtained using a threshold derived in all controls (n = 160) (mean +2 × SD) and applied to all participants with AD dementia (n = 179) and non-AD (n = 254) in the study, the Youden Index (55 with AD dementia vs 90 controls) in the Seoul cohort applied to participants with AD dementia (n = 124) and non-AD disorders (n = 165) from UCSF and BioFINDER cohorts, and the Youden Index (52 with AD dementia vs 66 controls) in BioFINDER cohort applied to participants with AD dementia (n = 127) and non-AD disorders (n = 181) from UCSF and Seoul cohorts.
Volume-weighted average of bilateral entorhinal, amygdala, fusiform, and inferior and middle temporal cortices.7,19,26
Based on a neuropathological staging system of neurofibrillary tangle pathology proposed by Braak and Braak.31 Stages I and II include (trans)entorhinal regions; III and IV, limbic areas; and V and VI, neocortical tangle pathology.
Figure 3. Receiver Operating Characteristic Analyses for Distinguishing Alzheimer Disease (AD) Dementia and Mild Cognitive Impairment (MCI) Due to AD From Non-AD Neurodegenerative Disorders.
Receiver operating characteristic curves of flortaucipir positron emission tomography (PET) and magnetic resonance imaging (MRI) measures for distinguishing AD dementia (A) or MCI due to AD (B) from non-AD neurodegenerative disorders. Voxelwise area under the curve (AUC) values for distinguishing AD dementia (C) or MCI due to AD (D) from non-AD neurodegenerative disorders using flortaucipir PET. ROI indicates regions of interest.
When comparing AD dementia with specific non-AD clinical syndromes, diagnostic performance within the temporal meta-ROI was consistent with results in the combined non-AD neurodegenerative disorders group, except for the lower specificity in the dementia with Lewy bodies (66.7% [95% CI, 44.7%-84.4%]) and semantic variant primary progressive aphasia (63.6% [95% CI, 30.8%-89.1%]) groups (Table 3). Across all ROIs, [18F]flortaucipir was positive in an autopsy-confirmed case with AD and negative in 4 of 5 autopsy-confirmed non-AD neurodegenerative disorders (the [18F]flortaucipir-positive non-AD case had secondary AD pathology).
Table 3. Diagnostic Performance of [18F]flortaucipir PET in Temporal Regions (Temporal Meta-ROI) for Distinguishing AD From Other Neurodegenerative Conditionsa.
| AUC (95% CI) | % (95% CI) | Positive Likelihood Ratio (95% CI) | Negative Likelihood Ratio (95% CI) | ||
|---|---|---|---|---|---|
| Accuracy | Specificity | ||||
| AD Dementia (n = 179) (Sensitivity: 89.9 [95% CI, 84.6-93.9]) | |||||
| vs All non-AD neurodegenerative disorders (n = 254) | 0.95 (0.93-0.97) | 90.3 (87.1-92.9) | 90.6 (86.3-93.9) | 9.5 (6.5-14.0) | 0.11 (0.07-0.17) |
| vs All amyloid-β–negative non-AD disorders (n = 161) | 0.97 (0.96-0.99) | 93.5 (90.4-95.9) | 97.5 (93.8-99.3) | 36.2 (13.7-95.4) | 0.10 (0.07-0.16) |
| vs All amyloid-β–positive non-AD disorders (n = 49) | 0.86 (0.79-0.92) | 85.1 (79.8-89.5) | 67.4 (52.5-80.1) | 2.8 (1.8-4.1) | 0.15 (0.09-0.24) |
| vs Frontotemporal dementia disorders (n = 62)b | 0.93 (0.89-0.97) | 88.8 (84.1-92.5) | 85.5 (74.2-93.1) | 6.2 (3.4-11.4) | 0.12 (0.08-0.18) |
| vs Movement disorder spectrum (n = 183)c | 0.96 (0.94-0.98) | 91.4 (88.1-94.1) | 92.9 (88.2-96.2) | 12.7 (7.5-21.4) | 0.11 (0.07-0.17) |
