Key Points
Question
What is the association between perioperative red blood cell transfusions and postoperative venous thromboembolism within 30 days of a surgical procedure?
Findings
In this registry study of 750 937 patients undergoing surgery, perioperative red blood cell transfusions (preoperative and intraoperative or postoperative) were significantly associated with higher risk for venous thromboembolism. The effect of this association was dose dependent, and the association remained robust with propensity score matching.
Meaning
These findings should reinforce the importance of rigorous perioperative patient blood management practices.
Abstract
Importance
Increasing evidence supports the role of red blood cells (RBCs) in physiological hemostasis and pathologic thrombosis. Red blood cells are commonly transfused in the perioperative period; however, their association with postoperative thrombotic events remains unclear.
Objective
To examine the association between perioperative RBC transfusions and postoperative venous thromboembolism (VTE) within 30 days of surgery.
Design, Setting, and Participants
This analysis used prospectively collected registry data from the American College of Surgery National Surgical Quality Improvement Program (ACS-NSQIP) database, a validated registry of 525 teaching and nonteaching hospitals in North America. Participants included patients in the ACS-NSQIP registry who underwent a surgical procedure from January 1 through December 31, 2014. Data were analyzed from July 1, 2016, through March 15, 2018.
Main Outcomes and Measures
Risk-adjusted odds ratios (aORs) were estimated using multivariable logistic regression. The primary outcome was the development of postoperative VTE (deep venous thrombosis [DVT] and pulmonary embolism [PE]) within 30 days of surgery that warranted therapeutic intervention; DVT and PE were also examined separately as secondary outcomes. Subgroup analyses were performed by surgical subtypes. Propensity score matching was performed for sensitivity analyses.
Results
Of 750 937 patients (56.8% women; median age, 58 years; interquartile range, 44-69 years), 47 410 (6.3%) received at least 1 perioperative RBC transfusion. Postoperative VTE occurred in 6309 patients (0.8%) (DVT in 4336 [0.6%]; PE in 2514 [0.3%]; both DVT and PE in 541 [0.1%]). Perioperative RBC transfusion was associated with higher odds of VTE (aOR, 2.1; 95% CI, 2.0-2.3), DVT (aOR, 2.2; 95% CI, 2.1-2.4), and PE (aOR, 1.9; 95% CI, 1.7-2.1), independent of various putative risk factors. A significant dose-response effect was observed with increased odds of VTE as the number of intraoperative and/or postoperative RBC transfusion events increased (aOR, 2.1 [95% CI, 2.0-2.3] for 1 event; 3.1 [95% CI, 1.7-5.7] for 2 events; and 4.5 [95% CI, 1.0-19.4] for ≥3 events vs no intraoperative or postoperative RBC transfusion; P < .001 for trend). In subgroup analyses, the association between any perioperative RBC transfusion and postoperative VTE remained statistically significant across all surgical subspecialties analyzed. The association between any perioperative RBC transfusion and the development of postoperative VTE also remained robust after 1:1 propensity score matching (47 142 matched pairs; matched OR, 1.9; 95% CI, 1.8-2.1).
Conclusions and Relevance
The results of this study suggest that perioperative RBC transfusions may be significantly associated with the development of new or progressive postoperative VTE, independent of several putative confounders. These findings, if validated, should reinforce the importance of rigorous perioperative management of blood transfusion practices.
This cohort study uses ACS-NSQUIP registry data to examine the association between perioperative red blood cell transfusions and postoperative venous thromboembolism within 30 days of surgery among North American patients undergoing surgery.
Introduction
Hospital-associated venous thromboembolism (VTE) is a major cause of morbidity and mortality.1 Venous thromboembolism contributes directly or indirectly to approximately 100 000 to 200 000 deaths annually, which represents 5% to 10% of all hospital deaths.2 Furthermore, as many as 70% of hospital-acquired VTE cases are considered to be preventable.3,4 Surgery is well recognized as a proinflammatory state and a prothrombotic stimulus for development of VTE.5,6,7 General anesthesia is an independent risk factor for postoperative thrombosis.8 Perioperative red blood cell (RBC) transfusions may also potentiate this postoperative prothrombotic state.5,7
Red blood cells are commonly transfused in the perioperative course of patients undergoing surgery. Increasing molecular and clinical evidence supports the role of RBCs in physiological hemostasis and pathologic thrombosis.9,10,11,12,13,14 Transfused RBCs have been proposed to modulate the inflammatory cascade.15,16,17 In addition, during the storage period, RBCs develop storage lesions, including metabolic, biochemical, and physical changes responsible for accumulation of bioreactive substances such as microparticles, which can have a prothrombogenic potential.18,19 Because inflammation and hypercoagulation are closely linked, the proinflammatory and immunomodulatory properties of RBC transfusion may further potentiate a hypercoagulable state.20
Recent evidence from studies in mice and an in vivo clot formation model suggests that transfused RBCs have a role in accelerating thrombus generation.21,22 Clinical studies have also suggested a role of RBC transfusions in thrombus development. In an analysis of various factors responsible for postoperative VTE, one of the reported independent factors was postoperative transfusion of more than 4 units of RBCs.7 In a separate study of hospitalized patients with cancer, RBC and platelet transfusions were associated with an increased risk of venous and arterial thrombotic events.14 In patients undergoing colorectal surgery for cancer resection, RBC transfusion has been associated with increased risk of VTE, but cancer can also be a predisposing factor for VTE.23,24 To date, no studies that we know of have primarily assessed the role of perioperative RBC transfusions in developing postoperative VTE among patients undergoing general surgery or surgery in various subspecialties. Using a large prospective, multicenter registry, this study examined the association between perioperative RBC transfusions and developing postoperative VTE.
Methods
Data Source
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database is a multicenter prospective registry of patients undergoing surgery and includes participation from 525 institutions (teaching and nonteaching hospitals) across North America. The ACS-NSQIP is well recognized as the leading nationally validated outcomes-based program to measure and improve the quality of surgical care; the accuracy and reproducibility of the data have also been previously demonstrated.25,26,27 The ACS-NSQIP data are collected in a standardized fashion by dedicated surgical clinical reviewers. Patients are followed up throughout their hospital course and after discharge from hospital to 30 postoperative days. Patients younger than 18 years, undergoing organ transplant, and with trauma are excluded from the ACS-NSQIP database.28,29 Participants in the present study included patients in the ACS-NSQIP registry who underwent a surgical procedure from January 1 through December 31, 2014. This study involved secondary analysis of a completely deidentified database and did not meet the definition of research with human subjects in accordance with the human research regulations (45 CFR part 46). Therefore, the Cornell institutional review board deemed the study be exempt from review by the institutional review board of Weill Cornell Medicine.
Outcomes and Risk Factors
The primary outcome was development of VTE up to 30 postoperative days. Cases were defined as new diagnoses of VTE (within 30 days of the surgical procedure) and were initially identified with diagnosis codes from the International Classification of Diseases, Ninth Revision, Clinical Modification, and verified by ACS-NSQIP data coordinators. Cases of VTE were confirmed by a definitive imaging modality (eg, duplex, venogram, computed tomographic scan, or other) or by direct pathologic examination on autopsy. Cases with known chronic deep venous thrombosis (DVT) were included only if they had documented postoperative progression (ie, chronic DVT events without documented postoperative progression were not included as a case). In addition, cases were required to have some therapeutic intervention with anticoagulation therapy and/or placement of a vena cava filter. Arterial clots were not documented as an outcome in the ACS-NSQIP database and thus could not be included in this analysis. In addition to a composite outcome of VTE, separate secondary outcomes were the occurrences of DVT and pulmonary embolism (PE) to 30 postoperative days.
The primary independent variable of interest was the occurrence of any perioperative RBC transfusion (≥1 RBC transfusion event from 72 hours before to 72 hours after surgery). The type and timing of perioperative RBC transfusion was also examined as follows: (1) none, (2) preoperative only (≤72 hours before surgery), (3) intraoperative or postoperative (from the start of surgery until 72 hours after), and (4) preoperative and intraoperative or postoperative. Although the ACS-NSQIP database did not document the exact number of RBC units transfused during the entire perioperative period, the total number of RBC transfusion events that occurred intraoperatively or postoperatively was recorded. We created a categorical and/or ordinal variable for intraoperative or postoperative RBC transfusion events (0, 1, 2, or ≥3). Intraoperative cell salvage was accounted for in the ACS-NSQIP study design with total dose transfused per case, with every 500-mL cell salvage considered equivalent to 1 unit of RBCs.
Statistical Analysis
Data were analyzed from July 1, 2016, through March 15, 2018. Descriptive statistics were used to compare characteristics of the study population by perioperative RBC transfusion status. The primary analysis examined the association of any perioperative RBC transfusion with the development of postoperative VTE. The reference group consisted of patients who never received a perioperative RBC transfusion. Univariate logistic regression was used to estimate odds ratios (ORs) and 95% CIs of postoperative VTE by perioperative RBC transfusion status. Other clinically relevant explanatory variables that were hypothesized to be potential confounders were also assessed for unadjusted associations with VTE, including age, sex, race, body mass index, baseline functional health status before surgery, the American Society of Anesthesiology severity class as a surrogate marker for the severity of underlying illness, concurrence of sepsis, the presence of disseminated cancer, relative value units of the surgical procedure as a surrogate for complexity of the surgery, use of mechanical ventilation, and hospital length of stay. Because all hypothesized potential confounders were significantly associated with VTE in univariate analysis, all variables were simultaneously included in a multivariable model; all covariates were retained in the final (fully adjusted) multivariable model because they remained significantly associated with VTE (P < .05) (eTable 1 in the Supplement). Statistical interactions were not examined.
In secondary analyses, development of postoperative DVT and PE were examined as separate outcomes. The associations between the time and type of perioperative RBC transfusion and the postoperative risk of VTE, DVT, and PE were also examined. A separate but consistent fully adjusted multivariable model was used for each exposure-outcome association.
The ACS-NSQIP database assigns a primary surgical subspecialty to all procedures included in the database. Subgroup analyses examining the association of any perioperative RBC transfusion with VTE were performed by stratifying by various surgical subspecialties, including general, cardiothoracic, gynecologic, neurologic, orthopedic, otolaryngologic, plastic, thoracic, urologic, and vascular surgery and interventional radiology. For subgroup analysis, full adjustment was made in the multivariable models as for the primary multivariable model.
An ancillary dose-response analysis was also conducted in which we assessed the association between the number of intraoperative or postoperative RBC transfusion events and the development of postoperative VTE. Similar to the primary analyses, the multivariable logistic regression model included full adjustment for all potential confounders. For this analysis, the number of RBC transfusion events was first modeled as a categorical variable. However, the 4 ordinal categories were also modeled as a continuous variable to test for a linear trend (P value for trend).
The multivariable models in the main analysis used a complete-case approach such that patients with any missing covariate data were excluded. All P values were 2-tailed, and statistical significance was set at P < .05. Data analysis was performed using Stata/MP software (version 15.1; StataCorp, Inc).
Two sensitivity analyses were performed. First, we examined missing data patterns and reanalyzed the data after using multiple imputation with chained equations. In addition to all variables in the primary analysis, the imputation model included ethnicity as an auxiliary variable. Twenty imputations were performed. A second sensitivity analysis was performed using propensity score matching, which can adjust for some baseline group differences and is a well-accepted method to account for identified confounding variables.30,31 Propensity scores of receiving any perioperative RBC transfusion (vs no RBC transfusion) were estimated by logistic regression using all covariates included in the primary analysis. For this analysis, binary indicators for missing data were included for each covariate when estimating the propensity scores. Patients who received any perioperative RBC transfusion were matched 1:1 (without replacement) on the logit of the propensity score using a nearest-neighbor matching approach and within a caliper equal to 0.2 of the SD of the logit of the propensity score.32 The balance of covariates between patients who received any perioperative RBC transfusion and matched control individuals was examined by calculating standardized percentage differences. In the propensity score-matched subsample, univariate conditional logistic regression models were used to estimate matched ORs of VTE.
Results
A total of 750 937 patients who underwent surgical procedures and were included in the ACS-NSQIP database for 2014 were analyzed (43.2% men and 56.8% women; median age, 58 years; interquartile range, 44-69 years). Perioperative transfusion of RBCs were documented in 47 410 patients (6.3%) (Table 1). Of these, 3605 patients (0.5%) received preoperative RBC transfusions only and 40 015 (5.3%) received intraoperative or postoperative RBC transfusions only. Preoperative and intraoperative or postoperative RBC transfusions occurred in 3790 patients (0.5%). A total of 6309 patients (0.8%) developed a postoperative VTE that warranted treatment. Deep venous thrombosis was documented in 4336 patients (0.6%); PE, 2514 patients (0.3%); and DVT and PE, 541 patients (0.1%). Missing data for each covariate was minimal (<2.5%) except for race, which was missing for 93 542 patients (12.5%).
Table 1. Patient Characteristics of the Study Sample by Perioperative Red Blood Cell Transfusion Statusa.
| Clinical Variable | No. (%) of Patients | ||
|---|---|---|---|
| Overall (N = 750 937) | Transfusion Group (n = 47 410) | Nontransfusion Group (n = 703 527) | |
| Age group, y | |||
| 18-44 | 188 796 (25.1) | 4609 (9.7) | 184 187 (26.2) |
| 45-60 | 236 902 (31.5) | 11 158 (23.5) | 225 744 (32.1) |
| >60 | 325 239 (43.3) | 31 643 (66.7) | 293 596 (41.7) |
| Sexb | |||
| Male | 324 209 (43.2) | 20 612 (43.5) | 303 597 (43.2) |
| Female | 426 727 (56.8) | 26 798 (56.5) | 399 929 (56.8) |
| Race | |||
| White | 551 935 (73.5) | 34 027 (71.8) | 517 908 (73.6) |
| Black or African American | 75 885 (10.1) | 6221 (13.1) | 69 664 (9.9) |
| Asian | 21 081 (2.8) | 1480 (3.1) | 19 601 (2.8) |
| Other | 8494 (1.1) | 460 (1.0) | 8034 (1.1) |
| Unknown | 93 542 (12.5) | 5222 (11.0) | 88 320 (12.6) |
| BMI | |||
| Underweight (<18.5) | 12 357 (1.6) | 1997 (4.2) | 10 360 (1.5) |
| Normal weight (18.5-24.9) | 179 533 (23.9) | 14 717 (31.0) | 164 816 (23.4) |
| Overweight (25.0-29.9) | 230 320 (30.7) | 13 666 (28.8) | 216 654 (30.8) |
| Obese | |||
| Class 1 (30.0-34.9) | 155 134 (20.7) | 8235 (17.4) | 146 899 (20.9) |
| Class 2 (35.0-39.9) | 81 949 (10.9) | 3950 (8.3) | 77 999 (11.1) |
| Class 3 (≥40.0) | 76 632 (10.2) | 3294 (6.9) | 73 338 (10.4) |
| Unknown | 15 012 (2.0) | 1551 (3.3) | 13 461 (1.9) |
| Functional health status before surgery | |||
| Independent | 726 360 (96.7) | 42 283 (89.2) | 684 077 (97.2) |
| Partially dependent | 16 006 (2.1) | 3755 (7.9) | 12 251 (1.7) |
| Totally dependent | 3184 (0.4) | 937 (2.0) | 2247 (0.3) |
| Unknown | 5387 (0.7) | 435 (0.9) | 4952 (0.7) |
| ASA class | |||
| No disturbance (1) | 69 685 (9.3) | 528 (1.1) | 69 157 (9.8) |
| Mild disturbance (2) | 337 688 (45.0) | 8742 (18.4) | 328 946 (46.8) |
| Severe disturbance (3) | 296 037 (39.4) | 25 493 (53.8) | 270 544 (38.5) |
| Life-threatening (4) | 44 179 (5.9) | 11 762 (24.8) | 32 417 (4.6) |
| Moribund (5) | 1343 (0.2) | 808 (1.7) | 535 (0.1) |
| Unknown | 2005 (0.3) | 77 (0.2) | 1928 (0.3) |
| Hospital length of stay, d | |||
| 0-1 | 372 952 (49.7) | 1316 (2.8) | 371 636 (52.8) |
| ≥2 | 377 369 (50.3) | 45 923 (96.9) | 331 446 (47.1) |
| Unknown | 616 (0.1) | 171 (0.4) | 445 (0.1) |
| Other medical complications | |||
| Concurrent sepsis | 13 307 (1.8) | 2886 (6.1) | 10 421 (1.5) |
| Ventilator dependence | 2557 (0.3) | 1385 (2.9) | 1172 (0.2) |
| Disseminated cancer | 17 850 (2.4) | 3688 (7.8) | 14 162 (2.0) |
| Work-related relative value units | |||
| Quartile 1 (<9.45) | 188 666 (25.1) | 2573 (5.4) | 186 093 (26.5) |
| Quartile 2 (9.45-15.38) | 191 609 (25.5) | 5185 (10.9) | 186 424 (26.5) |
| Quartile 3 (15.39-20.82) | 185 945 (24.8) | 15 285 (32.2) | 170 660 (24.3) |
| Quartile 4 (>20.82) | 184 717 (24.6) | 24 367 (51.4) | 160 350 (22.8) |
| Perioperative RBC transfusion | 47 410 (6.3) | 47 410 (100.0) | 0 |
| Preoperative only | 3605 (0.5) | 3605 (7.6) | 0 |
| Intraoperative or postoperative only | 40 015 (5.3) | 40 015 (84.4) | 0 |
| Preoperative and intraoperative or postoperative | 3790 (0.5) | 3790 (8.0) | 0 |
Abbreviations: ASA, American Society of Anesthesiology; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); RBC, red blood cell.
Data were derived from the American College of Surgeons’ National Surgical Quality Improvement Program database for 2014. Percentages have been rounded and may not total to 100.
One person had missing data for sex.
Patients receiving any perioperative RBC transfusion had significantly higher unadjusted odds of developing postoperative VTE (odds ratio [OR], 5.2; 95% CI, 4.9-5.5), DVT (OR, 5.8; 95% CI, 5.4-6.2), and PE (OR, 4.0; 95% CI, 3.6-4.4) compared with patients who never received an RBC transfusion (Table 2). In a multivariable model adjusted for age, sex, race, length of hospital stay, use of mechanical ventilation, presence of disseminated cancer, concurrent sepsis, body mass index, baseline functional status before surgery, American Society of Anesthesiology class, and work-related relative value units for the surgery, the odds of developing postoperative VTE were significantly higher among patients receiving any perioperative RBC transfusions vs no transfusions (adjusted OR [aOR], 2.1; 95% CI, 2.0-2.3) (Table 3 and eTable 1 in the Supplement). Data for the full model are detailed in eTable 1 in the Supplement. In separate multivariable models, any perioperative RBC transfusion remained significantly associated with developing postoperative DVT (aOR, 2.2; 95% CI, 2.1-2.4) and PE (aOR, 1.9; 95% CI, 1.7-2.1) (Table 3).
Table 2. Univariate Analysis of the Association Between Perioperative RBC Transfusion and the Development of Postoperative VTE, DVT, or PEa.
| Perioperative RBC Transfusion | No. of Patients (N = 750 937) | Thrombolic Eventb | |||||
|---|---|---|---|---|---|---|---|
| VTE | DVT | PE | |||||
| No. (%) | OR (95% CI) | No. (%) | OR (95% CI) | No. (%) | OR (95% CI) | ||
| Perioperative RBC transfusion | |||||||
| No | 703 527 | 4707 (0.7) | 1 [Reference] | 3141 (0.5) | 1 [Reference] | 1987 (0.3) | 1 [Reference] |
| Yes | 47 410 | 1602 (3.4) | 5.2 (4.9-5.5) | 1195 (2.5) | 5.8 (5.4-6.2) | 527 (1.1) | 4.0 (3.6-4.4) |
| Time of perioperative transfusion | |||||||
| None | 703 527 | 4707 (0.7) | 1 [Reference] | 3141 (0.5) | 1 [Reference] | 1987 (0.3) | 1 [Reference] |
| Preoperative only | 3605 | 105 (2.9) | 4.5 (3.7-5.4) | 88 (2.4) | 5.6 (4.5-6.9) | 30 (0.8) | 3.0 (2.1-4.3) |
| Intraoperative or postoperative only | 40 015 | 1295 (3.2) | 5.0 (4.7-5.3) | 940 (2.4) | 5.4 (5.0-5.8) | 452 (1.1) | 4.0 (3.6-4.5) |
| Preoperative and intraoperative or postoperative | 3790 | 202 (5.3) | 8.4 (7.2-9.7) | 167 (4.4) | 10.3 (8.8-12.0) | 45 (1.2) | 4.2 (3.2-5.7) |
Abbreviations: DVT, deep venous thrombosis; OR, odds ratio; PE, pulmonary embolism; RBC, red blood cell; VTE, venous thromboembolism.
Includes events within 30 d of a surgical procedure. Data were derived from the American College of Surgeons’ National Surgical Quality Improvement Program database for 2014.
Separate univariate models were used for each exposure-outcome association shown.
Table 3. Multivariable Risk-Adjusted Analysis of the Association Between Perioperative RBC Transfusion and the Development of Postoperative VTE, DVT, and PEa.
| Perioperative RBC Transfusion | Adjusted OR (95% CI)b | ||
|---|---|---|---|
| VTE | DVT | PE | |
| Perioperative RBC transfusion | |||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 2.1 (2.0-2.3) | 2.2 (2.1-2.4) | 1.9 (1.7-2.1) |
| Time of perioperative transfusion | |||
| None | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Preoperative only | 1.9 (1.5-2.3) | 2.1 (1.6-2.6) | 1.7 (1.2-2.5) |
| Intraoperative or postoperative only | 2.1 (1.9-2.2) | 2.2 (2.0-2.3) | 1.9 (1.7-2.1) |
| Preoperative and intraoperative or postoperative | 3.0 (2.5-3.5) | 3.3 (2.7-3.9) | 1.8 (1.3-2.6) |
Abbreviations: DVT, deep venous thrombosis; OR, odds ratio; PE, pulmonary embolism; RBC, red blood cell; VTE, venous thromboembolism.
Includes events within 30 d of a surgical procedure. Data were derived from the American College of Surgeons’ National Surgical Quality Improvement Program database for 2014.
This was a complete-case analysis of 642 946 patients. Separate multivariable models were used for each exposure-outcome association shown. Each multivariable model was adjusted for age, sex, race, body mass index, functional health status before surgery, the American Society of Anesthesiology severity class, hospital length of stay, occurrence of sepsis, mechanical ventilation dependence, disseminated cancer, and work-related relative value units (as a surrogate for complexity of surgery).
Patients receiving only preoperative RBC transfusions, only intraoperative or postoperative RBC transfusions, and both preoperative and intraoperative or postoperative RBC transfusions all had significantly higher unadjusted odds of developing VTE compared with patients who did not receive RBC transfusions (Table 2). In multivariable analysis, patients receiving only preoperative RBC transfusions (aOR, 1.9; 95% CI, 1.5-2.3) and only intraoperative or postoperative RBC transfusions (aOR, 2.1; 95% CI, 1.9-2.2) had significantly higher adjusted odds of developing postoperative VTE compared with patients who did not receive RBC transfusions, and the association was stronger for patients who received preoperative and intraoperative or postoperative RBC transfusions (aOR, 3.0; 95% CI, 2.5-3.5) (Table 3). Each type of perioperative RBC transfusion was also significantly associated with developing postoperative DVT and PE in secondary analyses (Tables 2 and 3).
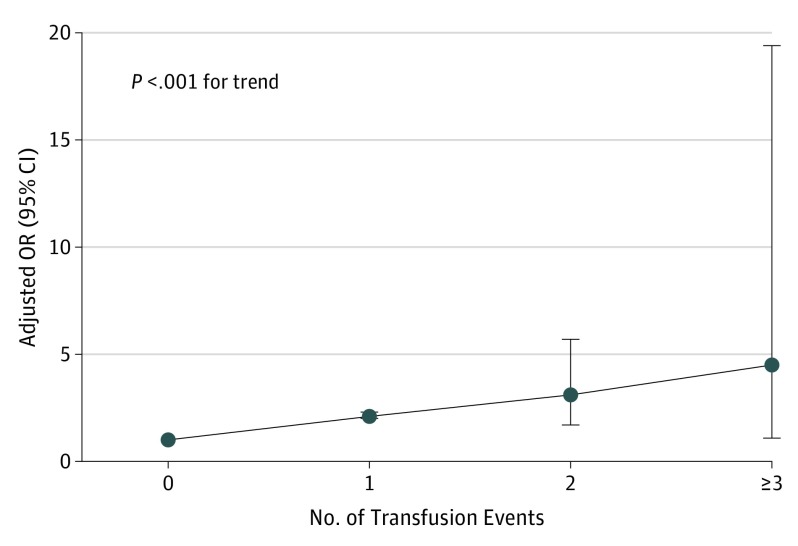
In a separate dose-response analysis, the odds of postoperative VTE increased significantly with an increasing number of intraoperative or postoperative RBC transfusion events (Figure). Compared with patients who did not receive an intraoperative or postoperative RBC transfusion (n = 707 132), patients with 1 (n = 43 490) (aOR, 2.1; 95% CI, 2.0-2.3), 2 (n = 275) (aOR, 3.1; 95% CI, 1.7-5.7), and 3 or more (n = 40) (aOR, 4.5; 95% CI, 1.0-19.4) RBC transfusion events during the intraoperative or postoperative period had increasing odds of developing postoperative VTE (P < .001 for trend).
Figure. Dose-Response Analysis.
Adjusted odds ratios (ORs) of 30-day postoperative venous thromboembolism with increased number of red blood cell (RBC) events intraoperatively or postoperatively vs no intraoperative or postoperative RBC transfusion are shown. The multivariable model was adjusted for age, sex, race, sepsis, length of stay, mechanical ventilation, disseminated cancer, body mass index, work-related relative value unit for the surgery (surrogate for complexity of surgery), and American Society of Anesthesiology severity class and functional status before surgery. Data were derived from the American College of Surgeons’ National Surgical Quality Improvement Program database for 2014.
In subgroup analyses stratified by various surgical subspecialties, significantly higher adjusted odds of developing VTE were found among patients receiving any perioperative RBC transfusions in various surgical subtypes, including general, neurologic, cardiothoracic, orthopedic, vascular, gynecologic, and urologic surgery (Table 4). Of note, interventional radiologic and otolaryngologic procedures were excluded from the subgroup analyses owing to an insufficient balance in sample sizes to allow the logistic regression model to converge.
Table 4. Subgroup Analysis by Surgical Subspecialty of the Association Between Any Perioperative RBC Transfusion and the Development of Postoperative VTEa.
| Surgical Subspecialtyb | Total No. of Patients | No. of Patients Receiving a Transfusion | No. (%) of Patients Who Developed VTE | OR (95% CI)c | Adjusted OR (95% CI) | |
|---|---|---|---|---|---|---|
| No Transfusion | Transfusion | |||||
| General surgery | 360 397 | 16 931 | 2242 (0.7) | 778 (4.6) | 7.3 (6.7-8.0) | 2.3 (2.1-2.5) |
| Neurosurgery | 37 442 | 1900 | 401 (1.1) | 97 (5.1) | 4.7 (3.8-5.9) | 2.4 (1.8-3.1) |
| Cardiothoracic surgery | 13 113 | 2764 | 123 (1.2) | 75 (2.7) | 2.3 (1.7-3.1) | 1.8 (1.2-2.5) |
| Orthopedic surgery | 153 320 | 12 641 | 1142 (0.8) | 280 (2.2) | 2.8 (2.4-3.2) | 1.7 (1.5-2.0) |
| Vascular surgery | 49 582 | 7197 | 274 (0.7) | 162 (2.3) | 3.5 (2.9-4.3) | 2.5 (2.0-3.2) |
| Gynecological surgery | 55 339 | 2933 | 188 (0.4) | 92 (3.1) | 9.0 (7.0-11.6) | 2.9 (2.1-4.0)d |
| Urological surgery | 39 632 | 2388 | 230 (0.6) | 101 (4.2) | 7.1 (5.6-9.0) | 2.9 (2.2-3.9)d |
Abbreviations: OR, odds ratio; RBC, red blood cell; VTE, venous thromboembolism.
Includes events within 30 d of a surgical procedure. Data were derived from the American College of Surgeons’ National Surgical Quality Improvement Program Database for 2014.
Remaining surgical subspecialty types were otolaryngology and interventional radiology, which were excluded owing to none or too few transfusions.
Unless specified otherwise, the multivariable model for each subspecialty was fully adjusted for age, sex, race, body mass index, functional health status before surgery, the American Society of Anesthesiology severity class, hospital length of stay, occurrence of sepsis, mechanical ventilation dependence, disseminated cancer, and work-related relative value units (as a surrogate for complexity of surgery). The multivariable models were limited to complete cases.
Mechanical ventilation use was not included in the multivariable model owing to multicollinearity.
Sensitivity Analyses
The results obtained using multiply imputed data mirrored estimates calculated in the primary (complete-case) multivariable analyses (eTable 2 in the Supplement). For instance, any perioperative RBC transfusion remained independently associated with VTE (aOR, 2.1; 95% CI, 2.0-2.3) after multiple imputation (n = 750 937).
In addition, using 1:1 propensity score matching, we generated a subsample of 47 142 patients who received any perioperative RBC transfusion and 47 142 matched controls who did not receive any RBC transfusions (eFigure in the Supplement). Patient characteristics of the original sample population and the matched subsample in this sensitivity analysis are shown in eTable 3 in the Supplement. The propensity score accounted for all the variables included in the multivariable model in the primary analysis, and these covariates were well balanced by perioperative RBC transfusion status in the matched subsample (eTable 3 in the Supplement). Receipt of any perioperative RBC transfusion was significantly associated with higher odds of VTE compared with patients who never received a transfusion (propensity-matched OR, 1.9; 95% CI, 1.8-2.1) (eTable 4 in the Supplement).
Discussion
This study examined the association of perioperative RBC transfusion with postoperative VTE. In this study of 750 937 patients undergoing surgical procedures, RBC transfusions were associated with an increased risk of VTE (including DVT and PE).
Increasing evidence suggests a role of RBCs in physiological hemostasis and pathologic thrombosis.9,10 Thrombosis is primarily considered to result from the interplay of endothelial cells, platelets, and soluble coagulation factors. Red blood cells are generally regarded as passive bystanders that coincidentally become entrapped in a developing thrombus as they flow through the vasculature. However, the initial published clinical observation to hypothesize that RBCs might play an active role in hemostasis was reported in 1910.33 The study noted that patients with thrombocytopenia showed an improvement in bleeding times after whole blood transfusion, although their platelet counts remained low. Although no direct evidence indicated that RBCs were the responsible component, the large increase in RBCs in the vasculature (despite no increase in platelet count) suggested that RBCs were the most likely candidate for improving the hemostatic parameters. Animal experiments and normal volunteer studies suggest that with as little as a 15% decrease in the hemoglobin level, a corresponding increase in bleeding time occurs.34 This observation led some clinicians to advocate for higher hemoglobin transfusion thresholds in patients with severe thrombocytopenia who are at increased risk of bleeding.35 This association has been reemphasized in recent studies.36 Conversely, excessive bleeding has been shown to be treated by elevation of hematocrit without increasing platelet counts.33,34,37 Abnormally high RBC counts and increased hematocrit (eg, in polycythemia vera) can also predispose a patient to thrombotic disease with hepatic, portal, cardiac, and cerebral thrombosis.38,39 Through the potential effect on blood viscosity, RBC transfusions can alter the rheologic variables that govern thrombosis and result in an elevated clotting potential.40
Red blood cells have also been shown to increase platelet responsiveness, especially at lower platelet counts.41 In the TRANSFUSION-2 (Impact of Transfusion of Red Blood Cells on Platelet Activation and Aggregation Studied With Flow Cytometry Use and Light Transmission Aggregometry) trial,42 the platelet reactivity increased in patients with acute coronary syndrome after an RBC transfusion. The authors concluded that the in vivo effect of RBC transfusion may account for the excess of ischemic events observed in the context of patients with acute coronary syndrome.42 In addition, murine model–based studies via iron trichloride–mediated thrombosis have revealed a previously unrecognized ability of RBCs to participate in thrombosis by mediating platelet adhesion to the intact endothelial surface.11
Recently, Walton et al21 showed that RBCs infused into healthy mice were associated with clot formation, thus isolating the contribution of the increased RBCs, and they suggested that elevated hematocrit may enhance thrombotic risk by increasing the number of platelets incorporated into the thrombus. That study21 also highlighted the effect of transfused RBCs on increasing the rate of thrombus formation beyond the native RBCs. Red blood cell storage lesions are responsible for accumulation of bioreactive substances43,44,45 such as microparticles.12,13 A few studies have provided evidence supporting an increase in levels of microparticles in stored RBCs and proposed a potential mechanistic role of these microparticles leading to venous and arterial thrombosis.44,45 In addition, storage of RBCs has been shown to increase endothelial adherence, which may also predispose toward thrombotic events. Thus, multiple mechanistic pathways link native and transfused RBCs to pathologic thrombotic outcomes for which our study provides supportive evidence.
Limitations
The ACS-NSQIP registry is the leading prospective, nationally validated surgical outcomes–based program and uses a standardized data collection algorithm; however, this study has limitations. Posttransfusion hematocrit (a possible surrogate marker of RBC mass) was not available in the database. A composite validated index of severity of illness, such as the Case-Mix Index, was not available. However, the results remained robust after adjustment for American Society of Anesthesiology classification of physical health, which is a widely accepted grading system for assessing preoperative health of the patients undergoing surgery, and relative value units, a surrogate marker for complexity of the surgical procedure. Because of the study design, cases of known chronic DVT could not be analyzed separately. The ACS-NSQIP database does not provide individual hospital-level information. Therefore, we could not evaluate the variation in outcomes by the hospital-wide practices in VTE prophylaxis and adherence to guidelines or account for clustering by hospital. Chronic DVT cases were included only with documented postoperative progression. Given the possible different physiologic and hematologic outcomes of transfusing cell-saver RBCs compared with stored RBCs, it would be ideal to conduct subgroup analyses of the patients with and without cell-saver transfusions. This analysis was not possible owing to the ACS-NSQIP registry not identifying these patients separately. Although the exact hemoglobin and/or hematocrit threshold for intraoperative or postoperative transfusion was unknown, the transfusions were administered in response to intraoperative bleeding. The exact details of the bleeding or anticipated blood loss and any associated hemodynamic changes are not known. Finally, future studies should account for additional potential confounders, such as concurrent medication use (eg, VTE prophylaxis) and family history of VTE or an underlying thrombophilia. The same association between perioperative RBC transfusion and development of VTE was observed in the propensity score–matched sensitivity analysis.
Conclusions
Perioperative RBC transfusions may be associated with development of new or progressive VTE warranting treatment within 30 days of surgery. The indications for transfusion (eg, hemodynamics, symptoms, and hemoglobin level and/or hematocrit), which may contribute to VTE, were not known. However, surgery is a well-recognized prothrombotic stimulus, and in a subset of patients receiving perioperative RBC transfusions, a synergistic and incremental dose-related risk for VTE development may exist. These results need prospective validation in cohort studies and randomized clinical trials; if proven, they underscore the continued need for more stringent and optimal perioperative blood management practices in addition to rigorous VTE prophylaxis in patients undergoing surgery.
eTable 1. Univariate and Multivariable Analysis of Factors Associated With the Development of VTE Within 30 Days Postoperatively in Patients Undergoing Surgical Procedures
eTable 2. Sensitivity Analysis of the Association Between Perioperative Red Blood Cell Transfusion and the Development of Postoperative VTE Within 30 Days of a Surgical Procedure Using Multiple Imputation With Chained Equations
eTable 3. Characteristics of Participants in the Full Sample and the Propensity-Matched Subsample by Perioperative Red Blood Cell Transfusion Status
eTable 4. Association of Perioperative Red Blood Cell Transfusion and the Development of VTE Within 30 Days Postoperatively in Propensity-Matched Patient Groups Undergoing Surgical Procedures
eFigure. Schema of Participants in the Original Sample and the Propensity-Matched Subsample
References
- 1.Centers for Disease Control and Prevention Venous thromboembolism (blood clots): data and statistics on HA-VTE. https://www.cdc.gov/ncbddd/dvt/ha-vte-data.html. Reviewed April 6, 2017. Accessed April 26, 2018.
- 2.Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23)(suppl 1):I9-I16. [DOI] [PubMed] [Google Scholar]
- 3.Lau BD, Haut ER. Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23(3):187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeidan AM, Streiff MB, Lau BD, et al. . Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545-549. [DOI] [PubMed] [Google Scholar]
- 5.Cook D, Crowther M, Meade M, et al. . Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. 2005;33(7):1565-1571. [DOI] [PubMed] [Google Scholar]
- 6.Fleming FJ, Kim MJ, Salloum RM, Young KC, Monson JR. How much do we need to worry about venous thromboembolism after hospital discharge? a study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53(10):1355-1360. [DOI] [PubMed] [Google Scholar]
- 7.Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vascular Surg. 2007;45(2):335-342. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld BA, Beattie C, Christopherson R, et al. ; Perioperative Ischemia Randomized Anesthesia Trial Study Group . The effects of different anesthetic regimens on fibrinolysis and the development of postoperative arterial thrombosis. Anesthesiology. 1993;79(3):435-443. [DOI] [PubMed] [Google Scholar]
- 9.Byrnes JR, Wolberg AS. Red blood cells in thrombosis. Blood. 2017;130(16):1795-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litvinov RI, Weisel JW. Role of red blood cells in haemostasis and thrombosis. ISBT Sci Ser. 2017;12(1):176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood. 2013;121(18):3733-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donadee C, Raat NJ, Kanias T, et al. . Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Zhao W, Christ GJ, Gladwin MT, Kim-Shapiro DB. Nitric oxide scavenging by red cell microparticles. Free Radic Biol Med. 2013;65:1164-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168(21):2377-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson RE, Grosse SD, Waitzman NJ, et al. . Using multiple sources of data for surveillance of postoperative venous thromboembolism among surgical patients treated in Department of Veterans Affairs hospitals, 2005-2010. Thromb Res. 2015;135(4):636-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hod EA, Godbey EA. The outsider adverse event in transfusion: inflammation. Presse Med. 2016;45(7-8, pt 2):e325-e329. [DOI] [PubMed] [Google Scholar]
- 17.Hod EA. Red blood cell transfusion-induced inflammation: myth or reality. ISBT Sci Ser. 2015;10(suppl 1):188-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zallen G, Moore EE, Ciesla DJ, Brown M, Biffl WL, Silliman CC. Stored red blood cells selectively activate human neutrophils to release IL-8 and secretory PLA2. Shock. 2000;13(1):29-33. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104(43):17058-17062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.L’Acqua C, Bandyopadhyay S, Francis RO, et al. . Red blood cell transfusion is associated with increased hemolysis and an acute phase response in a subset of critically ill children. Am J Hematol. 2015;90(10):915-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton BL, Lehmann M, Skorczewski T, et al. . Elevated hematocrit enhances platelet accumulation following vascular injury. Blood. 2017;129(18):2537-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machlus KR, Battinelli EM. RBCs pin platelets against the (thrombus) wall. Blood. 2017;129(18):2460-2461. [DOI] [PubMed] [Google Scholar]
- 23.Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res. 2012;129(5):568-572. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson KR, Berenholtz SM, Garrett-Mayer E, Dorman T, Klag MJ, Pronovost PJ. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg. 2007;142(2):126-132. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Cohen ME, Hall BL, Ko CY, Bilimoria KY. Evaluation and enhancement of calibration in the American College of Surgeons NSQIP surgical risk calculator. J Am Coll Surg. 2016;223(2):231-239. [DOI] [PubMed] [Google Scholar]
- 26.Mansmann U, Rieger A, Strahwald B, Crispin A. Risk calculators: methods, development, implementation, and validation. Int J Colorectal Dis. 2016;31(6):1111-1116. [DOI] [PubMed] [Google Scholar]
- 27.Kaafarani HM, Mavros MN, Hwabejire J, et al. . Derivation and validation of a novel severity classification for intraoperative adverse events. J Am Coll Surg. 2014;218(6):1120-1128. [DOI] [PubMed] [Google Scholar]
- 28.Khuri SF, Daley J, Henderson W, et al. ; National VA Surgical Quality Improvement Program . The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. Ann Surg. 1998;228(4):491-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daley J, Khuri SF, Henderson W, et al. . Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185(4):328-340. [PubMed] [Google Scholar]
- 30.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahlert J, Gribsholt SB, Gammelager H, Dekkers OM, Luta G. Control of confounding in the analysis phase: an overview for clinicians. Clin Epidemiol. 2017;9:195-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol. 2014;179(2):226-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duke WW. The relation of blood platelets to hemorrhagic disease. JAMA. 1910;55:1185-1192. [PubMed] [Google Scholar]
- 34.Blajchman MA, Bordin JO, Bardossy L, Heddle NM. The contribution of the haematocrit to thrombocytopenic bleeding in experimental animals. Br J Haematol. 1994;86(2):347-350. [DOI] [PubMed] [Google Scholar]
- 35.Valeri CR, Cassidy G, Pivacek LE, et al. . Anemia-induced increase in the bleeding time: implications for treatment of nonsurgical blood loss. Transfusion. 2001;41(8):977-983. [DOI] [PubMed] [Google Scholar]
- 36.Uhl L, Assmann SF, Hamza TH, Harrison RW, Gernsheimer T, Slichter SJ. Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood. 2017;130(10):1247-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho CH. The hemostatic effect of adequate red cell transfusion in patients with anemia and thrombocytopenia. Transfusion. 1996;36(3):290. [DOI] [PubMed] [Google Scholar]
- 38.Schafer AI. Bleeding and thrombosis in the myeloproliferative disorders. Blood. 1984;64(1):1-12. [PubMed] [Google Scholar]
- 39.Rossi C, Randi ML, Zerbinati P, Rinaldi V, Girolami A. Acute coronary disease in essential thrombocythemia and polycythemia vera. J Intern Med. 1998;244(1):49-53. [DOI] [PubMed] [Google Scholar]
- 40.Stoltz JF, Donner M. New trends in clinical hemorheology: an introduction to the concept of the hemorheological profile. Schweiz Med Wochenschr Suppl. 1991;43:41-49. [PubMed] [Google Scholar]
- 41.Valles J, Santos MT, Aznar J, et al. . Erythrocytes metabolically enhance collagen-induced platelet responsiveness via increased thromboxane production, adenosine diphosphate release, and recruitment. Blood. 1991;78(1):154-162. [PubMed] [Google Scholar]
- 42.Silvain J, Abtan J, Kerneis M, et al. . Impact of red blood cell transfusion on platelet aggregation and inflammatory response in anemic coronary and noncoronary patients: the TRANSFUSION-2 study (Impact of Transfusion of Red Blood Cell on Platelet Activation and Aggregation Studied With Flow Cytometry Use and Light Transmission Aggregometry). J Am Coll Cardiol. 2014;63(13):1289-1296. [DOI] [PubMed] [Google Scholar]
- 43.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96(2):93-103. [DOI] [PubMed] [Google Scholar]
- 44.Chin-Yee I, Arya N, d’Almeida MS. The red cell storage lesion and its implication for transfusion. Transfus Sci. 1997;18(3):447-458. [DOI] [PubMed] [Google Scholar]
- 45.Hess JR. Red cell storage. J Proteomics. 2010;73(3):368-373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Univariate and Multivariable Analysis of Factors Associated With the Development of VTE Within 30 Days Postoperatively in Patients Undergoing Surgical Procedures
eTable 2. Sensitivity Analysis of the Association Between Perioperative Red Blood Cell Transfusion and the Development of Postoperative VTE Within 30 Days of a Surgical Procedure Using Multiple Imputation With Chained Equations
eTable 3. Characteristics of Participants in the Full Sample and the Propensity-Matched Subsample by Perioperative Red Blood Cell Transfusion Status
eTable 4. Association of Perioperative Red Blood Cell Transfusion and the Development of VTE Within 30 Days Postoperatively in Propensity-Matched Patient Groups Undergoing Surgical Procedures
eFigure. Schema of Participants in the Original Sample and the Propensity-Matched Subsample