This cohort study evaluates the temporal and regional trends in age-adjusted amyloidosis-related mortality among men and women of various races in the United States.
Key Points
Question
Are there regional differences in reported amyloidosis-related mortality in the United States?
Findings
This cohort study found that amyloidosis-related mortality reported from US death certificates significantly increased from 1.77 to 3.96 per 1 000 000 between 1979 and 2015. The highest age-adjusted amyloidosis mortality rates were reported for black men and women; southern states reported the lowest US mortality rates despite having the highest proportions of black residents.
Meaning
Geographic and racial variations in reported amyloidosis mortality, which is most commonly due to cardiac amyloidosis, suggest the potential for regional underdiagnosis.
Abstract
Importance
Cardiac amyloidosis is an underdiagnosed disease and is highly fatal when untreated. Early diagnosis and treatment with the emerging novel therapies significantly improve survival. A comprehensive analysis of amyloidosis-related mortality is critical to appreciate the nature and distribution of underdiagnosis and improve disease detection.
Objective
To evaluate the temporal and regional trends in age-adjusted amyloidosis-related mortality among men and women of various races/ethnicities in the United States.
Design, Setting, and Participants
In this observational cohort study, death certificate information from the Centers for Disease Control and Prevention’s Wide-ranging ONline Data for Epidemiologic Research database and the National Vital Statistics System from 1979 to 2015 was analyzed. A total of 30 764 individuals in the United States with amyloidosis listed as the underlying cause of death and 26 591 individuals with amyloidosis listed as a contributing cause of death were analyzed.
Exposures
Region of residence.
Main Outcomes and Measures
Age-adjusted mortality rate from amyloidosis per 1 000 000 population stratified by year, sex, race/ethnicity, and state and county of residence.
Results
Of the 30 764 individuals with amyloidosis listed as the underlying cause of death, 17 421 (56.6%) were men and 27 312 (88.8%) were 55 years or older. From 1979 to 2015, the reported overall mean age-adjusted mortality rate from amyloidosis as the underlying cause of death doubled from 1.77 to 3.96 per 1 000 000 population (2.32 to 5.43 in men and 1.35 to 2.80 in women). Black men had the highest mortality rate (12.36 per 1 000 000), followed by black women (6.48 per 1 000 000). Amyloidosis contributed to age-adjusted mortality rates as high as 31.73 per 1 000 000 in certain counties. Most southern states reported the lowest US mortality rates despite having the highest proportions of black individuals.
Conclusions and Relevance
The increased reported mortality over time and in proximity to amyloidosis centers more likely reflects an overall increase in disease diagnosis rather than increased lethality. The reported amyloidosis mortality is highly variable in different US regions. The lack of higher reported mortality rates in states with a greater proportion of black residents suggests underdiagnosis of amyloidosis, including cardiac forms of the disease, in many areas of the United States. Better understanding of the determinants of geographic and racial disparity in the reporting of amyloidosis deaths are warranted.
Introduction
Systemic amyloidosis most commonly affects the heart and remains an underdiagnosed deadly disease.1 Untreated cardiac amyloidosis is highly fatal with a median survival of less than 1 year for light chain (AL) amyloidosis and 4 years for wild-type transthyretin (ATTR) amyloidosis.2,3 With the emergence of several novel therapies, early diagnosis and treatment (particularly for AL amyloidosis) significantly improve survival.4,5 Thus, there is now a critical need to better understand the epidemiology of amyloidosis. No study has evaluated US amyloidosis mortality since 1986, to our knowledge.6,7 Because most amyloidosis mortality is caused by cardiac involvement, death certificate data may be a powerful study tool. We aimed to comprehensively analyze US trends in reported amyloidosis mortality from 1979 to 2015 and highlight potential regions of underdetection.