| vs Behavioral variant frontotemporal dementia (n = 33) | 0.96 (0.93-0.98) | 89.6 (84.7-93.4) | 87.9 (71.8-96.6) | 7.4 (3.0-18.6) | 0.11 (0.07-0.18) |
| vs Non-fluent variant primary progressive aphasia (n = 17) | 0.97 (0.94-0.99) | 90.3 (85.3-94.1) | 94.1 (71.3-99.9) | 15.3 (2.3-102.5) | 0.11 (0.07-0.17) |
| vs Semantic variant primary progressive aphasia (n = 11) | 0.77 (0.61-0.94) | 88.4 (83.0-92.6) | 63.6 (30.8-89.1) | 2.5 (1.1-5.4) | 0.16 (0.08-0.25) |
| vs Dementia with Lewy bodies (n = 24) | 0.88 (0.82-0.90) | 87.2 (81.8-91.5) | 66.7 (44.7-84.4) | 2.7 (1.5-4.8) | 0.15 (0.09-0.25) |
| vs Parkinson disease with cognitive impairment (n = 70)d | 0.96 (0.93-0.99) | 90.8 (86.5-94.1) | 92.9 (84.1-97.6) | 12.6 (5.4-29.4) | 0.11 (0.07-0.17) |
| vs Progressive supranuclear palsy (n = 40) | 0.98 (0.96-0.99) | 91.8 (87.3-95.1) | 100 (91.2-100) | NCe | 0.10 (0.06-0.16) |
| vs Corticobasal syndrome (n = 23) | 0.98 (0.97-1.0) | 91.1 (86.3-94.6) | 100 (85.2-100) | NCe | 0.10 (0.06-0.16) |
| vs Controls (n = 160) | 0.97 (0.96-0.99) | 92.6 (89.3-95.2) | 95.6 (91.2-98.2) | 20.6 (10.0-42.5) | 0.11 (0.07-0.16) |
| MCI Due to AD (n = 83) (Sensitivity: 61.5 [95% CI, 50.1-71.9]) | |||||
| vs All non-AD neurodegenerative disorders (n = 254) | 0.82 (0.76-0.88) | 83.4 (79.0-87.2) | 90.6 (86.3-93.9) | 6.5 (4.3-9.9) | 0.43 (0.32-0.56) |
| vs All amyloid-β–negative non-AD disorders (n = 161) | 0.85 (0.80-0.91) | 85.3 (80.2-89.5) | 97.5 (93.8-99.3) | 24.7 (9.3-66.1) | 0.40 (0.30-0.52) |
| vs All amyloid-β–positive non-AD disorders (n = 49) | 0.67 (0.57-0.76) | 63.6 (54.8-71.8) | 67.4 (52.5-80.1) | 1.88 (1.2-2.9) | 0.57 (0.41-0.80) |
| vs Frontotemporal dementia disorders (n = 62)b | 0.76 (0.68-0.84) | 71.7 (63.7-78.9) | 85.5 (74.2-93.1) | 4.2 (2.3-7.9) | 0.45 (0.34-0.60) |
| vs Movement disorder spectrum (n = 183)c | 0.84 (0.78-0.89) | 83.1 (78.0-87.4) | 92.9 (88.2-96.2) | 8.7 (5.0-15.0) | 0.42 (0.32-0.55) |
| vs Behavioral variant frontotemporal dementia (n = 33) | 0.79 (0.72-0.87) | 69.0 (59.7-77.2) | 87.9 (71.8-96.6) | 5.1 (2.0-12.9) | 0.44 (0.33-0.59) |
| vs Non-fluent variant primary progressive aphasia (n = 17) | 0.84 (0.76-0.92) | 67.0 (56.9-76.1) | 94.1 (71.3-99.9) | 10.5 (1.6-70.5) | 0.41 (0.30-0.55) |
| vs Semantic variant primary progressive aphasia (n = 11) | 0.55 (0.36-0.74) | 61.7 (51.1-71.5) | 63.6 (30.8-89.1) | 1.7 (0.8-3.8) | 0.61 (0.36-1.02) |
| vs Dementia with Lewy bodies (n = 24) | 0.68 (0.57-0.79) | 62.6 (52.7-71.8) | 66.7 (44.7-84.4) | 1.8 (1.0-3.3) | 0.58 (0.39-0.86) |
| vs Parkinson disease with cognitive impairment (n = 70)d | 0.82 (0.76-0.89) | 75.8 (68.2-82.4) | 92.9 (84.1-97.6) | 8.6 (3.6-20.4) | 0.42 (0.31-0.55) |
| vs Progressive supranuclear palsy (n = 40) | 0.87 (0.81-0.93) | 74.0 (65.3-81.5) | 100 (91.2-100) | NCe | 0.39 (0.29-0.51) |
| vs Corticobasal syndrome (n = 23) | 0.89 (0.83-0.95) | 69.8 (60.1-78.4) | 100 (85.2-100) | NCe | 0.39 (0.29-0.51) |
| vs Controls (n = 160) | 0.86 (0.80-0.91) | 84.0 (78.7-88.3) | 95.6 (91.2-98.2) | 14.0 (6.7-29.6) | 0.40 (0.31-0.53) |
Abbreviations: AD, Alzheimer disease; AUC, area under the curve; MCI, mild cognitive impairment; NC, not calculated; PET, positron emission tomography; ROI, region of interest.
