Key Points
Question
Is it safe and efficacious to use dornase alfa for the treatment of clogged tympanostomy tubes (TTs) in children?
Findings
After establishing that dornase alfa is nonototoxic in a chinchilla model, a randomized clinical trial of 40 children was conducted. At the day-14 visit, the number of unclogged TTs was nonsignificantly higher in the dornase alfa group compared with the ofloxacin group.
Meaning
The pilot clinical trial likely showed safety but failed to show efficacy of dornase alfa to unclog tympanostomy tubes in children.
This pilot study investigates the ototoxic effects of dornase alfa in a chinchilla model and its efficacy vs ofloxacin in a randomized clinical trial of children with clogged tympanostomy tubes.
Abstract
Importance
Many treatments for clogged tympanostomy tubes (TTs) have been proposed, but none have met scientific rigor for safety and efficacy, including the popular empirical use of ototopical antibiotic drops. Dornase alfa, a recombinant molecule with the unique property of cleaving DNA, may be ideal in treating clogged TTs because both middle-ear effusion and the plug are abundant with DNA.
Objective
To investigate the ototoxic effects of dornase alfa in a chinchilla model and its efficacy in a clinical trial in children with clogged TTs.
Design, Setting, and Participants
The safety profiles of dornase alfa (full-strength and 1:10 strength) were evaluated in chinchilla middle ears using serial auditory brainstem response. The efficacy of ototopical dornase alfa (full-strength) was evaluated in children with clogged TTs in a prospective, single-blind randomized clinical trial. The animal study included 21 chinchillas and was conducted at Loma Linda University, Loma Linda, California, and the clinical trial was conducted at Children’s Hospital Colorado, Aurora. A total of 40 children (50 ears with tubes) were enrolled.
Interventions
In the animal study, chinchillas were assigned to 3 groups: controls (saline), full-strength dornase alfa, or 1:10 dornase alfa dilution. Children were randomly assigned to receive either topical dornase alfa or ofloxacin for clogged TT, 5 drops each ear twice a day for 7 days.
Main Outcomes and Measures
Animal study: Auditory brainstem responses. Randomized trial of children participants: The primary outcome was patency of TT at day 14 assessed by otoscopy and tympanometry.
Results
The chinchilla study showed similar auditory brainstem response degradation during a 6-hour period between the control (n = 5) and treatment groups (n = 21). In the clinical trial, a total of 40 clogged TTs (in 33 children, including 25 boys [76%]; mean age, 4.3 years; median [range] age, 3.4 [1.0-14.3] years) were analyzed. The number of unclogged TTs was higher in the dornase alfa group (13 [59%]) compared with the ofloxacin group (8 [44%]), with a difference of 15% (odds ratio, 1.8; 95% CI, 0.54-6.72).
Conclusions and Relevance
The chinchilla model suggests that dornase alfa is likely nonototoxic. The pilot clinical trial failed to show efficacy of dornase alfa to unclog TTs. With the difference seen between the treatment groups, a sample size estimate could be calculated for a future large-scale trial.
Trial Registration
ClinicalTrials.gov identifier: NCT00419380
Introduction
Tympanostomy tube (TT) placement is the treatment of choice for otitis media not responding to medical therapy. It is estimated that more than a million tubes are inserted annually in the United States. One of the main complications following TT placement is otorrhea, with a published frequency of 74.7% and a mean of 2.17 episodes during the first 12 months after tube insertion.1 In a portion of these patients with otorrhea, tubes become clogged with dried secretions, but the frequency is unknown. Various topical agents have been tried, including sodium bicarbonate and hydrogen peroxide, to unblock clogged tubes without any scientific credence or much success.2 Empirically, clinicians rely on ototopical antibiotic drops despite their relative lack of efficacy.
