Key Points
Question
Is the occurrence of live-born major congenital heart disease (CHD) changing?
Findings
In a nationwide, population-based study from 1996 to 2013 in Denmark, the live-born incidence of major CHD decreased from 0.22% to 0.14%. Prenatal detection rate increased, as did the proportion of terminated pregnancies, and when terminated pregnancies were included, the incidence of major CHD remained constant during the study.
Meaning
The increased prenatal detection of major CHD has led to an increased termination of pregnancy rate, with a subsequent decrease in live-birth incidence of major CHD.
Abstract
Importance
The occurrence of major congenital heart disease (CHD) is affected by several variables. Determining the development of the true incidence is critical to the establishment of proper treatment of these patients.
Objective
To evaluate time trends in incidence, detection rate, and termination of pregnancy (TOP) rate of major CHD in fetuses in Denmark and assess the influence of the introduction of general prenatal screening in 2004.
Design, Setting, and Participants
Nationwide, population-based, retrospective observational study in Denmark from 1996 to 2013 that included a consecutive sample of 14 688 live-born children and terminated fetuses diagnosed as having CHD. Patient records on TOP and children with major CHD were reviewed to validate the diagnoses. Major CHD included univentricular heart, transposition of the great arteries, congenitally corrected transposition of the great arteries, truncus arteriosus, interrupted aortic arch, atrioventricular septal defects, double outlet right ventricle, coarctatio of the aorta, Ebstein anomaly, pulmonary atresia with ventricular septal defect, pulmonary atresia with intact ventricular septum, and tetralogy of Fallot. Data were analyzed between January 2017 and March 2018.
Main Outcomes and Measures
Temporal changes in incidence, detection rate, and TOP of major CHD.
Results
Of 14 688 children and fetuses diagnosed with CHD, 2695 (18.4%; 95% CI, 17.8-19.1) had major CHD. A total of 7131 boys (1304 with major CHD) and 6926 girls (920 with major CHD) were included, with a median age of 11 years (interquartile range, 6-15 years). During the study period, the live-birth incidence of CHD was constant at 1.22% (95% CI, 1.18-1.26), whereas it decreased for major CHD. When including TOP, the incidence of major CHD did not change over time. The detection rate of major CHD increased from 4.5% (95% CI, 1.2-7.8) to 71.0% (95% CI, 63.3-78.7) (P < .001). At the end of the study, all cases of double outlet right ventricle, Ebstein anomaly, congenitally corrected transposition of the great arteries, and pulmonary atresia with ventricular septal defect were detected prenatally, whereas coarctation of the aorta had the lowest detection rate (21.7%; 95% CI, 3.5-40.0). The TOP rate increased from 0.6% (95% CI, −0.6 to 1.9) to 39.1% (95% CI, 30.9-47.4) (P < .001) among all major CHD. For prenatally diagnosed major CHD, 57.8% of cases were terminated and the proportion did not change significantly throughout the study. Diagnoses leading to TOP included all major CHD diagnoses.
Conclusions and Relevance
Detection rates of major CHD improved during the study. This has led to increased TOP rates, with a subsequent 39% decrease in the live-birth incidence of major CHD.
This population-based study evaluates time trends in incidence, detection rate, and termination of pregnancy rate of major congenital heart disease in fetuses in Denmark and assess the influence of the introduction of general prenatal screening in 2004.
Introduction
The reported incidence of congenital heart disease (CHD) varies from 0.8% to 1.5%.1,2,3 However, there are a limited number of nationwide population-based studies examining the incidence of CHD and even fewer that include termination of pregnancies (TOP). Termination of pregnancy rates vary according to cultural and religious differences, as well as prenatal detection rates for CHD. Different approaches to prenatal screening exist worldwide, ranging from on indication only to the routine practice of 3 ultrasonographic scans during pregnancy.4,5,6 Furthermore, access to routine scans may vary within a country owing to geographical, social, and economic limitations.
In Denmark, prior to 2004, the Danish Health Authority recommended that prenatal screening for fetal anomalies only be offered in high-risk pregnancies. In 2004, the Danish Health Authority changed the recommendations so that ultrasonography is offered universally, free of charge, with more than 95% of women in Denmark scanned during the pregnancy.
Prenatal diagnosis is pivotal because morbidity and mortality may be reduced when major CHD is detected prenatally7,8,9,10; however, the efficacy of routine screening has been questioned.4,11 Routine obstetric ultrasonography aims to identify suspected cases of CHD so these women can be referred for full fetal echocardiography by a specialist in fetal medicine, thereby detecting most CHD. Even so, it is unclear whether the detection rate of major CHD is acceptably high with screening of low-risk pregnancies because populations and techniques in previous studies have differed considerably. Thus, reported detection rates range from 23% to 68%.1,3,12,13 We aimed to evaluate the incidence, detection rates, and TOP rates of major CHD and assess their development over time as well as the influence of general prenatal screening.
Methods
We conducted a nationwide, retrospective study including all children born in Denmark with a CHD, as well as fetuses diagnosed with a CHD leading to TOP between January 1, 1996, and December 31, 2013. Our inclusion period ended in 2013 to allow time for the diagnosis of minor CHDs.