Diagnostic test measures of the temporal meta-ROI were obtained using a threshold derived in all controls (mean +2 × SD) and applied to all participants with AD dementia and non-AD disorders in the study, and all participants with MCI due to AD and those with non-AD disorders in the study.
Includes behavioral variant frontotemporal dementia, nonfluent variant primary progressive aphasia, and semantic variant primary progressive aphasia.
Includes Parkinson disease with and without cognitive impairment, progressive supranuclear palsy, dementia with Lewy bodies, and corticobasal syndrome.
Parkinson disease plus objective cognitive impairment (ie, MCI or dementia).
The positive likelihood ratio in patients with progressive supranuclear palsy or corticobasal syndrome could not be calculated due to 100% specificity rates.
eTables 8 and 9 in Supplement 1 show sensitivity analyses using partial volume-corrected ROIs and the Youden Index derived in AD dementia vs non-AD neurodegenerative disorders. eTables 9 and 10 in Supplement 1 show the diagnostic performance of [18F]flortaucipir determined when defining the cutoff using the SUVR closest to 95% sensitivity or 95% specificity for the contrast AD dementia vs controls in 1 cohort and then applied to the other 2 cohorts. The discriminative accuracy was not reduced when also including patients with Aβ-negative AD dementia (eg, temporal meta-ROI, AUC Aβ-positive AD dementia: 0.95 [95% CI, 0.93-0.97], AUC Aβ-positive and Aβ-negative AD dementia combined: 0.92 [95% CI, 0.90-0.95]; P for difference = .11), and AUCs for the 5 different ROIs ranged from 0.89 to 0.92 (eTable 11 in Supplement 1). eTable 12 in Supplement 1 shows the rates of tau positivity for each group by Aβ status.
MCI Due to AD vs Non-AD Neurodegenerative Disorders
The discriminative accuracy of [18F]flortaucipir in the MCI due to AD vs non-AD neurodegenerative conditions group was lower compared with the AD dementia group (Figure 3). The temporal meta-ROI, for example, yielded 83.4% (95% CI, 79.0%-87.2%) accuracy, 61.5% (95% CI, 50.1%-71.9%) sensitivity, and 90.6% (95% CI, 86.3%-93.9%) specificity using the threshold derived in controls (SUVR, 1.34) compared with all non-AD neurodegenerative conditions combined, and the AUC was 0.82 (95% CI, 0.76-0.88). The AUC ranged between 0.75 and 0.84 across the 5 [18F]flortaucipir ROIs. Voxelwise analyses in the MCI due to AD group yielded a pattern of AUC values characterized by an anteromedial temporal lobe predominance, which was spatially more confined compared with the pattern observed in patients with AD dementia (Figure 3D). Table 3 shows the diagnostic performance for distinguishing MCI due to AD from the different non-AD neurodegenerative conditions.
Factors Contributing to Flortaucipir Status
Bivariate binary logistic regression models in patients with AD dementia showed that tau positivity in the temporal meta-ROI was associated with lower odds for age (odds ratio [OR], 0.90 [95% CI, 0.84-0.96]; P = .001) and MMSE score (OR, 0.81 [95% CI, 0.71-0.93]; P = .002), but not with sex and APOE status (P > .05; eTable 13 in Supplement 1). Patients with late-onset AD dementia (≥65 years; 17/114 [14.9%] tau negative) were more often tau negative compared with patients with early-onset AD dementia (<65 years; 1/65 [1.5%] tau negative) (X2 = 8.2, P < .01; eTable 14 in Supplement 1). In patients with a non-AD neurodegenerative condition, tau positivity was associated with higher odds for age (OR, 1.14 [95% CI, 1.07-1.21]; P < .001) and Aβ positivity (OR, 2.08 [95% CI, 1.27-3.41]; P = .004), lower odds for MMSE (OR, 0.87 [95% CI, 0.82-0.93]; P < .001) and not with sex and APOE status (P > .05). Multivariable binary logistic regression models in both the original and imputed data set revealed the same significant predictors as the bivariate models (eTable 13 in Supplement 1). Hosmer and Lemeshow goodness-of-fit tests indicated adequate model fit for both AD dementia (X2 = 5.16, P = .74) and non-AD neurodegenerative disorder (X2 = 0.71, P > .99) groups.