Dornase alfa (Pulmozyme) is a recombinant human enzyme that cleaves DNA and was approved by the US Food and Drug Administration (FDA) in 1993 for treatment of cystic fibrosis; it is manufactured by Genentech. Its mechanism of action rests in hydrolyzing DNA in sputum and reducing sputum viscosity. Yang et al3 published a recent review summarizing the beneficial effects of dornase alfa on pulmonary functions in patients with cystic fibrosis. There have also been off-label uses of dornase alfa for treatment of non–cystic fibrosis lung diseases, such as respiratory syncytial virus bronchiolitis,4 empyema thoracis,5 and plastic bronchitis.6
One could postulate the theoretical effectiveness of using dornase alfa in the treatment of clogged TTs. The composition of TT plugs had been determined to be similar to mucoid effusions, so it had been suggested that treatments should be directed against components of mucoid effusion.7 More recently, Thornton et al8 studied middle-ear effusion samples from children with recurrent acute otitis media and found extensive, host-derived DNA representing neutrophil extracellular traps within which live bacteria in biofilm formations were present. Samples were incubated with dornase alfa, and fragmentation of the strands was found. The effects of pancreatic dornase were explored in 1962 by Loch and Loch.9 Although it was a poorly designed study, it noted favorable results in several types of otitis, including tympanic membrane perforations and fenestration cavities.
Our interest in the off-label use of dornase alfa stemmed from the compassionate use after signed parental consent on a single child with primary ciliary dysmotility with chronically clogged TTs. Dornase alfa was able to unclog both tubes and converted both ears to draining tubes but did not resolve the underlying otorrhea. The theoretical effectiveness and limited clinical experience formed the basis of the study hypothesis that dornase alfa might be efficacious in treating clogged tubes in a pediatric population through a randomized clinical trial given that no ototoxic effects could be shown in a chinchilla model.
Methods
Methods for Experiments in Chinchilla Ototoxic Effects
The use of the chinchilla model to assess ototoxic effects by using auditory brainstem response (ABR) is well established.10 Experiments in ototoxic effects were carried out in the laboratory of the senior author (T.T.K.J.) prior to a human trial following local institutional review board approval; the methodologic details are outlined by Jung et al.10
Pertinent to this study, 3 chinchilla groups were used: control (saline), full-strength dornase alfa, and 1:10 dornase alfa dilution. Baseline ABR recordings were taken before application of test substances on the round window membrane (RWM). The RWMs were exposed by the posteroinferior approach to the bulla. Saline and test substances were soaked into Gelfoam and applied to the RWM. Effects on hearing were monitored and recorded hourly by ABR testing for up to 8 hours.
Methods for Randomized Human Pilot Trial
The human dornase alfa trial was conducted after completion of the studies of ototoxic effects. An Investigational New Drug application was submitted to the FDA. The study was subsequently approved by the Colorado multiple institutional review board. All data were collected at Children’s Hospital Colorado. Written informed consent was obtained for all participants. The trial protocol is available in the Supplement.
The purpose of the study was to evaluate dornase alfa (full-strength) as an ototopical medication compared with a standard ototopical antibiotic drop, ofloxacin, in its ability to dissolve clogged TTs in a randomized, investigator-blinded clinical trial. Caregivers were not blinded to the treatment medications because stock bottles of ofloxacin from the manufacturer were used and the dornase alfa was transferred to sterile aftermarket bottles. Investigators were unblinded at the day-14 visit. Ototopical medications (5 drops twice daily for 7 days) were instilled to the affected ear(s). Patients were stratified into 2 subgroups: 20 with middle-ear effusion and 20 without. There was no attempt to perform a sample size calculation because of the pilot nature of the study and the lack of published efficacy data on the experimental compound for dissolving clogged TTs. The treatment randomization following stratification was based on randomized numbers in lots of 4 generated by a research pharmacist at Children’s Hospital Colorado. Inclusion criteria of the trial were children of both sexes ages 1 to 18 years with unilateral or bilateral clogged TTs placed within the previous 9 months. Exclusion criteria were signs and symptoms of acute otitis media (otalgia or otorrhea), sensorineural hearing loss, craniofacial syndromes, sensitivity to fluoroquinolones, and presence of granulation tissue in the lumen of the tube. Patients were evaluated at 14 days, 6 weeks, and 3 months following entry. Tympanometry and age-appropriate audiometry were performed at each of the follow-up visits. The primary outcome measure was patency of the TTs assessed as a clinical diagnosis based on a combination of otoscopy and tympanometry findings, and the secondary outcome measure was presence or absence of drainage at the day-14 visit. In the event of a narrow canal or cerumen making otoscopy difficult, the investigators had the option of removing cerumen as well as relying on tympanometry.
