This mouse study examines the effect of ciprofloxacin on aortic aneurysm and dissection development in mice.
Key Points
Question
Does ciprofloxacin increase susceptibility to aortic dissection and rupture in mice?
Findings
This study showed that ciprofloxacin significantly increased the incidence of aortic dissection and rupture in a mouse model of moderate, sporadic aortic aneurysm and dissection. In these mice, ciprofloxacin decreased lysyl oxidase expression and activity, increased MMP levels and activity, and increased elastic fiber fragmentation and cell injury, which may contribute to increased susceptibility to stress-induced aortic destruction.
Meaning
Ciprofloxacin should be used with caution in patients with aortic dilatation, as well as in those at high risk for aortic aneurysm and dissection.
Abstract
Importance
Fluoroquinolones are among the most commonly prescribed antibiotics. Recent clinical studies indicated an association between fluoroquinolone use and increased risk of aortic aneurysm and dissection (AAD). This alarming association has raised concern, especially in patients with AAD with risk of rupture and in individuals at risk for developing AAD.
Objective
To examine the effect of ciprofloxacin on AAD development in mice.
Design, Setting, and Participants
In a mouse model of moderate, sporadic AAD, 4-week-old male and female C57BL/6J mice were challenged with a high-fat diet and low-dose angiotensin infusion (1000 ng/min/kg). Control unchallenged mice were fed a normal diet and infused with saline. After randomization, challenged and unchallenged mice received ciprofloxacin (100 mg/kg/d) or vehicle through daily gavage during angiotensin or saline infusion. Aortic aneurysm and dissection development and aortic destruction were compared between mice. The direct effects of ciprofloxacin on aortic smooth muscle cells were examined in cultured cells.
Results
No notable aortic destruction was observed in unchallenged mice that received ciprofloxacin alone. Aortic challenge induced moderate aortic destruction with development of AAD in 17 of 38 mice (45%) and severe AAD in 9 (24%) but no rupture or death. However, challenged mice that received ciprofloxacin had severe aortic destruction and a significantly increased incidence of AAD (38 of 48 [79%]; P = .001; χ2 = 10.9), severe AAD (32 of 48 [67%]; P < .001; χ2 = 15.7), and rupture and premature death (7 of 48 [15%]; P = .01; χ2 = 6.0). The increased AAD incidence was observed in different aortic segments and was similar between male and female mice. Compared with aortic tissues from challenged control mice, those from challenged mice that received ciprofloxacin showed decreased expression of lysyl oxidase, an enzyme that is critical in the assembly and stabilization of elastic fibers and collagen. These aortas also showed increased matrix metalloproteinase levels and activity, elastic fiber fragmentation, and aortic cell injury. In cultured smooth muscle cells, ciprofloxacin treatment significantly reduced lysyl oxidase expression and activity, increased matrix metalloproteinase expression and activity, suppressed cell proliferation, and induced cell death. Furthermore, ciprofloxacin—a DNA topoisomerase inhibitor—caused nuclear and mitochondrial DNA damage and the release of DNA into the cytosol, subsequently inducing mitochondrial dysfunction, reactive oxygen species production, and activation of the cytosolic DNA sensor STING, which we further showed was involved in the suppression of lysyl oxidase expression and induction of matrix metalloproteinase expression.
Conclusions and Relevance
Ciprofloxacin increases susceptibility to aortic dissection and rupture in a mouse model of moderate, sporadic AAD. Ciprofloxacin should be used with caution in patients with aortic dilatation, as well as in those at high risk for AAD.
Introduction
Aortic aneurysm and dissection (AAD) are common disorders that carry a significant risk of mortality and mobidity.1 Avoiding factors that promote aortic damage, disease progression, and devastating rupture is critical in treating these patients. Fluoroquinolones are among the most commonly prescribed antibiotics worldwide2,3,4 and are used to treat a variety of bacterial infections,2,3,4 including infected aortic aneurysms.5,6,7 Since they were first introduced in the 1980s, fluoroquinolones became popular because of their broad antimicrobial spectrum and favorable pharmacokinetic and pharmacodynamic characteristics, such as excellent oral bioavailability, extensive tissue penetration, and moderate to long elimination half-lives.8 However, results of surveillance and clinical studies have indicated that fluoroquinolones are associated with several adverse effects,9 including tendinitis and tendon rupture.10,11,12 Because fluoroquinolones are associated with an increased risk of tendon rupture, the US Food and Drug Administration issued a black box warning in 2008 regarding the risk of tendon complications associated with fluoroquinolone use.13
Recently, the results of 2 observational clinical studies have suggested that fluoroquinolone use may be associated with an increased risk of AAD.14,15 Although the causal effects remain to be determined, this alarming association has raised concern, especially in patients with AAD with risk of rupture and in individuals with risk of developing AAD. Importantly, because of the limitations of the currently available observational reports, the US Food and Drug Administration released a statement in May 2017 clarifying that “published studies do not currently support reports that these medicines may result in aortic aneurysm and aortic dissection.”16 Therefore, given that rigorous prospective studies in patients are improbable and would be unethical, there is an acute need to study the risks of fluoroquinolones in experimental models.
To determine whether fluoroquinolone use can indeed cause AAD or increase the risk of AAD formation and progression, we examined the effect of ciprofloxacin—the most commonly used fluoroquinolone—on AAD development in a mouse model of moderate, sporadic AAD induced by challenging mice with a high-fat diet and angiotensin II infusion. We found that although ciprofloxacin treatment alone did not induce spontaneous AAD in unchallenged mice, it significantly increased the incidence of aortic dissection and rupture in the thoracic and abdominal aortic regions of challenged mice and showed similar effects in male and female mice. Our findings support that ciprofloxacin increases susceptibility to AAD formation and aortic rupture.
Methods
Detailed methods and methods for supplement data are described in the eMethods in the Supplement. The study began in October 2016 and ended in September 2017. Analyses began in September 2017.
Animal Studies
We evaluated the effects of ciprofloxacin on AAD development in a mouse model of moderate, sporadic AAD. Mice were randomly assigned to 1 of 4 treatment groups. Four-week-old wild-type (C57BL/6J) male and female mice were challenged with a combination of a high-fat diet for 8 weeks and subcutaneous angiotensin II infusion during the last 4 weeks. Unchallenged control mice were fed a normal chow diet and were infused with saline. Both groups also received either ciprofloxacin or vehicle through daily gavage during the infusion period. At the end of the study, mice were killed, and aortas were processed for AAD evaluation and tissue analysis. All animal experiments were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine in accordance with the guidelines of the National Institutes of Health.
Evaluation of Aortic Aneurysm, Dissection, and Rupture and AAD Severity
For each extracted aorta, we evaluated the diameter of the ascending, arch, descending, suprarenal, and infrarenal aortic segments. Detailed methods for measuring aortic diameters; defining aortic dilatation, aneurysm, and dissection; and classifying AAD severity are described in the eMethods in the Supplement. Aortic rupture and premature death were documented.