Data were extracted from the Danish National Patient Registry (NPR) and the Cause of Death Registry in January 2015. The NPR is a nationwide, population-based registry that prospectively records all diagnoses made during outpatient visits and hospital admissions, and the Cause of Death Registry logs all causes of death. All Danish residents are given a unique 10-digit registration number at birth or obtainment of residency permit, enabling identification of every person with possible relevance to the study.
Approval
The study was approved by the Danish Data Protection Agency (RH-2016-339) and the Danish Health Authority (3-3013-549/1). The study is a registry research study; therefore, approval from the research ethics committee, including the need for informed consent, was waived (protocol H-1-2013-FSP-033).
Screening Protocol
Between 1996 and 2004, prenatal screening was not universally offered.14 From 2004 on, all pregnant women were offered 2 ultrasonographies: 1 at 11 to 13 weeks to assess the nuchal fold and determine the risk of aneuploidy and a malformation scan at 18 to 20 weeks. The 4-chamber view was used for cardiac evaluation throughout the study, and some hospitals also included the 3-vessel and outflow-tract views. By 2010, these views were implemented nationwide.
If CHD was suspected, the woman was referred to a specialist in fetal medicine for fetal echocardiography. If the CHD necessitated treatment shortly after birth, delivery was set to take place at a tertiary center. There are 3 tertiary centers for the treatment of CHD in Denmark and 49 referral hospitals. Numbers of malformation scans performed at the individual institutions were derived from the annual report from FØTOdatabasen, a registry on prenatal screening in Denmark,15 or obtained directly from the institution.
Definitions
We defined major CHD as morphologically complex malformations of the heart and great arteries that usually necessitate intervention within the first year of life.1,2,12 Diagnoses modified from Hoffman and Kaplan16 included:
Univentricular hearts (UVH)
Congenitally corrected transposition of the great arteries (TGA)
Truncus arteriosus
TGA
Interrupted aortic arch
Atrioventricular septal defect (AVSD)
Double outlet right ventricle (DORV)
Coarctation of the aorta
Ebstein’s anomaly
Pulmonary atresia with ventricular septal defect (PA-VSD)
Pulmonary atresia with intact ventricular septum
Tetralogy of Fallot
Noncardiovascular malformation was defined as an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code describing a congenital malformation other than a CHD (DQ00-DQ19 and DQ26-DQ89). Isolated minor malformations, as defined by the European Surveillance of Congenital Anomalies,17 were excluded.
Live Births
Patients who at any time had been given an ICD-10 code corresponding to a CHD (DQ20-DQ25) were identified in the NPR. Extracted data included identification number, sex, place of birth, local hospital, and all ICD-10 and procedural codes given. Patients were excluded if they had only been diagnosed as having DQ25.0 Persistent Ductus Arteriosus or DQ21.1C Persistent Foramen Ovale.
We identified patients with possible major CHD based on the ICD-10 code, surgical or catheter-based intervention within the first year of life, or if the child had died. Patient records were examined, and only major CHD diagnoses verified postnatally by echocardiography, catheter angiography, during surgery, or at autopsy were included. Six patient records were unobtainable. These patients were included in the prevalence analyses of all CHD but not in the major CHD group.
Termination of Pregnancy
The NPR was searched for ICD-10 codes related to TOP after 12 weeks of gestation (DO04.0-DO07.9). Data were extracted from the NPR and the Register of Abortions and included the woman’s identification number, place, and date of the TOP. We reviewed all the records and noted any anomalies identified in each TOP. Only cases where the fetus was found to have a CHD were included. Thirty-one TOPs were excluded because patient records could not be obtained.
Analyses
The included patients and fetuses were grouped by CHD diagnosis, and those with multiple diagnoses were allocated to the lesion of the highest complexity by a hierarchy modified from Allan et al18 as exemplified in the list of diagnosis. Rates were calculated as follows: incidence of CHD as number of CHD (live-born and terminated) divided by the total number of fetuses, and live-birth incidence of CHD as number of CHD in live-born children divided by the total number of live births. Detection rates for major CHD were calculated as the number of prenatally diagnosed major CHD divided by the total number of fetuses (live-born and terminated) with major CHD. The TOP rates were calculated as the number of terminated fetuses with major CHD divided by the total number fetuses with major CHD (live-born and terminated). Categorical data are presented as percentages and 95% confidence intervals. We used χ2 analysis or Fisher exact test to test for differences in categorical probabilities.
Logistic regression analysis was used to assess changes over time, with splines to evaluate the effect of general prenatal screening on the temporal changes, given by odds ratio (OR). We examined whether detection rates were associated with the number of malformation scans performed at each institution.
All analyses were performed using SAS, version 9.4 (SAS Institute Inc). Hypothesis testing was 2-sided, and a P value less than .05 was considered significant. When multiple testing was undertaken, P values were adjusted using Bonferroni correction.
Results
In total, 17 864 children with possible CHD and 5931 terminated pregnancies after 12 weeks of gestation were identified from the NPR from 1996 to 2013. After excluding patients with unconfirmed CHD, isolated PDA or PFO, or incomplete medical records, as well as TOP without CHD, 14 057 children and 631 terminated fetuses with CHD were identified. Of these, 2224 children and 471 fetuses had major CHD.
Incidence
During the study, 1 150 907 children were born, resulting in a live-birth incidence of all CHD of 1.22% (95% CI, 1.18-1.26), with no significant change throughout the study. The live-birth incidence of major CHD decreased from 0.23% to 0.15% (OR, 0.68; 95% CI, 0.54-0.85; P < .001), with no influence of general prenatal screening (OR, 0.82; 95% CI, 0.42-1.62; P = .57) . Major CHD was more frequent in boys than in girls with an OR of 9.69 (95% CI, 2.56-36.64; P < .001). When including TOP, the incidence of CHD in all fetuses was 1.26% (95% CI, 1.22-1.30), with no significant changes over time.
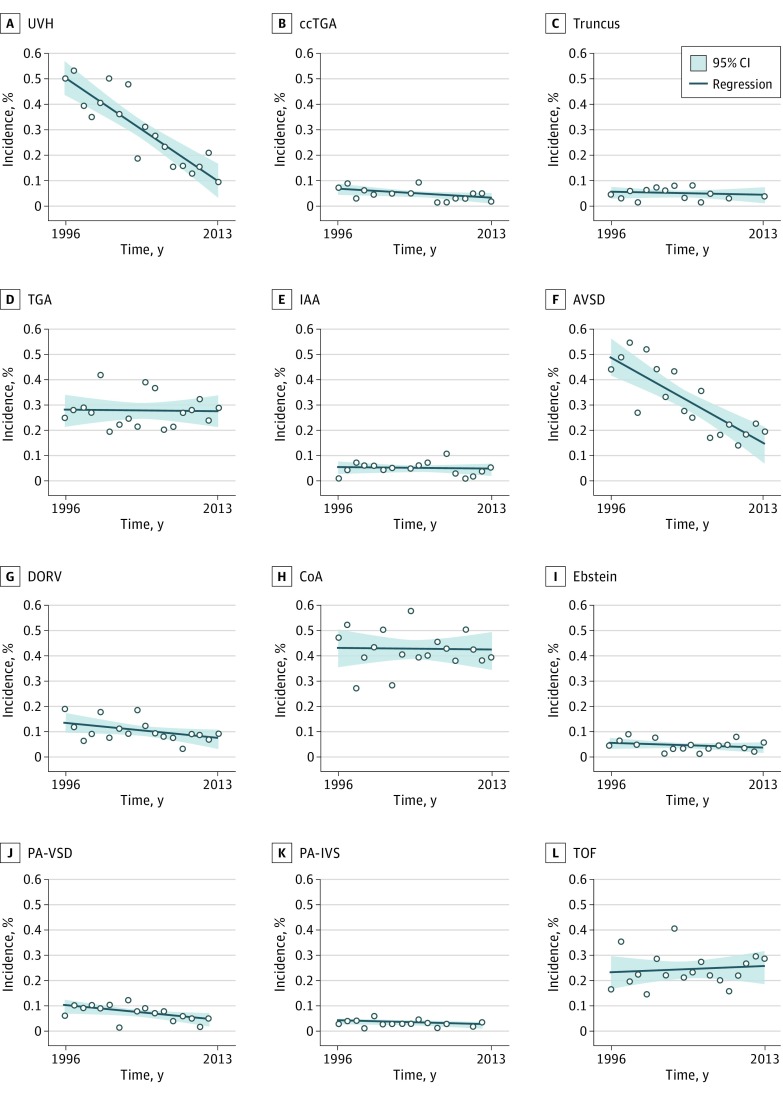
The individual live-birth incidences of major CHD are shown in Figure 1. The live-birth incidences of UVH (OR, 0.53; 95% CI, 0.39-0.72; P < .001), AVSD (OR, 0.63; 95% CI, 0.49-0.81; P < .001), and PA-VSD (OR, 0.80; 95% CI, 0.66-0.97; P = .03) decreased significantly during the study period. When TOP were included in the incidence, the decrease in AVSD and PA-VSD remained significant, whereas the incidence of UVH no longer decreased. However, once adjusted for multiple testing, only the decrease in live-birth incidence of UVH and AVSD remained significant.
Figure 1. Live-Birth Incidences of Major Congenital Heart Diseases.
AVSD indicates atrioventricular septal defects; ccTGA, congenitally corrected transposition of the great arteries; CoA, coarctation of the aorta; DORV, double outlet right ventricle; IAA, interrupted aortic arch; PA-IVS, pulmonary atresia with intact ventricle septum; PA-VSD, pulmonary atresia with ventricular septal defect; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; UVH, univentricular hearts.
An associated anomaly was found in 34.6% of live-born and terminated fetuses with major CHD (n = 934 of 2695; 95% CI, 32.8-36.4) (Table 1). The most common chromosomal anomalies were trisomy 21 (n = 30 of 82 [36.6%]) and trisomy 18 (n = 25 of 82 [30.5%]) in TOP cases, and trisomy 21 (n = 191 of 346 [55.2%]) and deletions (n = 66 of 346 [19.1%]) in live births. Correspondingly, the most common noncardiovascular malformations were hydronephrosis (n = 19 of 255 [7.4%]) and hydrocephalus (n = 17 of 255 [6.7%]) in TOP cases and orofacial cleft (n = 50 of 1204 [4.1%]) and cryptorchidism (n = 48 of 1204 [4.0%]) in live births.
Table 1. Characteristics of Major Congenital Heart Disease in Denmark.
| CHD | Prenatal Diagnosis, No./Total No. (%) | P Value | P Valuea | Associated Anomalies, No./Total No. (%) | ||
|---|---|---|---|---|---|---|
| No General Screening | General Screening | Chromosomal Anomaly | Noncardiovascular Malformation | |||
| UVH | 108/315 (34.3) | 269/323 (83.3) | <.001 | <.001 | 35/638 (5.5) | 141/638 (22.1) |
| ccTGA | 4/26 (15) | 16/25 (64) | <.001 | .005 | 1/51 (2) | 9/51 (18) |
| Truncus arteriosus | 8/37 (21) | 12/24 (50) | .02 | .27 | 12/61 (20) | 19/61 (31) |
| TGA | 7/163 (4.3) | 63/171 (36.8) | <.001 | <.001 | 4/334 (1.2) | 60/334 (18.0) |
| IAA | 1/26 (4) | 12/31 (39) | .002 | .02 | 17/57 (30) | 20/57 (35) |
| AVSD | 47/281 (16.7) | 91/180 (50.6) | <.001 | <.001 | 240/461 (52.1) | 153/461 (33.2) |
| DORV | 10/75 (13) | 31/64 (48) | <.001 | <.001 | 17/139 (12.2) | 46/139 (33.1) |
| CoA | 9/255 (3.5) | 29/235 (12.3) | <.001 | .004 | 40/490 (8.2) | 104/490 (21.2) |
| Ebstein anomaly | 5/28 (18) | 12/25 (48) | .02 | .25 | 3/53 (6) | 4/53 (8) |
| PA-VSD | 4/52 (8) | 11/34 (32) | .003 | .04 | 19/86 (22) | 43/86 (50) |
| PA-IVS | 4/23 (17) | 7/12 (58) | .02 | .29 | 1/35 (3) | 6/35 (17) |
| TOF | 7/147 (4.8) | 48/143 (33.6) | <.001 | <.001 | 39/290 (13.4) | 83/290 (28.6) |
| Total | 214/1428 (15.0) | 601/1267 (47.4) | <.001 | <.001 | 428/2695 (15.9) | 688/2695 (25.5) |
Abbreviations: AVSD, atrioventricular septal defects; ccTGA, congenitally corrected transposition of the great arteries; CoA, coarctation of the aorta; DORV, double outlet right ventricle; IAA, interrupted aortic arch; PA-IVS, pulmonary atresia with intact ventricle septum; PA-VSD, pulmonary atresia with ventricular septal defect; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; UVH, univentricular hearts.
Adjusted for multiple testing with Bonferroni Correction.
Detection Rates
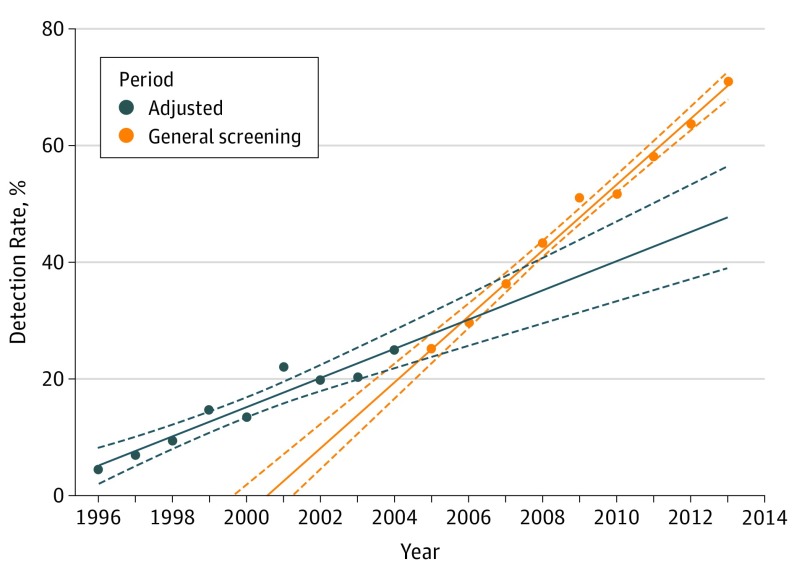
On average during the study period, 30.2% of children and fetuses with major CHD (95% CI, 28.5-32.0) were diagnosed prenatally, with no sex-specific differences. Detection rates increased significantly, both before the introduction of general screening (4.5%; 95% CI, 1.2-7.8 in 1996 to 25.0%; 95% CI, 18.0-32.0 in 2004; OR, 1.20; 95% CI, 1.14-1.26; P < .001) and after (25.2%; 95% CI, 18.3-32.0 in 2005 to 71.0%; 95% CI, 63.3-78.7 in 2013; OR, 1.25; 95% CI, 1.20-1.30; P < .001) (Figure 2). The increase following the introduction of general screening was not significantly greater than the increase before (OR, 1.04; 95% CI, 0.96-1.12; P = .35).
Figure 2. Detection Rates Before and After the Introduction of General Screening.
Regression line (full line) with 95% confidence interval (dashed lines).
Detection rates of the specific major CHD are shown in Table 1. In 2013, the lowest detection rate was in coarctation of the aorta (21.7%; 95% CI, 3.5-40.0) (Figure 3), whereas all known cases of congenitally corrected TGA, DORV, Ebstein anomaly, and PA-VSD, and 94.6% (95% CI, 87.0-100) of cases of UVH were detected. The presence of a chromosomal anomaly did not affect the likelihood of prenatal diagnosis (28.7%; 95% CI, 24.4-33.0 vs 30.5%; 95% CI, 28.6-32.4; P = .46), although the presence of a noncardiovascular anomaly increased the detection rate from 28.9% (95% CI, 26.8-30.8) to 34.3% (95% CI, 30.8-38.0) (P = .007).