All patients underwent tympanometric testing at entry and all 3 subsequent visit dates. Children younger than 3 years underwent visual reinforced audiometry with inserted earphones. Children ages 3 to 6 years underwent conditioned play audiometry. Children older than 6 years underwent conventional air and bone audiometry. A change of threshold greater than 15 dB in 2 frequencies was defined as a significant change.
To avoid confounding issues with TTs that might have been extruded at entry not diagnosable by otoscopy and tympanometry, patients who were found to have extruded TTs from their tympanic membranes at the day-14 visit were excluded from the analysis. Summary statistics were used to describe demographic characteristics of the study population, including mean, median, and range as continuous variables and number of patients and frequency as categorical variables. Frequency of tube patency and presence of drainage at the primary and secondary end points were summarized for the 2 study arms, and comparisons were made using χ2 test between the 2 arms at the day-14 visit. A generalized estimating equations approach was also used to estimate and compare the frequency of tube patency and presence of drainage between the 2 arms to account for the potential correlation between bilateral ears from the same patients. SAS statistical software, version 9.4 (SAS Institute Inc), was used for all the analyses.
Results
Study of Ototoxic Effects in Chinchillas
A total of 21 chinchillas were used in the study: 5 controls, 8 receiving full-strength dornase alfa, and 8 receiving 1:10 diluted-strength dornase alfa. The ABR measurements showed that hearing degradation was at its maximum 6 hours after dornase application on the RWM. The mean (SD) hearing degradations for the full-strength dornase and 1:10 dornase groups were 32 (2.9) dB and 13 (0.32) dB, respectively. Whether the hearing degradation between the 2 concentrations represented a dose response could not be ascertained, and this was not the intent of the chinchilla experiments of the study. When compared with the saline control group, hearing degradation was not different for either the full-strength or 1:10 dornase alfa group.
Human Clinical Trial
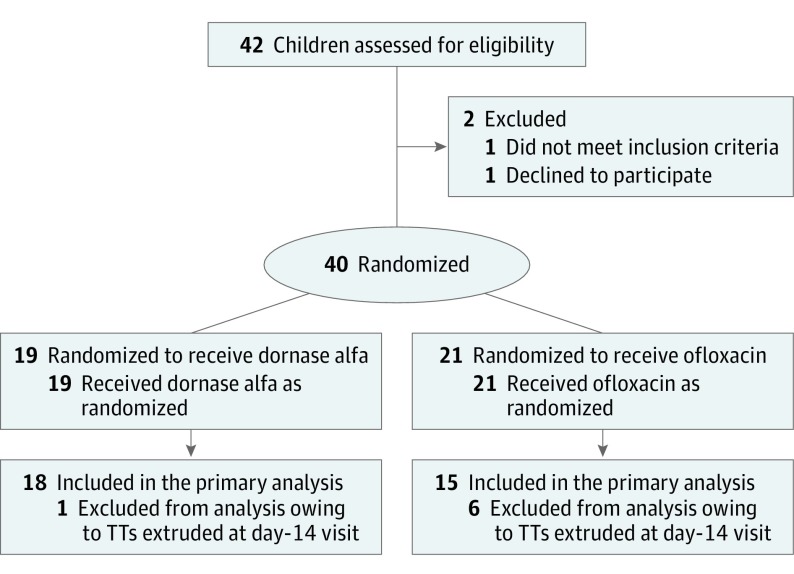
The flow of patients through the study is shown in the Figure. A total of 40 patients (50 ears with tubes) were enrolled. One patient was withdrawn from the study by the parents and another by the enrolling physician. Five patients (6 ears) were excluded from the analysis per protocol owing to findings of extruded tubes at the day-14 visit. A total of 40 clogged TTs (in 33 children, including 25 boys and 8 girls) were analyzed. The mean age of the patients was 4.3 years (median, 3.4; range, 1.0-14.3 years). Most of the cohort (28 [85%]) was white, with the white and Hispanic groups constituting 32 (97%). The remaining demographic characteristics of the 2 treatment groups are listed in Table 1. The mean (median) period between date of TT insertion and date of entry into the study was 92.6 (63.0) days. It is likely that the material clogging the TTs in the study patients was overwhelmingly dessicated middle-ear effusion rather than dried blood, which is generally encountered during the early period following tube placement.
Figure. CONSORT Flow Diagram of Participants in the Clinical Trial.
TT indicates tympanostomy tube.