Evaluation of Aortic Tissue
Aortic sections were stained with Verhoeff–van Gieson elastin staining, and elastic fiber fragmentation was scored. For immunofluorescence staining and quantification, aortic sections and treated cells were processed and analyzed as previously described.17 Antibodies are listed in the eTable in the Supplement. For the analysis of matrix metalloproteinase (MMP) activity, we performed in situ zymography with frozen aortic sections. Lysyl oxidase (LOX) activity was measured by using a Lysyl Oxidase Activity Assay Kit (fluorometric) (Abcam, ab112139). Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) staining was performed by using an in situ cell death detection kit (Roche Applied Science) as described previously.18 For the detection of reactive oxygen species (ROS), aortic tissue sections were stained with dihydroethidium (10μM, Thermo Fisher) at 37°C for 30 minutes in the dark. The presence of ROS was detected by using a fluorescence microscope (Olympus).
Statistical Analysis
Data were analyzed using SPSS software, version 22.0 (SPSS Inc). For all statistical analyses, 2-tailed probability values were used. A probability value of P less than .05 was considered significant.
Results
Increased Susceptibility to AAD Development in Mice That Received Ciprofloxacin
We examined the effect of ciprofloxacin on AAD formation in a mouse model of moderate, sporadic AAD. Sixty-seven male and 59 female C57BL/6J mice were randomly assigned into challenged (n = 86) and unchallenged groups (n = 40), aiming for an approximately 2:1 ratio. These groups were further randomized and also received ciprofloxacin (48 [55.8%] in the challenged group and 20 [50.0%] in the unchallenged group) or vehicle (38 [44.2%] in the challenged group and 20 [50.0%] in the unchallenged group).
In mice that did not receive aortic challenge, ciprofloxacin alone did not induce notable aortic destruction (Figure 1A), aortic enlargement (Figure 1B), or AAD formation, although aortic dilatation was observed in 4 of 20 mice (20%) (Figure 1C). In challenged mice given vehicle, aortic enlargement was observed in several aortic segments (Figure 1B); in addition, we observed aortic dilatation in 27 of 38 mice (71%), AAD in 17 of 38 (45%), and severe AAD in 9 of 38 (24%) but no aortic rupture or death (Figure 1C). However, in challenged mice given ciprofloxacin, we observed severe aortic disease (Figure 1A) with significantly increased incidences of dilatation (47 of 48 [98%]), AAD (38 of 48 [79%]), severe AAD (32 of 48 [67%]), and aortic rupture (Figure 1C) and premature death (7 of 48 [15%]) (Figure 1D). Our findings suggest that, rather than causing spontaneous AAD formation, ciprofloxacin significantly increases susceptibility to aortic aneurysm progression, dissection, and rupture in mice under aortic challenge.
Figure 1. Increased Susceptibility to Challenge-Induced Aortic Aneurysm and Dissection (AAD) Formation in Mice That Received Ciprofloxacin.
Wild-type mice were either unchallenged or challenged with a high-fat diet for 8 weeks and infused with angiotensin II during the last 4 weeks. Unchallenged and challenged mice received vehicle or ciprofloxacin during the last 4 weeks. A, Representative images of excised aortas showing gross differences between aortas from challenged mice that received vehicle and challenged mice that received ciprofloxacin. B, Comparison of aortic diameters in each aortic segment showing that aortic diameters were increased in both male and female challenged mice that received vehicle and were even further increased in challenged mice that received ciprofloxacin. C, Comparison of aortic lesion severity showing that the overall incidences of aortic dilatation, AAD (aneurysm, dissection, and rupture), severe AAD (dissection and rupture), and rupture were significantly increased in challenged mice that received ciprofloxacin compared with challenged mice that received vehicle. D, Kaplan-Meier survival analysis showing reduced survival in challenged mice that received ciprofloxacin compared with challenged mice that received vehicle.
Increased Susceptibility to AAD Development in Both Thoracic and Abdominal Aortic Segments of Mice That Received Ciprofloxacin
When we further analyzed disease formation in different aortic segments, we found that the incidences of AAD, severe AAD, and rupture were highest in the ascending aorta (38 of 48 [71%], 24 [48%], and 5 [10%], respectively) of challenged mice treated with ciprofloxacin, followed by the aortic arch (28 [58%], 18 [38%], and 3 [6%], respectively), the suprarenal aorta (21 [44%], 17 [35%], and 3 [6%], respectively), and the descending thoracic aorta (21 [44%], 3 [6%], and 1 [2%], respectively) (Figure 2). In the ascending aorta (Figure 2A) and aortic arch (Figure 2B), the incidence of AAD was more than 3 times higher and the incidence of severe AAD was more than 10 times higher in the challenged group given ciprofloxacin than in the challenged group given vehicle. The incidence of aortic rupture was also significantly higher in the ascending aorta of mice in the challenged group given ciprofloxacin (Figure 2A). In the descending thoracic aorta, the incidence of AAD was significantly higher in challenged mice given ciprofloxacin than in challenged mice given vehicle (Figure 2C). A similar pattern of AAD induction was also observed in the suprarenal abdominal aorta (Figure 2D) although with less fold induction than in the ascending aorta and aortic arch. Ciprofloxacin administration was not associated with a significantly increased incidence of dilatation or AAD in the infrarenal aortic region of challenged mice (data not shown). These data suggest that ciprofloxacin increases susceptibility to AAD formation in both the thoracic and abdominal aorta.
Figure 2. Increased Susceptibility to Challenged-Induced Aortic Aneurysm and Dissection (AAD) Formation in Both the Thoracic and Abdominal Aortic Segments of Mice That Received Ciprofloxacin.
Wild-type mice were either unchallenged or challenged with a high-fat diet for 8 weeks and infused with angiotensin II during the last 4 weeks. Unchallenged and challenged mice were given vehicle or ciprofloxacin during the last 4 weeks. A-D, Comparison of the incidences of aortic dilatation, AAD (aneurysm, dissection, and rupture), severe AAD (dissection and rupture), and rupture in the ascending aorta (A), aortic arch (B), descending thoracic aorta (C), and suprarenal abdominal aorta (D). The severity of aortic lesions was increased in challenged mice that received ciprofloxacin compared with challenged mice that received vehicle.
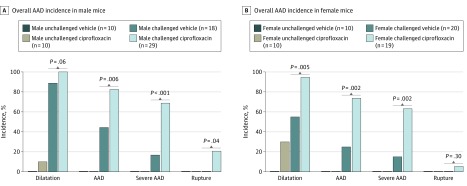
Similar Adverse Effects of Ciprofloxacin on AAD Development in Male and Female Mice
We also compared the effects of ciprofloxacin between male and female mice. In challenged mice given vehicle, the incidences of aortic dilatation (16 of 18 male mice [89%] vs 11 of 20 female mice [55%]; P = .02; χ2 = 5.3) and AAD (8 of 18 male mice [44%] vs 5 of 20 female mice [25%]; P = .20; χ2 = 1.6) were somewhat higher in male mice than in female mice, but the incidence of severe AAD (3 of 18 male mice [17%] vs 3 of 20 female mice [15%]) was similar between sexes (Figure 3). In male and female challenged mice, the addition of ciprofloxacin markedly increased the incidence of aortic dilatation, AAD, and severe AAD (Figure 3). Interestingly, the fold induction of dilatation and AAD in these mice was greater in female mice than in male mice, resulting in a similar overall incidence between sexes. A similar pattern was observed in the ascending, arch, descending thoracic, and suprarenal aortic segments (data not shown).
Figure 3. Similar Adverse Effects of Ciprofloxacin on Aortic Aneurysm and Dissection (AAD) Development in Male and Female Mice.
Wild-type mice were either unchallenged or challenged with a high-fat diet for 8 weeks and infused with angiotensin II during the last 4 weeks. Unchallenged and challenged mice were given vehicle or ciprofloxacin during the last 4 weeks. Comparison of the overall incidences of aortic dilatation, AAD (aneurysm, dissection, and rupture), severe AAD (dissection and rupture), and rupture in all aortic segments showing that ciprofloxacin treatment enhanced challenged-induced AAD formation and severity similarly in male and female mice.
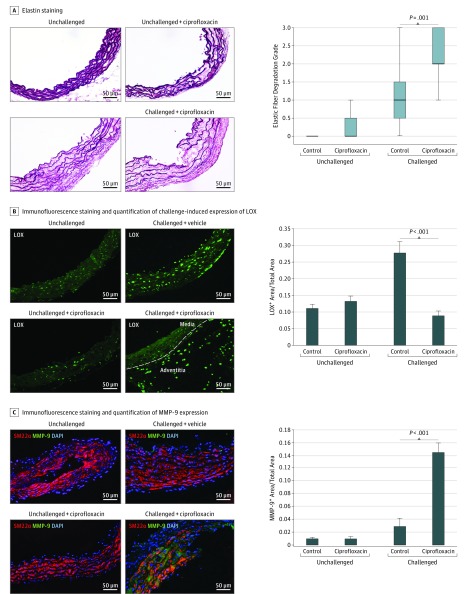
Decreased LOX Level and Increased MMP Levels and Activity in the Aortic Wall of Challenged Mice That Received Ciprofloxacin
We further characterized histologic changes in aortic tissues of unchallenged and challenged mice given ciprofloxacin or vehicle. In aortas of unchallenged mice given ciprofloxacin, hematoxylin-eosin staining and elastic fiber staining showed a low degree of aortic destruction (eFigure 1A in the Supplement) and elastic fiber fragmentation (Figure 4A), respectively, but no significant destruction. In contrast, aortic challenge increased aortic destruction (eFigure 1A in the Supplement) and elastic fiber fragmentation (Figure 4A), which were even more pronounced in challenged mice that received ciprofloxacin.
Figure 4. Decreased Lysyl Oxidase (LOX) Protein Expression and Increased Matrix Metalloproteinase (MMP) Expression and Activity in the Aortic Wall of Challenged Mice That Received Ciprofloxacin.
A, Representative images of Verhoeff–Van Gieson elastin staining and quantification showing the destruction of elastic lamellar architecture in the aortic wall of challenged mice that received ciprofloxacin. Representative images of immunofluorescence staining and quantification showing compromised LOX expression, especially in the medial layer (B), and increased MMP-9 expression in the aortic wall of challenged mice that received ciprofloxacin (C). DAPI indicates 4’,6-diamidino-2-phenylindole; SM22α, 22-kDa smooth muscle cell.
To further investigate the potential mechanisms underlying the increased elastin destruction observed in challenged mice that received ciprofloxacin, we examined extracellular matrix (ECM) protein expression. We observed no significant difference in collagen (I and III) levels in the aortic wall between challenged mice that received ciprofloxacin and those that received vehicle (data not shown). Interestingly, the level of LOX—a protein critical for cross-linking and stabilizing elastin and collagen, as well as for ECM organization—was significantly increased in the aortic media of challenged control mice compared with that of unchallenged mice (Figure 4B). However, in challenged mice that received ciprofloxacin, the challenge-induced increase in LOX level was markedly diminished in the aortic media (Figure 4B), particularly in smooth muscle cells (SMCs) (eFigure 1B in the Supplement). Interestingly, LOX expression was observed in macrophages in the adventitia of the diseased aortas (eFigure 1B in the Supplement).
We also examined the expression and activity of the proteins MMP-9—a key proteinase involved in ECM destruction—and tissue inhibitor of metalloproteinase 1 (TIMP-1). In the aortic tissue of challenged mice that received ciprofloxacin, TIMP-1 levels were not significantly decreased, but MMP-9 protein expression (Figure 4C) and MMP activity (eFigure 1C in the Supplement) were significantly increased, particularly in the aortic lesion segments. These data indicate that the aortic wall of challenged mice that received ciprofloxacin had significant aortic destruction that was accompanied by decreased LOX expression and increased MMP expression and activity.
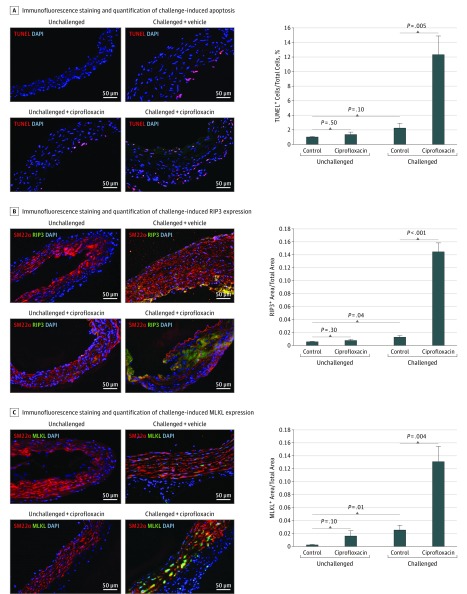
Increased Apoptosis and Necroptosis in the Aortic Wall of Challenged Mice That Received Ciprofloxacin
Because ciprofloxacin has been shown to suppress cell proliferation19,20,21,22,23,24,25 and induce cell death,24,26,27 we compared aortic cell proliferation and cell death among the groups of mice. We observed no significant difference in the detection of cell proliferation marker Ki67 between aortas of challenged mice given ciprofloxacin and those of challenged mice given vehicle (data not shown). However, the number of TUNEL-positive cells was significantly increased, particularly in lesion regions, in aortas of challenged mice given ciprofloxacin compared with challenged mice given vehicle (Figure 5A). Additionally, the levels of 2 key proteins in the necroptosis pathway,28,29,30,31 receptor-interacting protein kinase 332,33,34,35 (Figure 5B) and mixed lineage kinase domain-like36,37,38,39 (Figure 5C), were higher in aortas from challenged mice given ciprofloxacin than in those from challenged control mice. Furthermore, the level of ROS was higher in aortic tissues of challenged mice given ciprofloxacin than in those of challenged mice given vehicle (eFigure 1D in the Supplement). These data indicate that apoptosis and necroptosis are increased in the aortic wall of challenged mice given ciprofloxacin.
Figure 5. Increased Apoptosis and Necroptosis in the Aortic Wall of Challenged Mice That Received Ciprofloxacin.
A, Representative terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL)–stained images of the aortic wall and quantification. Apoptosis was significantly increased, particularly in the lesion areas, of challenged mice that received ciprofloxacin. Representative images of immunofluorescence staining and quantification showing the presence of receptor-interacting protein kinase 3 (RIP3) (B) and mixed lineage kinase domain-like (MLKL) (C) in aortic smooth muscle cells of challenged mice that received ciprofloxacin. DAPI indicates 4’,6-diamidino-2-phenylindole; SM22α, 22-kDa smooth muscle cell.
Dysregulated Gene Expression in Cultured Aortic SMCs Treated with Ciprofloxacin
To understand the adverse effects of ciprofloxacin on the aortic wall, we examined the direct effects of ciprofloxacin on gene expression and on cell proliferation and cell death in cultured aortic SMCs. The treatment of SMCs with ciprofloxacin for 24 hours dose-dependently reduced the levels of several ECM proteins including LOX protein (eFigure 2A in the Supplement). Ciprofloxacin decreased LOX messenger RNA (eFigure 2B in the Supplement) levels and LOX activity (eFigure 2C in the Supplement). Ciprofloxacin also decreased TIMP-1 levels and increased levels of MMP-1 and MMP-9 but not those of MMP-2 (eFigure 2D in the Supplement). Consistent with these observations, ciprofloxacin also increased MMP activity (eFigure 2E in the Supplement). These data indicate that ciprofloxacin may reduce ECM biosynthesis and stability while promoting MMP activation and ECM destruction.
Inhibition of Cell Proliferation and Induction of Cell Injury in Cultured Aortic SMCs Treated With Ciprofloxacin
We also examined the effects of ciprofloxacin on aortic SMC proliferation and cell death. Treating aortic SMCs with ciprofloxacin (ie, 0-200 μg/mL) inhibited cell cycle progression (eFigure 3A in the Supplement), particularly at the G2 phase (eFigure 3B in the Supplement). Furthermore, ciprofloxacin induced cell death (eFigure 3C in the Supplement), which was partially due to apoptosis, as indicated by the increased number of TUNEL-stained cells (eFigure 3D in the Supplement) and the cleavage of caspase-3 (eFigure 3E in the Supplement). Ciprofloxacin also induced the phosphorylation of mixed lineage kinase domain-like (eFigure 3E in the Supplement), indicating the activation of the necroptosis pathway. Thus, consistent with previously reported findings in other cell types,24,26,27 we showed that ciprofloxacin inhibits cell proliferation and induces cell death in aortic SMCs.
DNA Damage and Release of DNA to the Cytosol, Mitochondrial Dysfunction, and Activation of Cytosolic DNA Sensor Signaling Induced by Ciprofloxacin
We next investigated the mechanisms by which ciprofloxacin induces cell injury. As described in detail in eAppendix 1 in the Supplement, we found that ciprofloxacin induced the release of nuclear and mitochondrial DNA into the cytosol (eFigure 4A and B in the Supplement), mitochondrial dysfunction (eFigure 4C in the Supplement), ROS production (eFigure 4D in the Supplement), and activation of the STING (stimulator of interferon genes) cytosolic DNA sensor signaling pathway (eFigure 4E in the Supplement) in cultured aortic SMCs. We also found that silencing STING with small interfering RNA partially prevented the ciprofloxacin-induced decrease in LOX and increase in MMP-1 and MMP-9 protein expression (eFigure 4F in the Supplement). Together, these data suggest that ciprofloxacin causes mitochondrial DNA and nuclear DNA damage, leading to mitochondrial dysfunction and ROS production. Furthermore, these data indicate that the ciprofloxacin-induced disruption of ECM integrity may be mediated by the activation of the cytosolic DNA sensor STING.
Discussion
In this study, we showed that ciprofloxacin significantly increased the incidence of aortic dissection and rupture in a mouse model of moderate, sporadic AAD. These effects of ciprofloxacin were observed in both thoracic and abdominal aortic segments and were similar in male and female mice. Compared with aortic tissues from challenged control mice, those from challenged mice given ciprofloxacin showed decreased LOX expression and activity, increased MMP levels and activity, and increased elastic fiber fragmentation and cell injury. Although ciprofloxacin alone caused mild aortic elastic fiber breakage, it did not induce spontaneous AAD in the absence of exogenous stress. Our results suggest that ciprofloxacin use may increase susceptibility to AAD formation and lethal rupture.
Recently, authors of 2 independent observational clinical studies have suggested that fluoroquinolone use may be associated with an increased risk of AAD (eAppendix 2 in the Supplement).14,15 Although the causal effect cannot be determined by the results of these observational studies, this alarming association raises the concern that taking fluoroquinolones may promote AAD formation or increase the risk of disease progression and rupture in patients with AAD. We showed that although ciprofloxacin alone did not induce spontaneous AAD in the absence of exogenous stress, it significantly increased the incidence and severity of challenge-induced AAD, particularly with respect to dissection and rupture. Given that fluoroquinolones are widely used in the general population for a variety of common infections2,3,4 (and ironically in patients with infected AAD5,6,7,40,41,42,43), our findings should urge clinicians to use caution when treating particular patients with fluoroquinolones, especially those with aortic dilatation and those at high risk for AAD.44,45
Several mechanisms may account for the adverse effects of ciprofloxacin. The most likely mechanism—interfering with ECM integrity—may explain ciprofloxacin’s contribution to aortic dissection and rupture. Several studies have shown that fluoroquinolones inhibit collagen production in tenocytes46,47 and fibroblasts.20,48 Additionally, numerous studies have shown that ciprofloxacin inhibits TIMP-1 expression while inducing MMP expression in the cornea,49 in tendon cells and tissues,46,47,50,51,52 and in fibroblasts,53 leading to MMP activation and tissue destruction. Here, ciprofloxacin treatment significantly increased MMP-1 and MMP-9 expression and MMP activity in the aortas of challenged mice compared with those of vehicle-treated challenged mice. Furthermore, in cultured aortic SMCs, ciprofloxacin directly increased MMP-1 and MMP-9 levels, resulting in increased MMP activity. Ciprofloxacin treatment in cultured aortic SMCs reduced the levels of ECM proteins, including tropoelastin, collagen, and LOX. Notably, LOX oxidizes lysine residues in elastin and collagen to form covalent cross-links, and it plays a critical role in elastic fiber assembly and stabilization.54 Evidence from clinical55,56 and animal56 studies has indicated that LOX has an important role in maintaining aortic wall integrity. Therefore, the suppression of LOX expression by ciprofloxacin may conceivably affect elastic fiber stability in the aortic wall, therefore contributing to aortic dissection and rupture. Interestingly, in diseased aortas from challenged mice given ciprofloxacin, LOX expression was severely downregulated in SMCs in the aortic media but not in macrophages in the adventitia. The reasons for this remain unclear; further studies are needed to understand the mechanisms involved in the differential effects of ciprofloxacin on LOX expression in different cells. Regardless of the source of LOX production, our findings suggest that severely compromised LOX expression in the aortic media contributes to aortic dissection and rupture. The suppression of ECM biosynthesis and stability and the induction of ECM degradation may be key mechanisms underlying ciprofloxacin’s effects on aortic destruction, dissection, and rupture.
Because ciprofloxacin has been shown to potently inhibit cell proliferation and induce apoptosis in various cancer cells,57,58,59,60,61,62,63,64,65,66 ciprofloxacin derivatives have been developed for cancer chemotherapy.67,68,69,70 Ciprofloxacin has also been shown to inhibit proliferation in noncancer cells, including tenocytes,21,22 osteoblasts,23 and chondrocytes,19 and to induce cytotoxicity in various types of cells, including lens epithelial cells26 and tenocytes.46,52 We observed a significant increase in the number of TUNEL-positive cells in the aortic wall of challenged mice given ciprofloxacin compared with that of challenged mice given vehicle. Consistent with our in vivo findings, ciprofloxacin induced cell death in cultured aortic SMCs. These results suggest that the effect of ciprofloxacin on the induction of SMC injury may contribute to aortic destruction.
Although the toxicity of fluoroquinolone antibiotics in human cells has been increasingly recognized, the underlying mechanisms have not been fully elucidated. Quinolones are known to target bacterial DNA topoisomerase IV and DNA gyrase, yet numerous studies have shown that fluoroquinolones, including ciprofloxacin, inhibit human topoisomerases71,72,73,74,75,76,77,78,79,80 and induce cytotoxic effects in human cells. Human topoisomerases are also present in mitochondria81,82,83 and are critical for mitochondrial function. Here, we showed that ciprofloxacin induced DNA double-strand breaks in the nucleus (as indicated by TUNEL analysis) and the release of nuclear and mitochondrial DNA into the cytosol of aortic SMCs. As a result of mitochondrial DNA damage,80,84,85,86,87,88 ciprofloxacin-treated cells exhibited significant mitochondrial dysfunction and increased intracellular ROS production. Our findings suggest that, as a topoisomerase inhibitor, ciprofloxacin causes mitochondrial DNA and nuclear DNA damage and may lead to mitochondrial dysfunction, ROS production, cell dysfunction, and death. Finally, we observed that activation of STING,89,90,91,92,93 a proinflammatory cytosolic DNA sensor involved in cell dysfunction and tissue destruction,94,95 is involved in the ciprofloxacin-induced suppression of LOX expression and induction of MMP expression. Together, our findings point to a novel mechanism underlying the toxic cellular effects of ciprofloxacin.
Limitations
We acknowledge that our study has limitations. We did not test the effects of other fluoroquinolones. However, considering that fluoroquinolones exert their antibiotic effects through the same mechanism, we expect that different fluoroquinolones may have similar effects on the aortic wall. Additionally, we did not perform experiments to determine the time- and dose-dependent effects of ciprofloxacin in our model. Further studies are warranted to address these matters. The addition of periodic aortic ultrasound to evaluate rates of aortic dilatation would be useful in this context.
Conclusions
Although ciprofloxacin alone does not induce spontaneous AAD, it significantly increases susceptibility to challenge-induced aortic dissection and rupture in a mouse model of sporadic AAD. As a potent DNA topoisomerase inhibitor, ciprofloxacin may exert its adverse effects in human cells by inhibiting ECM protein biosynthesis and stability and inducing MMP activity and even cell death. Our findings support concerns raised in observational clinical studies regarding fluoroquinolone use and suggest that this drug should be used with caution in patients with aortic dilatation and those at high risk for AAD.
eMethods. Supplemental methods
eTable. Antibodies used for western blot and immunostaining experiments
eFigure 1. Increased aortic destruction, decreased LOX protein expression, increased MMP activity, and increased reactive oxygen species production in the aortic wall of challenged mice that received ciprofloxacin
eFigure 2. Downregulation of extracellular matrix proteins and upregulation of MMP expression and activity in cultured aortic smooth muscle cells (SMCs) treated with ciprofloxacin
eFigure 3. Inhibition of cell proliferation and induction of cell injury in cultured aortic SMCs treated with ciprofloxacin
eFigure 4. Increased DNA damage and its release into the cytosol and the activation of cytosolic DNA sensor signaling in aortic SMCs treated with ciprofloxacin
eAppendix 1. DNA damage and release to the cytosol, mitochondrial dysfunction, and activation of cytosolic DNA sensor signaling by ciprofloxacin
eAppendix 2. Two independent observational clinical studies suggesting that fluoroquinolone use may be associated with an increased risk of aortic aneurysm and dissection
References
- 1.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65(4):-. [PubMed] [Google Scholar]
- 2.Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med. 2005;118(3):259-268. doi: 10.1016/j.amjmed.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 3.Mamdani M, McNeely D, Evans G, et al. . Impact of a fluoroquinolone restriction policy in an elderly population. Am J Med. 2007;120(10):893-900. doi: 10.1016/j.amjmed.2007.02.028 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Steinman MA, Kaplan CM. Geographic variation in outpatient antibiotic prescribing among older adults. Arch Intern Med. 2012;172(19):1465-1471. doi: 10.1001/archinternmed.2012.3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laohapensang K, Rutherford RB, Arworn S. Mycotic abdominal aortic aneurysm due to Streptococcus suis: a case report. Surg Infect (Larchmt). 2010;11(2):179-181. doi: 10.1089/sur.2008.111 [DOI] [PubMed] [Google Scholar]
- 6.Risse J, Settembre N, Mandry D, et al. . Infected abdominal aortic aneurysm attributable to haemophilus influenzae: rapid changes of imaging findings. Circulation. 2015;132(7):613-615. doi: 10.1161/CIRCULATIONAHA.115.016323 [DOI] [PubMed] [Google Scholar]
- 7.Thompson PC, Wang L, Columbo J, Schanzer A, Robinson WP. Durable results with in situ graft repair of ruptured salmonella aneurysm in a patient with autoimmune deficiency syndrome. Int J Angiol. 2016;25(5):e131-e134. doi: 10.1055/s-0035-1556840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodvold KA, Neuhauser M. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Pharmacotherapy. 2001;21(10 Pt 2):233S-252S. doi: 10.1592/phco.21.16.233S.33992 [DOI] [PubMed] [Google Scholar]
- 9.Mehlhorn AJ, Brown DA. Safety concerns with fluoroquinolones. Ann Pharmacother. 2007;41(11):1859-1866. doi: 10.1345/aph.1K347 [DOI] [PubMed] [Google Scholar]
- 10.Huston KA. Achilles tendinitis and tendon rupture due to fluoroquinolone antibiotics. N Engl J Med. 1994;331(11):748. doi: 10.1056/NEJM199409153311116 [DOI] [PubMed] [Google Scholar]
- 11.Wise BL, Peloquin C, Choi H, Lane NE, Zhang Y. Impact of age, sex, obesity, and steroid use on quinolone-associated tendon disorders. Am J Med. 2012;125(12):1228.e23-1228.e28. doi: 10.1016/j.amjmed.2012.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wahl PM, Gagne JJ, Wasser TE, et al. . Early steps in the development of a claims-based targeted healthcare safety monitoring system and application to three empirical examples. Drug Saf. 2012;35(5):407-416. doi: 10.2165/11594770-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration Fluoroquinolone antimicrobial drugs information. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm346750.htm. Accessed June 26, 2018.
- 14.Lee CC, Lee MT, Chen YS, et al. . Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Intern Med. 2015;175(11):1839-1847. doi: 10.1001/jamainternmed.2015.5389 [DOI] [PubMed] [Google Scholar]
- 15.Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open. 2015;5(11):e010077. doi: 10.1136/bmjopen-2015-010077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Accessed February 13, 2018.
- 17.Shen YH, Zhang L, Ren P, et al. . AKT2 confers protection against aortic aneurysms and dissections. Circ Res. 2013;112(4):618-632. doi: 10.1161/CIRCRESAHA.112.300735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren P, Hughes M, Krishnamoorthy S, et al. . Critical role of ADAMTS-4 in the development of sporadic aortic aneurysm and dissection in mice. Sci Rep. 2017;7(1):12351. doi: 10.1038/s41598-017-12248-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams RJ III, Attia E, Wickiewicz TL, Hannafin JA. The effect of ciprofloxacin on tendon, paratenon, and capsular fibroblast metabolism. Am J Sports Med. 2000;28(3):364-369. doi: 10.1177/03635465000280031401 [DOI] [PubMed] [Google Scholar]
- 20.Tsai WC, Hsu CC, Tang FT, Wong AM, Chen YC, Pang JH. Ciprofloxacin-mediated cell proliferation inhibition and G2/M cell cycle arrest in rat tendon cells. Arthritis Rheum. 2008;58(6):1657-1663. doi: 10.1002/art.23518 [DOI] [PubMed] [Google Scholar]
- 21.Tsai WC, Hsu CC, Chen HC, et al. . Ciprofloxacin-mediated inhibition of tenocyte migration and down-regulation of focal adhesion kinase phosphorylation. Eur J Pharmacol. 2009;607(1-3):23-26. doi: 10.1016/j.ejphar.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 22.Holtom PD, Pavkovic SA, Bravos PD, Patzakis MJ, Shepherd LE, Frenkel B. Inhibitory effects of the quinolone antibiotics trovafloxacin, ciprofloxacin, and levofloxacin on osteoblastic cells in vitro. J Orthop Res. 2000;18(5):721-727. doi: 10.1002/jor.1100180507 [DOI] [PubMed] [Google Scholar]
- 23.Li P, Cheng NN, Chen BY, Wang YM. In vivo and in vitro chondrotoxicity of ciprofloxacin in juvenile rats. Acta Pharmacol Sin. 2004;25(10):1262-1266. [PubMed] [Google Scholar]
- 24.Somekh E, Douer D, Shaked N, Rubinstein E. In vitro effects of ciprofloxacin and pefloxacin on growth of normal human hematopoietic progenitor cells and on leukemic cell lines. J Pharmacol Exp Ther. 1989;248(1):415-418. [PubMed] [Google Scholar]
- 25.Somekh E, Lev B, Schwartz E, Barzilai A, Rubinstein E. The effect of ciprofloxacin and pefloxacin on bone marrow engraftment in the spleen of mice. J Antimicrob Chemother. 1989;23(2):247-251. doi: 10.1093/jac/23.2.247 [DOI] [PubMed] [Google Scholar]
- 26.Zhao B, Chignell CF, Rammal M, et al. . Detection and prevention of ocular phototoxicity of ciprofloxacin and other fluoroquinolone antibiotics. Photochem Photobiol. 2010;86(4):798-805. doi: 10.1111/j.1751-1097.2010.00755.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamocki K, Nör JE, Bottino MC. Effects of ciprofloxacin-containing antimicrobial scaffolds on dental pulp stem cell viability-In vitro studies. Arch Oral Biol. 2015;60(8):1131-1137. doi: 10.1016/j.archoralbio.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700-714. doi: 10.1038/nrm2970 [DOI] [PubMed] [Google Scholar]
- 29.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38(2):209-223. doi: 10.1016/j.immuni.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 30.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15(2):135-147. doi: 10.1038/nrm3737 [DOI] [PubMed] [Google Scholar]
- 31.Zhou W, Yuan J. SnapShot: necroptosis. Cell. 2014;158(2):464-464.e1. doi: 10.1016/j.cell.2014.06.041 [DOI] [PubMed] [Google Scholar]
- 32.Linkermann A, Bräsen JH, Darding M, et al. . Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110(29):12024-12029. doi: 10.1073/pnas.1305538110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton K, Dugger DL, Wickliffe KE, et al. . Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343(6177):1357-1360. doi: 10.1126/science.1249361 [DOI] [PubMed] [Google Scholar]
- 34.Remijsen Q, Goossens V, Grootjans S, et al. . Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis. Cell Death Dis. 2014;5:e1004. doi: 10.1038/cddis.2013.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang DW, Shao J, Lin J, et al. . RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332-336. doi: 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]
- 36.Sun L, Wang H, Wang Z, et al. . Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1-2):213-227. doi: 10.1016/j.cell.2011.11.031 [DOI] [PubMed] [Google Scholar]
- 37.Zhao J, Jitkaew S, Cai Z, et al. . Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109(14):5322-5327. doi: 10.1073/pnas.1200012109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dondelinger Y, Declercq W, Montessuit S, et al. . MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7(4):971-981. doi: 10.1016/j.celrep.2014.04.026 [DOI] [PubMed] [Google Scholar]
- 39.Galluzzi L, Kepp O, Kroemer G. MLKL regulates necrotic plasma membrane permeabilization. Cell Res. 2014;24(2):139-140. doi: 10.1038/cr.2014.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czerny M, Zimpfer D, Fleck T, et al. . Successful treatment of an aortoesophageal fistula after emergency endovascular thoracic aortic stent-graft placement. Ann Thorac Surg. 2005;80(3):1117-1120. doi: 10.1016/j.athoracsur.2004.02.136 [DOI] [PubMed] [Google Scholar]
- 41.Viswanathan R, Khee TK, Chong CF. Perigraft infections due to salmonella after abdominal aortic aneurysm repair. Singapore Med J. 2008;49(7):e183-e185. [PubMed] [Google Scholar]
- 42.Choi SJ, Lee JS, Cheong MH, Byun SS, Hyun IY. F-18 FDG PET/CT in the management of infected abdominal aortic aneurysm due to salmonella. Clin Nucl Med. 2008;33(7):492-495. doi: 10.1097/RLU.0b013e31817793a0 [DOI] [PubMed] [Google Scholar]
- 43.Lemaire X, Dehecq C, Cattoen C, et al. . Spondylodiscitis and an aortic aneurysm due to Campylobacter coli. Ann Clin Microbiol Antimicrob. 2010;9:8. doi: 10.1186/1476-0711-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging. 2010;27(3):193-209. doi: 10.2165/11531490-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 45.Stahlmann R, Lode HM. Risks associated with the therapeutic use of fluoroquinolones. Expert Opin Drug Saf. 2013;12(4):497-505. doi: 10.1517/14740338.2013.796362 [DOI] [PubMed] [Google Scholar]
- 46.Sendzik J, Shakibaei M, Schäfer-Korting M, Lode H, Stahlmann R. Synergistic effects of dexamethasone and quinolones on human-derived tendon cells. Int J Antimicrob Agents. 2010;35(4):366-374. doi: 10.1016/j.ijantimicag.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 47.Menon A, Pettinari L, Martinelli C, et al. . New insights in extracellular matrix remodeling and collagen turnover related pathways in cultured human tenocytes after ciprofloxacin administration. Muscles Ligaments Tendons J. 2013;3(3):122-131. [PMC free article] [PubMed] [Google Scholar]
- 48.Orobello NC, Dirain CO, Schultz G, Milne-Davies BA, Ng MR, Antonelli PJ. Ciprofloxacin decreases collagen in mouse tympanic membrane fibroblasts. Otolaryngol Head Neck Surg. 2016;155(1):127-132. doi: 10.1177/0194599816633671 [DOI] [PubMed] [Google Scholar]
- 49.Sharma C, Velpandian T, Baskar Singh S, Ranjan Biswas N, Bihari Vajpayee R, Ghose S. Effect of fluoroquinolones on the expression of matrix metalloproteinase in debrided cornea of rats. Toxicol Mech Methods. 2011;21(1):6-12. doi: 10.3109/15376516.2010.529183 [DOI] [PubMed] [Google Scholar]
- 50.Shakibaei M, Stahlmann R. Ultrastructure of Achilles tendon from rats after treatment with fleroxacin. Arch Toxicol. 2001;75(2):97-102. doi: 10.1007/s002040000203 [DOI] [PubMed] [Google Scholar]
- 51.Corps AN, Harrall RL, Curry VA, Fenwick SA, Hazleman BL, Riley GP. Ciprofloxacin enhances the stimulation of matrix metalloproteinase 3 expression by interleukin-1beta in human tendon-derived cells: a potential mechanism of fluoroquinolone-induced tendinopathy. Arthritis Rheum. 2002;46(11):3034-3040. doi: 10.1002/art.10617 [DOI] [PubMed] [Google Scholar]
- 52.Tsai WC, Hsu CC, Chen CP, et al. . Ciprofloxacin up-regulates tendon cells to express matrix metalloproteinase-2 with degradation of type I collagen. J Orthop Res. 2011;29(1):67-73. doi: 10.1002/jor.21196 [DOI] [PubMed] [Google Scholar]
- 53.Bujor AM, Haines P, Padilla C, et al. . Ciprofloxacin has antifibrotic effects in scleroderma fibroblasts via downregulation of Dnmt1 and upregulation of Fli1. Int J Mol Med. 2012;30(6):1473-1480. doi: 10.3892/ijmm.2012.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88(4):660-672. doi: 10.1002/jcb.10413 [DOI] [PubMed] [Google Scholar]
- 55.Guo DC, Regalado ES, Gong L, et al. ; University of Washington Center for Mendelian Genomics . LOX mutations predispose to thoracic aortic aneurysms and dissections. Circ Res. 2016;118(6):928-934. doi: 10.1161/CIRCRESAHA.115.307130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee VS, Halabi CM, Hoffman EP, et al. ; Brigham Genomic Medicine . Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc Natl Acad Sci U S A. 2016;113(31):8759-8764. doi: 10.1073/pnas.1601442113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rabau M, Nyska A, Dayan D. In vitro effect of ciprofloxacin on HT-29 human colon carcinoma cell line: assessment of cell proliferation by thymidine uptake and silver nucleolar organizer regions (AgNOR) histomorphometry. Arch Toxicol. 1995;70(2):124-126. doi: 10.1007/BF02733673 [DOI] [PubMed] [Google Scholar]
- 58.Miclau T, Edin ML, Lester GE, Lindsey RW, Dahners LE. Effect of ciprofloxacin on the proliferation of osteoblast-like MG-63 human osteosarcoma cells in vitro. J Orthop Res. 1998;16(4):509-512. doi: 10.1002/jor.1100160417 [DOI] [PubMed] [Google Scholar]
- 59.Aranha O, Wood DP Jr, Sarkar FH. Ciprofloxacin mediated cell growth inhibition, S/G2-M cell cycle arrest, and apoptosis in a human transitional cell carcinoma of the bladder cell line. Clin Cancer Res. 2000;6(3):891-900. [PubMed] [Google Scholar]
- 60.Herold C, Ocker M, Ganslmayer M, Gerauer H, Hahn EG, Schuppan D. Ciprofloxacin induces apoptosis and inhibits proliferation of human colorectal carcinoma cells. Br J Cancer. 2002;86(3):443-448. doi: 10.1038/sj.bjc.6600079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Rayes BF, Grignon R, Aslam N, Aranha O, Sarkar FH. Ciprofloxacin inhibits cell growth and synergises the effect of etoposide in hormone resistant prostate cancer cells. Int J Oncol. 2002;21(1):207-211. [PubMed] [Google Scholar]
- 62.Aranha O, Grignon R, Fernandes N, McDonnell TJ, Wood DP Jr, Sarkar FH. Suppression of human prostate cancer cell growth by ciprofloxacin is associated with cell cycle arrest and apoptosis. Int J Oncol. 2003;22(4):787-794. [PubMed] [Google Scholar]
- 63.Smart DJ, Halicka HD, Traganos F, Darzynkiewicz Z, Williams GM. Ciprofloxacin-induced G2 arrest and apoptosis in TK6 lymphoblastoid cells is not dependent on DNA double-strand break formation. Cancer Biol Ther. 2008;7(1):113-119. doi: 10.4161/cbt.7.1.5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bourikas LA, Kolios G, Valatas V, et al. . Ciprofloxacin decreases survival in HT-29 cells via the induction of TGF-beta1 secretion and enhances the anti-proliferative effect of 5-fluorouracil. Br J Pharmacol. 2009;157(3):362-370. doi: 10.1111/j.1476-5381.2009.00161.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koziel R, Szczepanowska J, Magalska A, Piwocka K, Duszynski J, Zablocki K. Ciprofloxacin inhibits proliferation and promotes generation of aneuploidy in Jurkat cells. J Physiol Pharmacol. 2010;61(2):233-239. [PubMed] [Google Scholar]
- 66.Phiboonchaiyanan PP, Kiratipaiboon C, Chanvorachote P. Ciprofloxacin mediates cancer stem cell phenotypes in lung cancer cells through caveolin-1-dependent mechanism. Chem Biol Interact. 2016;250:1-11. doi: 10.1016/j.cbi.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 67.Azéma J, Guidetti B, Dewelle J, et al. . 7-((4-Substituted)piperazin-1-yl) derivatives of ciprofloxacin: synthesis and in vitro biological evaluation as potential antitumor agents. Bioorg Med Chem. 2009;17(15):5396-5407. doi: 10.1016/j.bmc.2009.06.053 [DOI] [PubMed] [Google Scholar]
- 68.Suresh N, Nagesh HN, Sekhar KV, Kumar A, Shirazi AN, Parang K. Synthesis of novel ciprofloxacin analogues and evaluation of their anti-proliferative effect on human cancer cell lines. Bioorg Med Chem Lett. 2013;23(23):6292-6295. doi: 10.1016/j.bmcl.2013.09.077 [DOI] [PubMed] [Google Scholar]
- 69.Ude Z, Romero-Canelón I, Twamley B, Fitzgerald Hughes D, Sadler PJ, Marmion CJ. A novel dual-functioning ruthenium(II)-arene complex of an anti-microbial ciprofloxacin derivative: anti-proliferative and anti-microbial activity. J Inorg Biochem. 2016;160:210-217. doi: 10.1016/j.jinorgbio.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 70.Mohammed HHH, Abd El-Hafeez AA, Abbas SH, Abdelhafez EMN, Abuo-Rahma GEA. New antiproliferative 7-(4-(N-substituted carbamoylmethyl)piperazin-1-yl) derivatives of ciprofloxacin induce cell cycle arrest at G2/M phase. Bioorg Med Chem. 2016;24(19):4636-4646. doi: 10.1016/j.bmc.2016.07.070 [DOI] [PubMed] [Google Scholar]
- 71.Hussy P, Maass G, Tümmler B, Grosse F, Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts. Antimicrob Agents Chemother. 1986;29(6):1073-1078. doi: 10.1128/AAC.29.6.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oomori Y, Yasue T, Aoyama H, Hirai K, Suzue S, Yokota T. Effects of fleroxacin on HeLa cell functions and topoisomerase II. J Antimicrob Chemother. 1988;22(suppl D):91-97. doi: 10.1093/jac/22.Supplement_D.91 [DOI] [PubMed] [Google Scholar]
- 73.Pessina A, Neri MG, Muschiato A, Mineo E, Cocuzza G. Effect of fluoroquinolones on the in-vitro proliferation of myeloid precursor cells. J Antimicrob Chemother. 1989;24(2):203-208. doi: 10.1093/jac/24.2.203 [DOI] [PubMed] [Google Scholar]
- 74.Bredberg A, Brant M, Jaszyk M. Ciprofloxacin-induced inhibition of topoisomerase II in human lymphoblastoid cells. Antimicrob Agents Chemother. 1991;35(3):448-450. doi: 10.1128/AAC.35.3.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elsea SH, McGuirk PR, Gootz TD, Moynihan M, Osheroff N. Drug features that contribute to the activity of quinolones against mammalian topoisomerase II and cultured cells: correlation between enhancement of enzyme-mediated DNA cleavage in vitro and cytotoxic potential. Antimicrob Agents Chemother. 1993;37(10):2179-2186. doi: 10.1128/AAC.37.10.2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perrone CE, Takahashi KC, Williams GM. Inhibition of human topoisomerase IIalpha by fluoroquinolones and ultraviolet A irradiation. Toxicol Sci. 2002;69(1):16-22. doi: 10.1093/toxsci/69.1.16 [DOI] [PubMed] [Google Scholar]
- 77.Bromberg KD, Burgin AB, Osheroff N. Quinolone action against human topoisomerase IIalpha: stimulation of enzyme-mediated double-stranded DNA cleavage. Biochemistry. 2003;42(12):3393-3398. doi: 10.1021/bi027383t [DOI] [PubMed] [Google Scholar]
- 78.Kloskowski T, Gurtowska N, Olkowska J, et al. . Ciprofloxacin is a potential topoisomerase II inhibitor for the treatment of NSCLC. Int J Oncol. 2012;41(6):1943-1949. doi: 10.3892/ijo.2012.1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bisacchi GS, Hale MRA. “Double-edged” scaffold: antitumor power within the antibacterial quinolone. Curr Med Chem. 2016;23(6):520-577. doi: 10.2174/0929867323666151223095839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu Y, Yang Y, Zhou S, et al. . Ciprofloxacin containing Mannich base and its copper complex induce antitumor activity via different mechanism of action. Int J Oncol. 2014;45(5):2092-2100. doi: 10.3892/ijo.2014.2611 [DOI] [PubMed] [Google Scholar]
- 81.Wang JC. DNA topoisomerases. Annu Rev Biochem. 1996;65:635-692. doi: 10.1146/annurev.bi.65.070196.003223 [DOI] [PubMed] [Google Scholar]
- 82.Zhang H, Zhang YW, Yasukawa T, Dalla Rosa I, Khiati S, Pommier Y. Increased negative supercoiling of mtDNA in TOP1mt knockout mice and presence of topoisomerases IIα and IIβ in vertebrate mitochondria. Nucleic Acids Res. 2014;42(11):7259-7267. doi: 10.1093/nar/gku384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kasiviswanathan R, Collins TR, Copeland WC. The interface of transcription and DNA replication in the mitochondria. Biochim Biophys Acta. 2012;1819(9-10):970-978. doi: 10.1016/j.bbagrm.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aranha O, Zhu L, Alhasan S, Wood DP Jr, Kuo TH, Sarkar FH. Role of mitochondria in ciprofloxacin induced apoptosis in bladder cancer cells. J Urol. 2002;167(3):1288-1294. doi: 10.1016/S0022-5347(05)65283-4 [DOI] [PubMed] [Google Scholar]
- 85.Pouzaud F, Dutot M, Martin C, Debray M, Warnet JM, Rat P. Age-dependent effects on redox status, oxidative stress, mitochondrial activity and toxicity induced by fluoroquinolones on primary cultures of rabbit tendon cells. Comp Biochem Physiol C Toxicol Pharmacol. 2006;143(2):232-241. doi: 10.1016/j.cbpc.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 86.Barnhill AE, Brewer MT, Carlson SA. Adverse effects of antimicrobials via predictable or idiosyncratic inhibition of host mitochondrial components. Antimicrob Agents Chemother. 2012;56(8):4046-4051. doi: 10.1128/AAC.00678-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu M, Li R, Zhang J. Repositioning of antibiotic levofloxacin as a mitochondrial biogenesis inhibitor to target breast cancer. Biochem Biophys Res Commun. 2016;471(4):639-645. doi: 10.1016/j.bbrc.2016.02.072 [DOI] [PubMed] [Google Scholar]
- 88.Song M, Wu H, Wu S, et al. . Antibiotic drug levofloxacin inhibits proliferation and induces apoptosis of lung cancer cells through inducing mitochondrial dysfunction and oxidative damage. Biomed Pharmacother. 2016;84:1137-1143. doi: 10.1016/j.biopha.2016.10.034 [DOI] [PubMed] [Google Scholar]
- 89.Wu J, Sun L, Chen X, et al. . Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826-830. doi: 10.1126/science.1229963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ablasser A, Goldeck M, Cavlar T, et al. . cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498(7454):380-384. doi: 10.1038/nature12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang X, Shi H, Wu J, et al. . Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51(2):226-235. doi: 10.1016/j.molcel.2013.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ouyang S, Song X, Wang Y, et al. . Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity. 2012;36(6):1073-1086. doi: 10.1016/j.immuni.2012.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786-791. doi: 10.1126/science.1232458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y, Jesus AA, Marrero B, et al. . Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371(6):507-518. doi: 10.1056/NEJMoa1312625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeremiah N, Neven B, Gentili M, et al. . Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124(12):5516-5520. doi: 10.1172/JCI79100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental methods
eTable. Antibodies used for western blot and immunostaining experiments
eFigure 1. Increased aortic destruction, decreased LOX protein expression, increased MMP activity, and increased reactive oxygen species production in the aortic wall of challenged mice that received ciprofloxacin
eFigure 2. Downregulation of extracellular matrix proteins and upregulation of MMP expression and activity in cultured aortic smooth muscle cells (SMCs) treated with ciprofloxacin
eFigure 3. Inhibition of cell proliferation and induction of cell injury in cultured aortic SMCs treated with ciprofloxacin
eFigure 4. Increased DNA damage and its release into the cytosol and the activation of cytosolic DNA sensor signaling in aortic SMCs treated with ciprofloxacin
eAppendix 1. DNA damage and release to the cytosol, mitochondrial dysfunction, and activation of cytosolic DNA sensor signaling by ciprofloxacin
eAppendix 2. Two independent observational clinical studies suggesting that fluoroquinolone use may be associated with an increased risk of aortic aneurysm and dissection