Methods
Death certificate data from the Centers for Disease Control and Prevention’s Wide-ranging ONline Data for Epidemiologic Research database from 1979 to 2015 were used (http://wonder.cdc.gov/). Multiple cause of death files were downloaded from the National Vital Statistics System of the National Center for Health Statistics for 1999 to 2015. The diagnosis of amyloidosis was established using the International Classification of Diseases, Ninth Revision (ICD-9) for 1979 to 1998 (277.3x) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) for 1999 to 2015 (E85.x). Analyses were conducted in June 2017.
Race and ethnicity were defined per the Office of Management and Budget as American Indian or Alaskan Native, Asian/Pacific Islander, black or African American, white, Hispanic, or non-Hispanic. Amyloidosis mortality was analyzed by the state and county of the decedent’s legal residence. This study did not require institutional review board approval as the analyses were performed using publicly available, deidentified data. Therefore, consent from study individuals also was not required.
Mortality from amyloidosis either as an underlying or contributing cause of death formed the primary end points. Data for 1979 to 1998 were only available for the underlying cause of death. Hence, any analyses including these data only report on amyloidosis as the underlying cause of death. All other analyses use multiple cause of death data for amyloidosis as a contributing cause of death for 1999 to 2015. For these data, amyloidosis may be listed as the underlying cause or 1 of up to 20 additional contributing causes of death. Comorbid conditions were estimated using the ICD-10 codes heart failure (I50.x), renal diseases (N00-N99), and multiple myeloma (C90.0). Centers listed on the Amyloidosis Foundation (http://amyloidosis.org/) or Amyloidosis Research Consortium (http://www.arci.org/) websites were considered amyloidosis referral centers.
Age-adjusted mortality rates per 1 000 000 with 95% confidence intervals were calculated. Linear regressions were used when appropriate (StataCorp 2015). Statistical significance was defined as a 2-sided P value less than .05. Tableau Public (version 10.3) software was used to display the geographic data. Interactive maps are available at https://goo.gl/RCdBV8.
Results
Nationwide Amyloidosis Mortality Trends
From 1979 to 2015, amyloidosis was the underlying cause of death in 30 764 individuals (Table). Most deaths occurred in older individuals, with 88.8% (27 312) of the deaths reported in those 55 years or older. The reported number of amyloidosis deaths increased from 367 in 1979 to 1454 in 2015. The age-adjusted amyloidosis death rate increased from 1.77 per 1 000 000 (95% CI, 1.58-1.95) in 1979 to 3.96 per 1 000 000 (95% CI, 3.75-4.17) in 2015 (P < .001) (eFigure 1A in the Supplement).
Table. Demographics of Individuals With Reported Amyloidosis-Related Death.
| Characteristica | 1979-1998 | 1999-2015 | 1979-2015 |
|---|---|---|---|
| Underlying Cause of Death | |||
| Total deaths | 12 845 | 17 919 | 30 764 |
| Male | 7149 (55.7) | 10 272 (57.3) | 17 421 (56.6) |
| Female | 5696 (44.3) | 7647 (42.7) | 13 343 (43.4) |
| Race/ethnicity | |||
| White | 10 740 (83.6) | 14 569 (81.3) | 25 309 (82.3) |
| Black or African American | 1952 (15.2) | 2907 (16.2) | 4859 (15.8) |
| Asian/Pacific Islander | NA | 387 (2.2) | NA |
| American Indian/Alaskan Native | NA | 56 (0.3) | NA |
| Other | 153 (1.2) | NA | NA |
| Hispanic | NA | 835 (4.7) | NA |
| Non-Hispanic | NA | 17 045 (95.1) | NA |
| Not stated | NA | 39 (0.2) | NA |
| Age, y | |||
| <25 | 33 (0.3) | 18 (0.1) | 51 (0.1) |
| 25-34 | 78 (0.6) | 47 (0.3) | 125 (0.4) |
| 35-44 | 373 (2.9) | 358 (2.0) | 731 (2.4) |
| 45-54 | 1200 (9.3) | 1345 (7.5) | 2545 (8.3) |
| 55-64 | 2676 (20.8) | 3040 (17.0) | 5716 (18.6) |
| 65-74 | 4394 (34.2) | 4951 (27.6) | 9345 (30.4) |
| 75-84 | 3233 (25.2) | 5859 (32.7) | 9092 (29.6) |
| >85 | 858 (6.7) | 2301 (12.8) | 3159 (10.3) |
| Contributing Cause of Death | |||
| Total deaths | NA | 26 591 | NA |
| Male | NA | 15 324 (57.6) | NA |
| Female | NA | 11 267 (42.4) | NA |
| Race/ethnicity | |||
| White | NA | 21 461 (80.7) | NA |
| Black or African American | NA | 4461 (16.8) | NA |
| Asian/Pacific Islander | NA | 583 (2.2) | NA |
| American Indian/Alaskan Native | NA | 86 (0.3) | NA |
| Hispanic | NA | 1313 (4.9) | NA |
| Non-Hispanic | NA | 25 218 (94.8) | NA |
| Not stated | NA | 60 (0.2) | NA |
| Age, median (IQR), y | NA | 73 (63-80) | NA |
| Age, y | |||
| <25 | NA | 23 (0.1) | NA |
| 25-34 | NA | 76 (0.3) | NA |
| 35-44 | NA | 548 (2.1) | NA |
| 45-54 | NA | 2082 (7.8) | NA |
| 55-64 | NA | 4645 (17.5) | NA |
| 65-74 | NA | 7501 (28.2) | NA |
| 75-84 | NA | 8420 (31.7) | NA |
| >85 | NA | 3296 (12.4) | NA |
Abbreviations: IQR, interquartile range; NA, not available.
Data are reported as number (percentage) unless otherwise noted.
During 1999 to 2015, amyloidosis was listed as a contributing cause of death in 26 591 individuals (Table). This mortality rate increased from 4.95 per 1 000 000 (95% CI, 4.68-5.21) in 1999 to 5.69 per 1 000 000 (95% CI, 5.45-5.94) in 2015 but did not reach statistical significance (P = .07) (eFigure 1A in the Supplement).
Sex Differences in Amyloidosis Mortality
For amyloidosis as the underlying cause of death, there was a greater increase in the number of deaths reported in men compared with women from 1979 to 2015 (314.69% increase [211 to 875 deaths] vs 271.15% increase [156 to 579 deaths], respectively; P < .001) (eFigure 1B in the Supplement). The age-adjusted amyloidosis mortality rate significantly increased for both men (2.32 per 1 000 000 [95% CI, 2.00-2.65] in 1979 to 5.43 per 1 000 000 [95% CI, 5.06-5.80] in 2015; P < .001) and women (1.35 per 1 000 000 [95% CI, 1.14-1.57] in 1979 to 2.80 per 1 000 000 [95% CI, 2.56-3.03] in 2015; P < .001).
Racial Differences in Amyloidosis Mortality
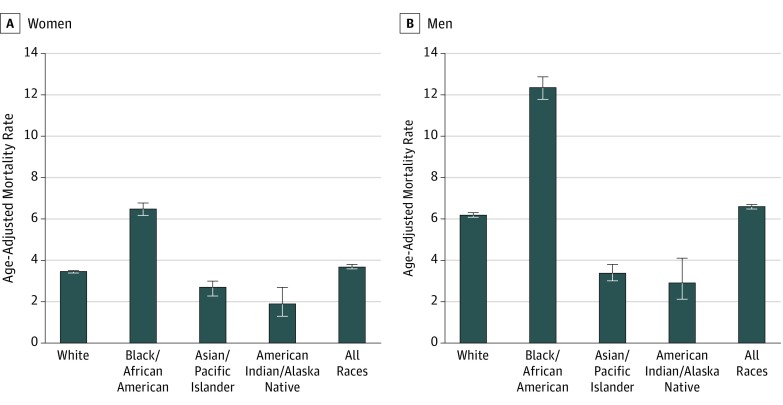
During 1999 to 2015, black men had the highest reported age-adjusted mortality rate at 12.36 per 1 000 000 (95% CI, 11.85-12.87), nearly twice the rate for white men (6.20 per 1 000 000 [95% CI, 6.09-6.31]) (Figure 1). Black women had the second highest amyloidosis mortality rate (6.48 per 1 000 000 [95% CI, 6.19-6.77]).
Figure 1. Reported Age-Adjusted Death Rates for Amyloidosis Deaths.
Data are shown for 1999 to 2015. Rates are reported per 1 000 000 persons. Black bars indicate 95% confidence intervals.
Geographic Variation in Amyloidosis Mortality
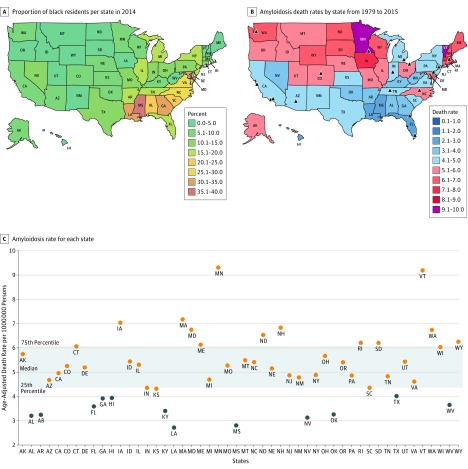
Interstate differences were evident throughout the study period (eFigure 2A and B in the Supplement and interactive maps, available at https://goo.gl/RCdBV8) with most states reporting an increased mortality over time (eFigure 2C in the Supplement). During 1979 to 1998, Minnesota reported the highest mortality rate (4.56 per 1 000 000 [95% CI, 4.10-5.02] vs the national mean of 2.75 per 1 000 000 [95% CI, 2.70-2.79]). During 1999 to 2015, Vermont reported the highest mortality rate (6.25 per 1 000 000 [95% CI, 4.90-7.86] vs the national mean of 3.34 per 1 000 000 [95% CI, 3.29-3.39]). Notably, the southern states, which have the highest proportions of black individuals (Figure 2A), reported the lowest mortality rates (Figure 2B and C).
Figure 2. Geographic Disparities in Reported Age-Adjusted Death Rates for Amyloidosis in the United States.
Proportion of black residents per state (A) in 2014 compared with the geographic distribution of reported age-adjusted death rates per 1 000 000 persons for amyloidosis as a contributing cause of death during 1999 to 2015 (B). Amyloidosis referral centers are marked with an arrowhead. Age-adjusted death rates per 1 000 000 persons for each state (1999-2015) is listed in alphabetical order (C). Most states, except for 1, below the 25th percentile ranking for reporting of amyloidosis mortality did not have an amyloidosis referral center (B). US territories and Washington, DC, are not included in these maps. Interactive online Tableau figures are available at https://goo.gl/RCdBV8.
We then assessed county data for amyloidosis as a contributing cause of death. The 5 counties with the highest reported amyloidosis mortality were located near (<400 km) an amyloidosis referral center: Rochester, Minnesota (Mower County: 31.73 per 1 000 000 [95% CI, 21.41-45.30]; Olmsted County: 25.45 per 1 000 000 [95% CI, 19.46-32.69]), Iowa City/Cedar Rapids, Iowa (Johnson County: 14.81 per 1 000 000 [95% CI, 9.28-22.42]; Linn County: 13.54 per 1 000 000 [95% CI, 10.02-17.90]), and Boston, Massachusetts (Suffolk County: 12.84 per 1 000 000 [95% CI, 10.65-15.03]) (eTable in the Supplement). These death rates were considerably higher than the overall national mortality rate (4.95 per 1 000 000 [95% CI, 4.89-5.01]). Notably, Washington, DC (neither a state nor a county), reported a high mortality rate (10.47 per 1 000 000 [95% CI, 8.42-12.52]).
Comorbidities in Individuals With Amyloidosis Mortality
Amyloidosis represents a range of protein misfolding diseases with multiorgan involvement and frequent delays in diagnosis. To identify associated comorbidities, we examined other reported contributing causes of death in 26 591 individuals with amyloidosis-related mortality (eFigure 3 in the Supplement). Amyloidosis was the underlying (ie, primary) cause of death in most cases (17 965 [67.4%]), followed by multiple myeloma (2575 [9.66%]) and cardiovascular diseases (2539 [9.52%]). We examined comorbidities clinically associated with amyloidosis, including heart failure, renal disease, and multiple myeloma (eFigures 4 and 5 in the Supplement). Men and black individuals had a higher frequency of congestive heart failure compared with women and white individuals (eFigures 4A and 5A in the Supplement). No differences were observed for renal disease or multiple myeloma.
Discussion
We conducted an analysis of the largest and most racially diverse epidemiological data on reported US amyloidosis deaths to date. We discovered the following major findings: (1) the overall reported age-adjusted amyloidosis mortality rate has more than doubled from 1979 to 2015; (2) black men had the highest mortality rate that was 2-fold higher compared with other men; and (3) wide geographic variation in reported mortality raises the possibility of amyloidosis underdetection in many areas.
Importantly, the mortality rates reported in this study reflect ascertainment of amyloidosis based on death certificates rather than necessarily indicating that the disease has become more lethal. The increasing death rates most likely represent increased disease recognition over time. For instance, counties with better access to amyloidosis centers reported the highest amyloidosis mortality rates. Areas around Rochester and Boston, home to the oldest US amyloidosis centers (Mayo Clinic and Boston University, respectively), had the first and third highest amyloidosis mortality rates. Of note, the Centers for Disease Control and Prevention’s Wide-ranging ONline Data for Epidemiologic Research database records the county of residence of the decedent and thus reflects the mortality in the local population surrounding amyloidosis centers rather than mortality from patients referred to these centers. A similar inverse association was observed between amyloidosis mortality and distance to the National Amyloidosis Center in the United Kingdom.8 These findings suggest an underdiagnosis of amyloidosis in locations with less access to specialized amyloidosis care.
Moreover, we observed that black individuals were overrepresented in amyloidosis mortality, consistent with a previous study6 and also reported for ATTR amyloidosis.9 It could therefore be anticipated that areas with a high proportion of black residents (ie, southern states) would have higher amyloidosis mortality rates. Our contrary finding suggests that underdiagnosis is present. Wild-type transthyretin cardiomyopathy is responsible for a significant, underrecognized heart failure burden in black individuals and warrants targeted interventions. Approximately 3% to 4% of black individuals carry the amyloidogenic transthyretin gene mutation (valine-to-isoleucine substitution at codon 122), which confers higher lifetime risk of heart failure.10 Furthermore, this type of variant ATTR cardiomyopathy was recently reported as the fourth leading cause of heart failure in individuals of African descent.11
Regions with underdiagnosis have options to improve. If clinically suspected, AL amyloidosis can now be diagnosed accurately at most medical centers using serum immunofixation and serum-free light chain measurements to identify abnormal paraproteins.12 Cardiac amyloidosis can be suspected by echocardiography demonstrating a characteristic longitudinal strain pattern.13 Advanced techniques, including cardiac magnetic resonance imaging with T1 mapping, technetium 99m pyrophosphate single-photon emission tomography, and fluorine 18–labeled amyloid imaging with positron emission tomography, can detect cardiac amyloidosis.13,14,15
Limitations
Despite its scope, our study has several limitations. The analyses are affected by the accuracy of ICD coding, including regional variations in death certificate reporting and race ascertainment. However, amyloidosis has well-defined clinicopathological characteristics, decreasing the chance of false positive diagnoses. ICD codes lack detail of the amyloidosis subtype, such as AL or ATTR amyloidosis. However, in this mortality study, the study individuals more likely represent severe cases (ie, cardiac amyloidosis). Indeed, cardiovascular disease was frequently listed as a cause of death on the amyloidosis death certificates. Despite these limitations, death certificates provide comprehensive information on an uncommon disease, such as amyloidosis, that is not otherwise feasible.
Conclusions
During 1979 to 2015, the reported US amyloidosis mortality increased and varied by sex, race/ethnicity, and geography. The clustering of mortality in regions with amyloidosis centers and the lack of higher reported mortality rates in states with a greater proportion of black residents support possible underdiagnosis of cardiac amyloidosis in these regions. Future studies are warranted to better understand these disparities in reported amyloidosis mortality.
eTable. Fifteen US Counties With the Highest Reported Death Rates During 1999-2015
eFigure 1. Temporal Trends in Reported Amyloidosis Mortality
eFigure 2. Geographic Trends in Reported Amyloidosis Mortality
eFigure 3. Underlying Cause of Death in Individuals with Reported Amyloidosis Mortality
eFigure 4. Reported Amyloidosis-associated Comorbidities Stratified by Sex
eFigure 5. Reported Amyloidosis-associated Comorbidities Stratified by Race
References
- 1.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337(13):898-909. doi: 10.1056/NEJM199709253371306 [DOI] [PubMed] [Google Scholar]
- 2.Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91(2):141-157. doi: 10.1093/qjmed/91.2.141 [DOI] [PubMed] [Google Scholar]
- 3.Connors LH, Sam F, Skinner M, et al. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander KM, Singh A, Falk RH. Novel pharmacotherapies for cardiac amyloidosis. Pharmacol Ther. 2017;180:129-138. doi: 10.1016/j.pharmthera.2017.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gertz MA. Immunoglobulin light chain amyloidosis: 2016 update on diagnosis, prognosis, and treatment. Am J Hematol. 2016;91(9):947-956. doi: 10.1002/ajh.24433 [DOI] [PubMed] [Google Scholar]
- 6.Simms RW, Prout MN, Cohen AS. The epidemiology of AL and AA amyloidosis. Baillieres Clin Rheumatol. 1994;8(3):627-634. doi: 10.1016/S0950-3579(05)80119-0 [DOI] [PubMed] [Google Scholar]
- 7.Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79(7):1817-1822. [PubMed] [Google Scholar]
- 8.Pinney JH, Smith CJ, Taube JB, et al. Systemic amyloidosis in England: an epidemiological study. Br J Haematol. 2013;161(4):525-532. doi: 10.1111/bjh.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah KB, Mankad AK, Castano A, et al. Transthyretin cardiac amyloidosis in black Americans. Circ Heart Fail. 2016;9(6):e002558. doi: 10.1161/CIRCHEARTFAILURE.115.002558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quarta CC, Buxbaum JN, Shah AM, et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. 2015;372(1):21-29. doi: 10.1056/NEJMoa1404852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dungu JN, Papadopoulou SA, Wykes K, et al. Afro-Caribbean heart failure in the United Kingdom: cause, outcomes, and ATTR V122I cardiac amyloidosis. Circ Heart Fail. 2016;9(9):e003352. doi: 10.1161/CIRCHEARTFAILURE.116.003352 [DOI] [PubMed] [Google Scholar]
- 12.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362-1369. doi: 10.1056/NEJMoa054494 [DOI] [PubMed] [Google Scholar]
- 13.Falk RH, Quarta CC, Dorbala S. How to image cardiac amyloidosis. Circ Cardiovasc Imaging. 2014;7(3):552-562. doi: 10.1161/CIRCIMAGING.113.001396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banypersad SM, Sado DM, Flett AS, et al. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2013;6(1):34-39. doi: 10.1161/CIRCIMAGING.112.978627 [DOI] [PubMed] [Google Scholar]
- 15.Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404-2412. doi: 10.1161/CIRCULATIONAHA.116.021612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Fifteen US Counties With the Highest Reported Death Rates During 1999-2015
eFigure 1. Temporal Trends in Reported Amyloidosis Mortality
eFigure 2. Geographic Trends in Reported Amyloidosis Mortality
eFigure 3. Underlying Cause of Death in Individuals with Reported Amyloidosis Mortality
eFigure 4. Reported Amyloidosis-associated Comorbidities Stratified by Sex
eFigure 5. Reported Amyloidosis-associated Comorbidities Stratified by Race