Table 1. Demographic Characteristics.
| Characteristic | All Patients (N = 33) | Dornase Alfa (n = 18) | Ofloxacin (n = 15) |
|---|---|---|---|
| Total ears, No. | 40 | 22 | 18 |
| Sex, No. (%) | |||
| Male | 25 (76) | 12 (67) | 13 (87) |
| Female | 8 (24) | 6 (33) | 2 (13) |
| Age, y | |||
| Mean | 4.3 | 4.5 | 4.1 |
| Median (range) | 3.4 (1.0-14.3) | 3.4 (1.0-14.3) | 3.5 (1.1-9.6) |
| Ethnicity, No. (%) | |||
| White | 28 (85) | 14 (78) | 14 (93) |
| Hispanic or Latino | 4 (12) | 4 (22) | 0 |
| American Indian or Alaska Native | 1 (3) | 0 | 1 (7) |
| Laterality of clogged tubes, No. | |||
| Unilateral | 26 | 14 | 12 |
| Bilateral | 7 | 4 | 3 |
| Middle ear effusion | 15 (45) | 7 (39) | 8 (53) |
Tube patency rate at day-14 visit was assessed based on effusion at study entry and treatment groups (Table 2). At study entry, 19 (48%) of TTs presented with effusion and 21 (52%) without effusion. At the day-14 visit, a 15% difference was seen in the tube patency rates. Thirteen (59%) TTs in the dornase alfa group were patent, while 8 (44%) were patent in the ofloxacin group (OR, 1.8; 95% CI, 0.54-6.72). Similar results were obtained by carrying out the analyses using generalized estimating equations. The patency status of the TTs did not remain stable throughout the study period. By the 6-week visit, 2 patent TTs from each of the study groups, which were 15% and 19%, respectively, for the dornase alfa and ofloxacin groups, became clogged. Furthermore, by the 3-month visit, 13 tubes (59%) from the dornase alfa and 8 tubes (44%) from the ofloxacin group had become dislodged.
Table 2. Tympanostomy Tube Patency at the Day-14 Visit Following Treatment Based on Presence or Absence of Effusion at Entry.
| Effusion Status | Tube Patency at Day 14, No. (% of Row Total) | Total, No. | OR (95% CI) | |
|---|---|---|---|---|
| Open | Closed | |||
| Dornase alfa | ||||
| Present | 5 (50) | 5 (50) | 10 | 0.5 (0.09-10.50) |
| Absent | 8 (67) | 4 (33) | 12 | |
| Total | 13 | 9 | 22 | |
| Ofloxacin | ||||
| Present | 5 (56) | 4 (44) | 9 | 2.5 (0.42-13.30) |
| Absent | 3 (33) | 6 (67) | 9 | |
| Total | 8 | 10 | 18 | |
Abbreviation: OR, odds ratio.
Eleven patients underwent TT replacement owing to the study’s failure to unclog their TTs. One patient (1 tube) was found to have granulation tissue in the lumen of the TTs. The outcomes of these analyses did not change when adjusted for the clogged tube due to granulation tissue.
Adverse events (number of patients) during the study period included hearing loss (7), upper respiratory tract infections (6), otitis media (2), streptococcal tonsillitis (1), and cervical lymphadenitis (1). None was related to the treatment. Specifically, transient conductive hearing loss related to the underlying disease will not be explained further. No serious adverse events occurred during the conduct of the study.
Sensorineural hearing stability of the cohort was maintained throughout the duration of the study for all but 1 patient. This child, initially enrolled in the dornase alfa group with a unilateral clogged TT that failed to unclog during treatment after confirming prestudy normal hearing threshold in both ears, was found to have bilateral mild sensorineural hearing loss (SNHL) at the 4-week visit. His adverse event was not attributed to the treatment medication because his TT remained clogged throughout the study and the subsequent SNHL was bilateral. It was concluded that this was a case of SNHL that had developed during the study period, and his hearing loss has remained stable since.
The study points out a natural history aspect of children with clogged TTs, namely, the recurrence of clogging: 2 (15%) and 2 (25%) of the unclogged tubes became clogged between the day-14 and 6-week visits for the dornase alfa and ofloxacin groups, respectively. Clinicians are to be reminded not to be complacent in the clinical setting and not to view long-term patency as a given once a TT has been unclogged by whatever means.