Flortaucipir PET vs MRI Markers
We compared the discriminative accuracy of [18F]flortaucipir vs state-of-the-art MRI measures of brain atrophy. Figure 3A shows that AUCs for [18F]flortaucipir (>0.9 for all ROIs) were higher compared with the MRI measures for distinguishing AD dementia from non-AD neurodegenerative disorders (AUC hippocampal volumes: 0.63 [95% CI, 0.57-0.68], P < .001 for difference with AUC [18F]flortaucipir temporal meta-ROI: 0.95 [95% CI, 0.93-0.97]; AD-signature thickness: 0.75 [95% CI, 0.71-0.80], P < .001; whole-brain cortical thickness: 0.71 [95% CI, 0.66-0.76], P < .001). For the contrast MCI due to AD vs non-AD neurodegenerative disorders, AUC for [18F]flortaucipir in the temporal meta-ROI (0.82 [95% CI, 0.76-0.88]) was higher compared with all MRI measures (AUC hippocampal volumes: 0.59 [95% CI, 0.52-0.66], P < .001 for difference with AUC [18F]flortaucipir temporal meta-ROI; AD-signature thickness: 0.56 [95% CI, 0.49-0.64], P < .001; whole-brain cortical thickness: 0.49 [95% CI, 0.41-0.64], P < .001) (Figure 3B).
Combined assessment of temporal meta-ROI [18F]flortaucipir and MRI measures did not improve discriminative accuracy in AD dementia or MCI due to AD groups (all P > .73; eTable 15 in Supplement 1).
Flortaucipir PET vs Aβ Status
Although PET and CSF Aβ measures had 100% sensitivity in this study based on our definition of (Aβ-positive) AD dementia according to NIA-AA criteria,2,16 there is a potential lack of specificity as the prevalence of Aβ positivity is 20% to 40% in healthy elderly and non-AD populations.3,4 Bootstrapped specificity for the contrast AD dementia vs non-AD neurodegenerative disorders was higher for [18F]flortaucipir SUVR in the temporal meta-ROI (90.4% [95 CI, 86.2%-94.3%]) than for Aβ status (76.1% [95% CI, 70.5%-81.9%]; difference, 14.3% [95% CI, 9.0%-19.5%], P for difference < .001; eTable 16 and eFigure 4 in Supplement 1). For the contrast AD dementia vs controls, the bootstrapped specificity was higher for [18F]flortaucipir SUVR in the temporal meta-ROI (95.6% [95% CI, 91.9%-98.8%]) than for Aβ status (73.8% [95% CI, 66.9%-80.6%]; difference, 21.8% [95% CI, 15.0%-28.1%], P for difference < .001; eTable 16 and eFigure 4 in Supplement 1). Bootstrapped specificity for [18F]flortaucipir vs Aβ status also differed for the other 4 ROIs, with the differences ranging between 13.8% and 17.7% in non-AD neurodegenerative disorders and 21.1% and 23.0% in controls (all P < .001; eTable 16 in Supplement 1). When these analyses were performed in cases 69 years of age and older (based on median split), the difference in specificity for [18F]flortaucipir vs Aβ status was even more pronounced in both non-AD neurodegenerative disorders (range, 17.8%-23.3% increase for [18F]flortaucipir vs Aβ status, P < .001) and controls (range, 29.5%-31.6% increase for [18F]flortaucipir vs Aβ status, P < .001; eTable 17 in Supplement 1).
Discussion
The main finding of the present study was that [18F]flortaucipir PET was able to discriminate AD from other neurodegenerative diseases among patients with established diagnoses at a memory disorder clinic. [18F]flortaucipir outperformed established structural MRI markers for AD, and showed higher specificity for the diagnosis AD dementia compared with Aβ biomarkers, resulting in lower rates of false-positives in non-AD neurodegenerative disorders. [18F]flortaucipir showed moderate discriminative accuracy for patients with MCI due to AD vs non-AD neurodegenerative disorders.
Whereas MRI, Aβ PET, and CSF are increasingly used as add-ons to clinical examination in patients with cognitive impairment,2,15 the utility of [18F]flortaucipir PET as a diagnostic biomarker has yet to be defined. Because Aβ is assumed to become abnormal approximately 15 to 30 years prior to dementia onset,4 many patients with a non-AD neurodegenerative disorder, especially at older age, will be in an Aβ latency period, wherein Aβ is detected by PET or CSF but is not the primary etiology underlying the clinical symptoms.17 Due to the limited specificity, Aβ biomarkers are therefore often used to rule out rather than rule in a diagnosis of AD.32 An important finding in this study was that [18F]flortaucipir showed high sensitivity and specificity for distinguishing AD dementia from non-AD neurodegenerative disorders. Due to the tight temporal association of AD-like tau pathology with clinical manifestation of the disease,8,33 [18F]flortaucipir may have higher discriminative accuracy in older populations compared with Aβ biomarkers. This is supported by the current study showing higher specificity for [18F]flortaucipir in discriminating AD dementia from non-AD cases and controls compared with Aβ status. Accordingly, an intended clinical use of [18F]flortaucipir PET might be to improve the diagnostic workup as an add-on test to Aβ biomarkers in patients with early-onset dementia and possibly as a triage or even replacement test in patients with late-onset dementia in whom incidental Aβ pathology is common.
The discriminative accuracy of [18F]flortaucipir dropped substantially at the prodromal stage of AD because the extent of [18F]flortaucipir uptake was less pronounced in patients with MCI due to AD compared with AD dementia (Figure 3). Also, there were only few (approximately 4%) cognitively normal controls with a positive [18F]flortaucipir scan. [18F]flortaucipir PET may thus be most valuable for differential diagnosis rather than early disease detection. In contrast, Aβ biomarkers are highly sensitive at prodromal and even preclinical stages of AD,15,34 and may therefore be most useful for early detection. Future studies will have to determine whether tau PET has added value over tau measurements in CSF, thereby also considering the higher costs and technical requirements associated with PET. An initial study pointed toward better discriminative accuracy for [18F]flortaucipir PET compared with CSF tau in mild-to-moderate AD,35 while another found equivalent performance for discriminating AD from non-AD neurodegenerative disorders.36 The superior performance of [18F]flortaucipir compared with established MRI markers suggests that [18F]flortaucipir could precede structural MRI when the differential diagnosis includes AD dementia. In case of a negative [18F]flortaucipir scan, the atrophy pattern might help determine the type of non-AD dementia.
In the AD dementia group, approximately 10% were classified as tau negative, which was associated with older age and higher MMSE scores (eTable 13 in Supplement 1). Certain elderly individuals may develop clinical AD dementia in the presence of a lower tau burden due to age-related reductions in cognitive reserve and/or the development of multiple comorbid pathologies.25,37 Higher MMSE scores (ie, better general cognitive performance) indicate that patients in less advanced stages of AD dementia may not have accumulated sufficient tau to exceed the threshold, which is in line with lower rates of tau positivity in MCI due to AD compared with AD dementia (Figure 1). Another possible explanation for the absence of [18F]flortaucipir signal is that Aβ was present as comorbid pathology in addition to a primary pathology (eg, hippocampal sclerosis, vascular lesions, or argyrophilic grain disease) that is typically not associated with AD-like tauopathy.38
Although specificity of [18F]flortaucipir was high, about 5% to 10% of patients with a non-AD neurodegenerative disorder were classified as tau positive. The rate of Aβ positivity in the whole non-AD disorder group was 23.8%, which is in line with prevalence estimates of Aβ positivity in this age range in normal populations and non-AD syndromes.3,4 A proportion of the tau-positive cases may have been clinically misdiagnosed as having a non-AD disorder, with AD as underlying pathological substrate for their symptoms. Alternatively, paired helical filament-tau may have been present as a secondary pathology whereas the clinical syndrome was driven by non-AD pathologies. In this study, the strongest predictor for tau positivity in non-AD cases was Aβ positivity (eTable 13 in Supplement 1). This was observed across all non-AD neurodegenerative disorders, but was especially pronounced in the Lewy body dementia group.
In line with neuropathological data, roughly 10% of patients with Aβ-negative Lewy body dementia were tau positive, while more than half of Aβ-positive patients were also tau positive (Figure 1; eFigure 3 in Supplement 1).39 Another explanation for tau-positive non-AD cases is that [18F]flortaucipir may not only bind to paired helical filaments of tau, but also weakly to other targets like straight or coiled filaments associated with non-AD tauopathies, TAR DNA–binding protein 43, monoamine oxidase B, or vascular lesions.6 However, the mean SUVR images indicate that, although there may occur some binding in non-AD tauopathies and other proteinopathies, the regional distribution of [18F]flortaucipir uptake at a group level clearly differentiates AD from non-AD neurodegenerative disorders (Figure 2). There were some individual exceptions, including 3 patients diagnosed as having semantic variant primary progressive aphasia (typically caused by TAR DNA–binding protein 43 type-C pathology) with strongly elevated neocortical [18F]flortaucipir uptake (Figure 1). Neuropathological examinations of such cases will enhance our understanding of the exact binding properties of [18F]flortaucipir.
Recently, an NIA-AA research framework was proposed that defines AD as a biological entity (ie, presence of Aβ plaques and tau neurofibrillary tangles) rather than a clinical syndrome.16 Accordingly, irrespective of cognitive status (ie, cognitively normal or dementia) or degree of neurodegeneration, individuals with abnormal Aβ biomarkers have “Alzheimer pathological changes,” and if these persons additionally harbor tau pathology, they have “Alzheimer disease.” Positivity on [18F]flortaucipir PET (and/or CSF phosphorylated-tau) is required to meet criteria for AD, and—given its high specificity—tau PET will consequently play a major role in the classification of participants in research studies and enrollment in clinical trials. The cutoffs derived using standard threshold approaches yielded lower sensitivity at the MCI stage of AD and only few cognitively normal individuals were considered tau positive. This might be a limitation for investigating AD in prodromal or preclinical stages, although eTable 9 in Supplement 1 indicates that the sensitivity can be improved by lowering cutoffs. Another topic for future investigation is whether and how [18F]flortaucipir PET and CSF phosphorylated-tau can be used interchangeably in this novel framework.
Limitations
This study has several limitations. First, there is a potential selection bias because participants were recruited from academic memory disorder clinics and had already established diagnoses at time of [18F]flortaucipir PET scanning. Ideally, the reference standard (diagnosis) and index test ([18F]flortaucipir) are assessed simultaneously. This caveat could potentially have resulted in an overestimation of the test sensitivity and specificity. Second, the clinical diagnosis served as reference standard, as there were only limited (n = 6 cases) autopsy data available. Future studies on clinicopathological relationships are essential, especially in tau-negative AD dementia and tau-positive non-AD disorders. Third, there is currently no consensus on the optimal methodology for determining tau positivity. As visual read metrics still need to be developed, the analyses were based on dichotomous classification (positive/negative) applying thresholds to quantitative tau PET. Discriminative accuracy was consistent across methods and ROIs, however, and thresholds for tau positivity were comparable with an independent study.7 Fourth, the multicenter approach has some inherent disadvantages related to lack of harmonization of both clinical and neuroimaging data acquisition. This could also be considered an advantage in terms of generalizability, as the consistency of results between sites supports the robustness of the findings. Fifth, results obtained with [18F]flortaucipir might not be directly generalizable to other tau PET tracers given the differences in specificity and affinity between tracers.40
Conclusions
Among patients with established diagnoses at a memory disorder clinic, [18F]flortaucipir PET was able to discriminate AD from other neurodegenerative diseases. The accuracy and potential utility of this test in patient care require further research in clinically more representative populations.
eTable 1. Diagnostic criteria
eTable 2. Methods to determine Aβ-positivity across centers
eTable 3. PET and MRI protocols
eTable 4. Youden index to derive [18F]flortaucipir cut-offs
eTable 5. Subject characteristics by diagnostic group
eTable 6. Subject characteristics for each center
eTable 7. Diagnostic performance of partial volume corrected [18F]flortaucipir PET in distinguishing AD from non-AD neurodegenerative disease
eTable 8. Diagnostic performance of [18F]flortaucipir PET using a cut-off derived from the Youden Index in AD dementia vs non-AD neurodegenerative disorders
eTable 9. Diagnostic performance of [18F]flortaucipir PET using the closest cut-off at 95% sensitivity in AD dementia vs controls
eTable 10. Diagnostic performance of [18F]flortaucipir PET using the closest cut-off at 95% specificity in AD dementia vs controls
eTable 11. Diagnostic accuracy in combined Aβ-positive and Aβ-negative AD dementia patients vs non-AD neurodegenerative disorders
eTable 12. Tau-positivity in the temporal Meta-ROI by Aβ status
eTable 13. Factors contributing to tau-negativity in AD dementia and tau positivity in non-AD neurodegenerative conditions in the temporal meta-ROI.
eTable 14. Tau-negativity in the temporal Meta-ROI in AD dementia by age
eTable 15. Combined assessment of temporal Meta-ROI flortaucipir and MRI measures
eTable 16. Specificity for [18F]flortaucipir in AD dementia versus non-AD neurodegenerative disorders and controls
eTable 17. Specificity for [18F]flortaucipir versus non-AD disorders and controls in younger and older patient groups
eFigure 1. Flow diagram of participant inclusion
eFigure 2. [18F]flortaucipir uptake in predefined ROIs per diagnostic group
(A. Entorhinal, B. Inferior temporal, C. Temporoparietal, D. Braak stage V/VI)
eFigure 3. [18F]flortaucipir SUVR in Aβ-positive (A) and Aβ-negative (B) participants
eFigure 4. Differences in specificity for [18F]flortaucipir PET versus Aβ status
Data Sharing Statement
References
- 1.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266-273. doi: 10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. ; Amyloid PET Study Group . Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939-1949. doi: 10.1001/jama.2015.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen WJ, Ossenkoppele R, Knol DL, et al. ; Amyloid Biomarker Study Group . Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924-1938. doi: 10.1001/jama.2015.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexopoulos P, Kriett L, Haller B, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Limited agreement between biomarkers of neuronal injury at different stages of Alzheimer’s disease. Alzheimers Dement. 2014;10(6):684-689. doi: 10.1016/j.jalz.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Marquié M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787-800. doi: 10.1002/ana.24517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50-95 years: a cross-sectional study. Lancet Neurol. 2017;16(6):435-444. doi: 10.1016/S1474-4422(17)30077-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(pt 5):1551-1567. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanseeuw BJ, Betensky RA, Schultz AP, et al. Fluorodeoxyglucose metabolism associated with tau-amyloid interaction predicts memory decline. Ann Neurol. 2017;81(4):583-596. doi: 10.1002/ana.24910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971-982. doi: 10.1016/j.neuron.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110-119. doi: 10.1002/ana.24546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schonhaut DR, McMillan CT, Spina S, et al. 18F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: a multicenter study. Ann Neurol. 2017;82(4):622-634. doi: 10.1002/ana.25060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevan-Jones WR, Cope TE, Jones PS, et al. [18F]AV-1451 binding in vivo mirrors the expected distribution of TDP-43 pathology in the semantic variant of primary progressive aphasia [published online September 14, 2017]. J Neurol Neurosurg Psychiatry. doi: 10.1136/jnnp-2017-316402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitwell JL, Ahlskog JE, Tosakulwong N, et al. Pittsburgh Compound B and AV-1451 positron emission tomography assessment of molecular pathologies of Alzheimer’s disease in progressive supranuclear palsy. Parkinsonism Relat Disord. 2018;48:3-9. doi: 10.1016/j.parkreldis.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR Jr, Bennett DA, Blennow K, et al. ; Contributors . NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergeron D, Ossenkoppele R, Jr Laforce R. Evidence-based interpretation of amyloid-β pet results: a clinician’s tool. Alzheimer Dis Assoc Disord. 2018;32(1):28-34. doi: 10.1097/WAD.0000000000000239 [DOI] [PubMed] [Google Scholar]
- 18.Jessen F, Amariglio RE, van Boxtel M, et al. ; Subjective Cognitive Decline Initiative (SCD-I) Working Group . A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10(6):844-852. doi: 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho H, Choi JY, Hwang MS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80(2):247-258. doi: 10.1002/ana.24711 [DOI] [PubMed] [Google Scholar]
- 20.Ossenkoppele R, Cohn-Sheehy BI, La Joie R, et al. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Hum Brain Mapp. 2015;36(11):4421-4437. doi: 10.1002/hbm.22927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustavsson AM, Stomrud E, Abul-Kasim K, et al. Cerebral microbleeds and white matter hyperintensities in cognitively healthy elderly: a cross-sectional cohort study evaluating the effect of arterial stiffness. Cerebrovasc Dis Extra. 2015;5(2):41-51. doi: 10.1159/000377710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattsson N, Schöll M, Strandberg O, et al. 18F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer’s disease. EMBO Mol Med. 2017;9(9):1212-1223. doi: 10.15252/emmm.201707809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maass A, Landau S, Baker SL, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage. 2017;157:448-463. doi: 10.1016/j.neuroimage.2017.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39(5):904-911. [PubMed] [Google Scholar]
- 25.Schöll M, Ossenkoppele R, Strandberg O, et al. ; Swedish BioFINDER study . Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain. 2017;140(9):2286-2294. doi: 10.1093/brain/awx171 [DOI] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017;13(3):205-216. doi: 10.1016/j.jalz.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villemagne VL, Dore V, Bourgeat P, et al. Generating continuous and categorical measures from tau imaging studies with 18F-AV1451 and 18F-THK5351: The Tau MeTeR scale. Alzheimers Dement. 2016;12(7):244. doi: 10.1016/j.jalz.2016.06.43726218444 [DOI] [Google Scholar]
- 28.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32-35. doi: [DOI] [PubMed] [Google Scholar]
- 29.Mathotaarachchi S, Wang S, Shin M, et al. VoxelStats: a MATLAB package for multi-modal voxel-wise brain image analysis. Front Neuroinform. 2016;10:20. doi: 10.3389/fninf.2016.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattsson N, Groot C, Jansen WJ, et al. Prevalence of the apolipoprotein E ε4 allele in amyloid β positive subjects across the spectrum of Alzheimer’s disease. Alzheimers Dement. 2018;14(7):913-924. doi: 10.1016/j.jalz.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 31.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. doi: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 32.Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. J Nucl Med. 2013;54(7):1011-1013. doi: 10.2967/jnumed.113.127068 [DOI] [PubMed] [Google Scholar]
- 33.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362-381. doi: 10.1097/NEN.0b013e31825018f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattsson N, Smith R, Strandberg O, et al. Comparing 18F-AV-1451 with CSF t-tau and p-tau for diagnosis of Alzheimer disease. Neurology. 2018;90(5):e388-e395. doi: 10.1212/WNL.0000000000004887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.La Joie R, Bejanin A, Fagan AM, et al. Associations between [18F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology. 2018;90(4):e282-e290. doi: 10.1212/WNL.0000000000004860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitwell JL, Graff-Radford J, Tosakulwong N, et al. [18 F]AV-1451 clustering of entorhinal and cortical uptake in Alzheimer’s disease. Ann Neurol. 2018;83(2):248-257. doi: 10.1002/ana.25142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botha H, Mantyh WG, Murray ME, et al. FDG-PET in tau-negative amnestic dementia resembles that of autopsy-proven hippocampal sclerosis. Brain. 2018;141(4):1201-1217. doi: 10.1093/brain/awy049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. doi: 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol. 2015;14(1):114-124. doi: 10.1016/S1474-4422(14)70252-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnostic criteria
eTable 2. Methods to determine Aβ-positivity across centers
eTable 3. PET and MRI protocols
eTable 4. Youden index to derive [18F]flortaucipir cut-offs
eTable 5. Subject characteristics by diagnostic group
eTable 6. Subject characteristics for each center
eTable 7. Diagnostic performance of partial volume corrected [18F]flortaucipir PET in distinguishing AD from non-AD neurodegenerative disease
eTable 8. Diagnostic performance of [18F]flortaucipir PET using a cut-off derived from the Youden Index in AD dementia vs non-AD neurodegenerative disorders
eTable 9. Diagnostic performance of [18F]flortaucipir PET using the closest cut-off at 95% sensitivity in AD dementia vs controls
eTable 10. Diagnostic performance of [18F]flortaucipir PET using the closest cut-off at 95% specificity in AD dementia vs controls
eTable 11. Diagnostic accuracy in combined Aβ-positive and Aβ-negative AD dementia patients vs non-AD neurodegenerative disorders
eTable 12. Tau-positivity in the temporal Meta-ROI by Aβ status
eTable 13. Factors contributing to tau-negativity in AD dementia and tau positivity in non-AD neurodegenerative conditions in the temporal meta-ROI.
eTable 14. Tau-negativity in the temporal Meta-ROI in AD dementia by age
eTable 15. Combined assessment of temporal Meta-ROI flortaucipir and MRI measures
eTable 16. Specificity for [18F]flortaucipir in AD dementia versus non-AD neurodegenerative disorders and controls
eTable 17. Specificity for [18F]flortaucipir versus non-AD disorders and controls in younger and older patient groups
eFigure 1. Flow diagram of participant inclusion
eFigure 2. [18F]flortaucipir uptake in predefined ROIs per diagnostic group
(A. Entorhinal, B. Inferior temporal, C. Temporoparietal, D. Braak stage V/VI)
eFigure 3. [18F]flortaucipir SUVR in Aβ-positive (A) and Aβ-negative (B) participants
eFigure 4. Differences in specificity for [18F]flortaucipir PET versus Aβ status
Data Sharing Statement