Figure 3. Detection Rates of Major Congenital Heart Diseases Over Time Among All Fetuses.
AVSD indicates atrioventricular septal defects; ccTGA, congenitally corrected transposition of the great arteries; CoA, coarctation of the aorta; DORV, double outlet right ventricle; IAA, interrupted aortic arch; PA-IVS, pulmonary atresia with intact ventricle septum; PA-VSD, pulmonary atresia with ventricular septal defect; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; UVH, univentricular hearts.
By the end of the study, there was a significant difference in detection rates at tertiary centers vs referral hospitals (82.2% vs 67.0%; P = .04). Furthermore, the number of malformation scans performed by an institution was associated with higher detection rates (OR, 0.88; 95% CI, 0.81-0.95; P < .001). All institutions performing more than 4000 malformation scans per year had detection rates greater than 70%.
Termination of Pregnancy
During the study period, 17.5% (95% CI, 16.0-18.9) of all pregnancies with a major CHD in the fetus were terminated. This increased from 0.6% (95% CI, −0.6 to 1.9) in 1996 to 39.1% (95% CI, 30.9-47.4) in 2013 (P < .001), with no significant difference before and after the introduction of general screening (OR, 1.08; 95% CI, 0.99-1.17; P = .09). For pregnancies with prenatally diagnosed major CHD, 57.8% (95% CI, 54.4-61.2; P = .74) were terminated and the proportion did not change over time (Table 2). Associated anomalies did not increase the risk of TOP for chromosomal anomaly (relative risk, 1.12; 95% CI, 0.90-1.38; P = .31) or for noncardiovascular malformations (RR, 1.17; 95% CI, 0.98-1.40; P = .09).
Table 2. Termination of Pregnancy in Major Congenital Heart Disease in Denmark.
| CHD | Total, No./Total No. (%) | P Valuea | Prenatally Diagnosed, No./Total No. (%) | P Valuea | ||||
|---|---|---|---|---|---|---|---|---|
| No General Screening | General Screening | P Value | No General Screening | General Screening | P Value | |||
| UVH | 70/315 (22.2) | 216/323 (66.9) | <.001 | <.001 | 70/108 (64.8) | 216/269 (80.3) | .002 | .03 |
| ccTGA | 0/26 | 6/25 (24) | .01 | .13 | 0/4 | 6/16 (38) | .27 | >.99 |
| Truncus arteriosus | 7/37 (19) | 11/24 (67) | .02 | .32 | 7/8 (88) | 11/12 (2) | >.99 | >.99 |
| TGA | 5/163 (3.1) | 11/171 (10.1) | .15 | >.99 | 5/7 (71) | 11/63 (18) | .006 | .07 |
| IAA | 0/26 | 6/31 (19) | .03 | .35 | 0/1 | 6/12 (50) | >.99 | >.99 |
| AVSD | 33/281 (11.7) | 60/180 (33.3) | <.001 | <.001 | 33/47 (70) | 60/91 (66) | .61 | >.99 |
| DORV | 2/75 (3) | 18/64 (28) | <.001 | <.001 | 2/10 (20) | 18/31 (58) | .07 | .87 |
| CoA | 1/255 (0.4) | 2/235 (0.9) | .61 | >.99 | 1/9 (11) | 2/29 (7) | >.99 | >.99 |
| Ebstein anomaly | 2/28 (7) | 2/25 (8) | >.99 | >.99 | 2/5 (40) | 2/12 (17) | .54 | >.99 |
| PA-VSD | 1/52 (2) | 4/34 (12) | .08 | >.99 | 1/4 (25) | 4/11 (36) | >.99 | >.99 |
| PA-IVS | 2/23 (9) | 1/12 (8) | >.99 | >.99 | 2/4 (50) | 1/7 (14) | .49 | >.99 |
| TOF | 1/147 (0.7) | 10/143 (7.0) | .005 | .06 | 1/7 (14) | 10/48 (21) | >.99 | >.99 |
| Total | 124/1428 (8.7) | 347/1267 (27.4) | <.001 | <.001 | 124/214 (57.9) | 347/601 (57.7) | .96 | >.99 |
Abbreviations: AVSD, atrioventricular septal defects; ccTGA, congenitally corrected transposition of the great arteries; CHD, congenital heart disease; CoA, coarctation of the aorta; DORV, double outlet right ventricle; IAA, interrupted aortic arch; PA-IVS, pulmonary atresia with intact ventricle septum; PA-VSD, pulmonary atresia with ventricular septal defect; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; UVH, univentricular hearts.
Adjusted for multiple testing with Bonferroni Correction.
Univentricular hearts were most frequently associated with TOP (286 of 471 TOP [60.7%]; 95% CI, 56.3-65.1), with 86.5% (95% CI, 74.9-98.0) of all pregnancies with UVH terminated in 2013. However, throughout the study, TOP was performed in 90.0% (18 of 20; 95% CI, 75.6-104.4) of cases of prenatally diagnosed truncus arteriosus compared with 75.8% (286 of 377; 95% CI, 71.5-80.2) of prenatally diagnosed UVH.
Discussion
To our knowledge, this is the first study to describe the time trends in the incidence, detection rates, and TOP rates for major CHD in a nationwide cohort. The main findings were that while the live-birth incidence decreased significantly, the incidence was stationary when TOPs were included. Furthermore, there was an overall increase in prenatal diagnoses, from 4.5% to 71.0%, and a 65-fold increase in TOP rates. Thus, currently TOP is performed in 39% of all fetuses with major CHD.
The overall incidence was 1.26% for all CHD. This is higher than most estimates1,2,6,16,19 yet not extraordinary. A Norwegian study20 of similar design found an incidence of 1.23%, and in a review of 62 reports,16 9 reported even higher incidences. The high incidence in our study may be explained by the unique identification numbers allocated to all newborns in Denmark and the systematic registration of codes in the NPR, previously shown to accurately relay other incidences.21 This permitted complete, nationwide inclusion.
The reported incidences may vary owing to temporal change. There remains controversy concerning time trends of the incidence of CHD22,23,24; however, we did not find significant change over time in the overall incidence. Furthermore, higher incidences have been reported in countries where TOP is not performed, although this difference diminished when stratified for TOP.25 This is consistent with our finding that the decrease in live-birth incidence of major CHD follows increased TOP rate, which is supported by Idorn et al,26 who demonstrated a decreasing live-birth incidence of UVH owing to increasing TOP.26
We did find a decrease in overall incidence of AVSD and PA-VSD, although insignificant when adjusted for multiple testing. Atrioventricular septal defect is highly associated with chromosomal anomalies, and because aneuploidy is generally diagnosed in the first trimester, before CHD is commonly detected, the decrease can be attributed to increased TOP prior to cardiac diagnosis. In Denmark before 2004, women older than 35 years were offered amniocentesis/chorion villus biopsy to test for chromosomal anomalies. The general offer of prenatal screening introduced in 2004 includes an estimate of the risk of aneuploidy in the first trimester. It detects 92% of all fetuses with trisomy 21,15 and 91% of these are terminated.27 Because the detection rate of trisomy 21 is close to 100%, the decrease in AVSD prevalence will likely stabilize in the near future. Similarly, half of the cases with PA-VSD had a noncardiovascular malformation, and increased detection of these malformations may also lead to TOP before the CHD is diagnosed. Accordingly, Jicinska et al28 found that the presence of associated anomalies and TOP rates were higher in fetuses diagnosed in the first trimester compared with those diagnosed in the second trimester.
Detection rates increased, and the detection rate at the end of our study is consistent with reports conducted within the last decade.29,30 It is currently not feasible to reach a 100% detection rate of major CHD. Coarctation of the aorta is exceedingly difficult to diagnose in fetuses owing to the open ductus arteriosus. However, other diagnoses are more readily detected and, at the end of the study, all known cases of congenitally corrected TGA, DORV, Ebstein anomaly, and PA-VSD, as well as most cases with UVH, were detected prenatally.
The introduction of general prenatal screening did not significantly improve detection rates. Therefore, the reason for the improvement must lie elsewhere. It may be attributed to the development in obstetric ultrasonographic equipment and technique as well as increased awareness and referral for fetal echocardiography. Furthermore, the implementation of the 3-vessel and outflow-tract views has improved the detectability of TGA, DORV, and TOF, and special focus has been placed on the training of sonographers. Nevertheless, it is reasonable to assume that without general prenatal screening, detection rates would eventually have reached a plateau because most CHD occurs in pregnancies with no risk factors.31
Our findings, as well of those of others,32 have shown that clinical experience of the sonographer has an effect on prenatal detection. Furthermore, tertiary centers had a higher detection rate in first-time scanned fetuses than referral hospitals. It is imperative to ensure high-quality examinations across hospitals. The complete implementation of 3-vessel and outflow tracts views enhances the detectability of major CHD, but further centralization of malformation scans may further improve detection rates.
Finally, we found that TOP rates increased during the study, and 58% of prenatally diagnosed major CHD led to TOP, thereby reducing live-birth incidence of major CHD by almost 40%. When considering prenatally diagnosed major CHDs individually, a significant change in TOP rate was only observed for UVH, which increased, illustrating the severity of the disease. Nevertheless, there was a decrease in TOP of TGA by a factor of 4, although this was not significant when adjusted for multiple testing. In the beginning of the study, the arterial switch had shown remarkable results, but the 1-year mortality was still reportedly 8%,33 which by some parents will be perceived as unacceptable. Currently, the 25-year postoperative survival rate after arterial switch surpasses 96%.34 The ensuing optimism in the counselling specialist has likely affected the parents’ decision.
Increased TOP rates led to decreased live-birth incidence of major CHD and therefore lower patient flow at tertiary centers. This raises the discussion of centralization of the surgical and interventional treatment of these patients because high procedural experience is crucial for the invasive treatment of major CHD.
Limitations
Major CHD is often defined as those with a need for invasive intervention within the first year of life or death of the child.1,2,12 This excludes complex but balanced CHD and includes lesions that are severe in that they necessitate intervention but are mild morphologically. Furthermore, some mild lesions are difficult to evaluate prenatally as the gradients cannot be estimated until after birth. We therefore only included morphologically complex CHD to define major CHD, as previously suggested.16,23
Not all CHDs are diagnosed prior to death, and 5 infants in whom major CHD was suspected died before echocardiography was possible, and autopsy was not undertaken. These children were excluded from the study. Furthermore, not all terminated fetuses undergo autopsy, and miscarriages were not included in this study. Therefore, we may have missed some fetuses with major CHD.
All data were derived from the NPR. Although we validated data, only patient records from children who died, had an ICD-10 code of major CHD, or underwent surgery within the first year of life were reviewed. It is possible that children with an ICD-10 code for mild CHD had major CHD instead. Nonetheless, because children with major CHD will have numerous contacts with the health care system and receive at least 1 ICD-10 code for each contact, it is unlikely that the correct diagnosis is not made on at least 1 occasion.
Our results are based on an environment with universally available, free of charge, prenatal screening and health care services including TOP. Therefore, they may not be directly applicable to countries with different settings.
Conclusions
The incidences of fetuses with any CHD as well as major CHD have not changed significantly from 1996 to 2013. Prenatal detection of major CHD has increased significantly, currently approximating 70% in Denmark. The consequence is that the pregnancy is terminated in 39% of all fetuses with major CHD. Additional centralization of malformation scans may further improve detection rates, and further studies on the outcomes of major CHD are warranted to prevent TOP in cases with a positive prognosis.
References
- 1.Oster ME, Kim CH, Kusano AS, et al. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol. 2014;113(6):1036-1040. doi: 10.1016/j.amjcard.2013.11.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sainz JA, Zurita MJ, Guillen I, et al. Prenatal screening of congenital heart defects in population at low risk of congenital defects: a reality today. An Pediatr (Barc). 2015;82(1):27-34. doi: 10.1016/j.anpedi.2013.10.039 [DOI] [PubMed] [Google Scholar]
- 3.Tegnander E, Williams W, Johansen OJ, Blaas HG, Eik-Nes SH. Prenatal detection of heart defects in a non-selected population of 30,149 fetuses: detection rates and outcome. Ultrasound Obstet Gynecol. 2006;27(3):252-265. doi: 10.1002/uog.2710 [DOI] [PubMed] [Google Scholar]
- 4.Buskens E, Steyerberg EW, Hess J, Wladimiroff JW, Grobbee DE. Routine prenatal screening for congenital heart disease: what can be expected? a decision-analytic approach. Am J Public Health. 1997;87(6):962-967. doi: 10.2105/AJPH.87.6.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Germanakis I, Sifakis S. The impact of fetal echocardiography on the prevalence of liveborn congenital heart disease. Pediatr Cardiol. 2006;27(4):465-472. doi: 10.1007/s00246-006-1291-6 [DOI] [PubMed] [Google Scholar]
- 6.Garne E, Stoll C, Clementi M; Euroscan Group . Evaluation of prenatal diagnosis of congenital heart diseases by ultrasound: experience from 20 European registries. Ultrasound Obstet Gynecol. 2001;17(5):386-391. doi: 10.1046/j.1469-0705.2001.00385.x [DOI] [PubMed] [Google Scholar]
- 7.Tworetzky W, McElhinney DB, Reddy VM, Brook MM, Hanley FL, Silverman NH. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation. 2001;103(9):1269-1273. doi: 10.1161/01.CIR.103.9.1269 [DOI] [PubMed] [Google Scholar]
- 8.Bonnet D, Coltri A, Butera G, et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999;99(7):916-918. doi: 10.1161/01.CIR.99.7.916 [DOI] [PubMed] [Google Scholar]
- 9.van Velzen CL, Haak MC, Reijnders G, et al. Prenatal detection of transposition of the great arteries reduces mortality and morbidity. Ultrasound Obstet Gynecol. 2015;45(3):320-325. doi: 10.1002/uog.14689 [DOI] [PubMed] [Google Scholar]
- 10.Morris SA, Ethen MK, Penny DJ, et al. Prenatal diagnosis, birth location, surgical center, and neonatal mortality in infants with hypoplastic left heart syndrome. Circulation. 2014;129(3):285-292. doi: 10.1161/CIRCULATIONAHA.113.003711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copel JA, Tan AS, Kleinman CS. Does a prenatal diagnosis of congenital heart disease alter short-term outcome? Ultrasound Obstet Gynecol. 1997;10(4):237-241. doi: 10.1046/j.1469-0705.1997.10040237.x [DOI] [PubMed] [Google Scholar]
- 12.Bull C; British Paediatric Cardiac Association . Current and potential impact of fetal diagnosis on prevalence and spectrum of serious congenital heart disease at term in the UK. Lancet. 1999;354(9186):1242-1247. doi: 10.1016/S0140-6736(99)01167-8 [DOI] [PubMed] [Google Scholar]
- 13.Landis BJ, Levey A, Levasseur SM, et al. Prenatal diagnosis of congenital heart disease and birth outcomes. Pediatr Cardiol. 2013;34(3):597-605. doi: 10.1007/s00246-012-0504-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jørgensen FS. Organization of obstetric ultrasound in Denmark 2000. Description of the development since 1990. Ugeskr Laeger. 2003;165(46):4404-4409. [PubMed] [Google Scholar]
- 15.National database for fetal medicine: FØTO Databasen: national annual report 2013. http://docplayer.dk/5883862-Dansk-foetalmedicinsk-database-foetodatabasen-www-dfms-dk-national-aarsrapport-2013.html. Published 2013. Accessed October 22, 2017.
- 16.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890-1900. doi: 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 17.EUROCAT Guide 1.3: instructions for the registration of congenital anomalies: EUROCAT central registry, university of ulster. http://www.eurocat-network.eu/content/EUROCAT-Guide-1.3.pdf. Accessed July 4, 2017.
- 18.Allan LD, Crawford DC, Anderson RH, Tynan M. Spectrum of congenital heart disease detected echocardiographically in prenatal life. Br Heart J. 1985;54(5):523-526. doi: 10.1136/hrt.54.5.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jørgensen DE, Vejlstrup N, Jørgensen C, et al. Prenatal detection of congenital heart disease in a low risk population undergoing first and second trimester screening. Prenat Diagn. 2015;35(4):325-330. doi: 10.1002/pd.4525 [DOI] [PubMed] [Google Scholar]
- 20.Leirgul E, Fomina T, Brodwall K, et al. Birth prevalence of congenital heart defects in Norway 1994-2009: a nationwide study. Am Heart J. 2014;168(6):956-964. doi: 10.1016/j.ahj.2014.07.030 [DOI] [PubMed] [Google Scholar]
- 21.Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women. Am J Epidemiol. 2007;166(2):117-124. doi: 10.1093/aje/kwm139 [DOI] [PubMed] [Google Scholar]
- 22.Allan LD, Sharland GK, Milburn A, et al. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol. 1994;23(6):1452-1458. doi: 10.1016/0735-1097(94)90391-3 [DOI] [PubMed] [Google Scholar]
- 23.Egbe A, Uppu S, Lee S, Ho D, Srivastava S. Changing prevalence of severe congenital heart disease: a population-based study. Pediatr Cardiol. 2014;35(7):1232-1238. doi: 10.1007/s00246-014-0921-7 [DOI] [PubMed] [Google Scholar]
- 24.Oyen N, Poulsen G, Boyd HA, Wohlfahrt J, Jensen PK, Melbye M. National time trends in congenital heart defects, Denmark, 1977-2005. Am Heart J. 2009;157(3):467-473.e1. doi: 10.1016/j.ahj.2008.10.017 [DOI] [PubMed] [Google Scholar]
- 25.Daubeney PE, Sharland GK, Cook AC, Keeton BR, Anderson RH, Webber SA; UK and Eire Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum . Pulmonary atresia with intact ventricular septum: impact of fetal echocardiography on incidence at birth and postnatal outcome. Circulation. 1998;98(6):562-566. doi: 10.1161/01.CIR.98.6.562 [DOI] [PubMed] [Google Scholar]
- 26.Idorn L, Olsen M, Jensen AS, et al. Univentricular hearts in Denmark 1977 to 2009: incidence and survival. Int J Cardiol. 2013;167(4):1311-1316. doi: 10.1016/j.ijcard.2012.03.182 [DOI] [PubMed] [Google Scholar]
- 27.Viuff MH, Stochholm K, Uldbjerg N, Nielsen BB, Gravholt CH; Danish Fetal Medicine Study Group . Only a minority of sex chromosome abnormalities are detected by a national prenatal screening program for Down syndrome. Hum Reprod. 2015;30(10):2419-2426. doi: 10.1093/humrep/dev192 [DOI] [PubMed] [Google Scholar]
- 28.Jicinska H, Vlasin P, Jicinsky M, et al. Does first-trimester screening modify the natural history of congenital heart disease? analysis of outcome of regional cardiac screening at 2 different time periods. Circulation. 2017;135(11):1045-1055. doi: 10.1161/CIRCULATIONAHA.115.020864 [DOI] [PubMed] [Google Scholar]
- 29.Gardiner HM, Kovacevic A, van der Heijden LB, et al. Prenatal screening for major congenital heart disease: assessing performance by combining national cardiac audit with maternity data. Heart. 2014;100(5):375-382. doi: 10.1136/heartjnl-2013-304640 [DOI] [PubMed] [Google Scholar]
- 30.Galindo A, Herraiz I, Escribano D, Lora D, Melchor JC, de la Cruz J. Prenatal detection of congenital heart defects: a survey on clinical practice in Spain. Fetal Diagn Ther. 2011;29(4):287-295. doi: 10.1159/000322519 [DOI] [PubMed] [Google Scholar]
- 31.Stümpflen I, Stümpflen A, Wimmer M, Bernaschek G. Effect of detailed fetal echocardiography as part of routine prenatal ultrasonographic screening on detection of congenital heart disease. Lancet. 1996;348(9031):854-857. doi: 10.1016/S0140-6736(96)04069-X [DOI] [PubMed] [Google Scholar]
- 32.Tegnander E, Eik-Nes SH. The examiner’s ultrasound experience has a significant impact on the detection rate of congenital heart defects at the second-trimester fetal examination. Ultrasound Obstet Gynecol. 2006;28(1):8-14. doi: 10.1002/uog.2804 [DOI] [PubMed] [Google Scholar]
- 33.Wernovsky G, Mayer JE Jr, Jonas RA, et al. Factors influencing early and late outcome of the arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg. 1995;109(2):289-301. doi: 10.1016/S0022-5223(95)70391-8 [DOI] [PubMed] [Google Scholar]
- 34.Khairy P, Clair M, Fernandes SM, et al. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation. 2013;127(3):331-339. doi: 10.1161/CIRCULATIONAHA.112.135046 [DOI] [PubMed] [Google Scholar]