Limitations
The major deficiency of the clinical trial is the 12% of TT extrusions (6 of 50 ears) that were noted at the day-14 visit. The attempt to minimize this issue by setting the inclusion criterion to enroll only children with tubes that had been inserted fewer than 9 months prior to study enrollment proved not to be stringent enough. Indeed, the patients with extruded tubes at the day-14 visit (n = 5) had a mean (SD) post-TT duration of 8 (2) months compared with 3 (3) months in the remainder of the cohort (n = 33). The other confounding issue that was not controlled for was the type of TTs included in the study, even though tubes intended for long-term use were excluded. This no doubt played a role in tube extrusion because certain tubes are known to have a shorter life span. The main deficiency of the toxic effects study in animals is the lack of ultrastructural morphologic analysis of not only the cochlea but also the vestibular system.
We are not advocating the clinical off-label use of dornase alfa in the clinical setting of clogged TTs in children but are only reporting our experimental experience. If a subsequent dornase alfa human trial is to be conducted, the unclogging rate of dornase alfa from this investigation could serve as pilot data. Based on a power of 0.8, 1-tail comparison, and P = .05, the sample size per treatment group is calculated to be 135 tubes. Certainly, we would highly recommend adjusting the inclusion criterion to study only children who are 4 months from tube insertion as the maximum time length for entry into the study.
Conclusions
To our knowledge, this study represents the first attempt based on sound scientific principles to explore the effectiveness of dornase alfa in resolving clogged TTs. Dornase alfa was shown to be nonototoxic in the chinchilla model, and no adverse events attributable to dornase alfa were found during the human trial. The human trial failed to show a difference between the effectiveness of dornase alfa and ofloxacin otic.
Trial Protocol
References
- 1.Ah-Tye C, Paradise JL, Colborn DK. Otorrhea in young children after tympanostomy-tube placement for persistent middle-ear effusion: prevalence, incidence, and duration. Pediatrics. 2001;107(6):1251-1258. doi: 10.1542/peds.107.6.1251 [DOI] [PubMed] [Google Scholar]
- 2.Spraggs PD, Robinson PJ, Ryan R, East CA, Graham JM. A prospective randomised trial of the use of sodium bicarbonate and hydrogen peroxide ear drops to clear a blocked tympanostomy tube. Int J Pediatr Otorhinolaryngol. 1995;31(2-3):207-214. doi: 10.1016/0165-5876(94)01105-7 [DOI] [PubMed] [Google Scholar]
- 3.Yang CL, Chilvers M, Montgomery M, Nolan SJ. Dornase alfa for cystic fibrosis. Paediatr Respir Rev. 2017;21:65-67. doi: 10.1016/j.prrv.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 4.Nasr SZ, Strouse PJ, Soskolne E, et al. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest. 2001;120(1):203-208. doi: 10.1378/chest.120.1.203 [DOI] [PubMed] [Google Scholar]
- 5.Simpson G, Roomes D, Reeves B. Successful treatment of empyema thoracis with human recombinant deoxyribonuclease. Thorax. 2003;58(4):365-366. doi: 10.1136/thorax.58.4.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manna SS, Shaw J, Tibby SM, Durward A. Treatment of plastic bronchitis in acute chest syndrome of sickle cell disease with intratracheal rhDNase. Arch Dis Child. 2003;88(7):626-627. doi: 10.1136/adc.88.7.626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westine JG, Giannoni CM, Antonelli PJ. Defining tympanostomy tube plugs. Laryngoscope. 2002;112(6):951-954. doi: 10.1097/00005537-200206000-00003 [DOI] [PubMed] [Google Scholar]
- 8.Thornton RB, Wiertsema SP, Kirkham LA, et al. Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media: a potential treatment target. PLoS One. 2013;8(2):e53837. doi: 10.1371/journal.pone.0053837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loch WE, Loch MH. Enzyme treatment of ear infections: local use of pancreatic dornase. Laryngoscope. 1962;72:598-607. doi: 10.1288/00005537-196205000-00005 [DOI] [PubMed] [Google Scholar]
- 10.Jung TTK, Park YM, Miller SK, Rozehnal S, Woo HY, Baer W. Effect of exogenous arachidonic acid metabolites applied on round window membrane on hearing and their levels in the perilymph. Acta Otolaryngol Suppl. 1992;493:171-176. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol