Abstract
Background
With the increase of primary lung transplantation across major centers worldwide, over the last several years the need of lung retransplant (ReTX) is likely to increase. Therefore, characterization of ReTX patients is prudent and necessary. Our study aimed to investigate and characterize the covariates and outcomes associated with lung ReTX survival in a single large U.S. transplant center.
Methods
Demographic, clinical diagnoses, and comorbidities were analyzed. Kaplan-Meier statistics were used to calculate and predict survival for 30 days and up to 3 years. Cox proportional modeling was used to determine the variables associated with mortality.
Results
Of included 684 lung transplants performed at the Houston Methodist Hospital between January 2009 and December 2015, 49 were lung ReTX. Median age of primary lung transplant (non-ReTX) and ReTx recipients was 62 and 49 years, respectively. Chronic graft rejection in the form of restrictive chronic lung allograft dysfunction and bronchiolitis obliterans syndrome was the main indications for ReTX. Compared with non-ReTX patients, ReTX patients had higher median lung allocation score (46.2 vs 37.0, respectively) and higher mortality after 6 months posttransplant. ReTX, older age, female sex, hospitalization 15 days or longer, estimated glomerular filtration rate less than 60, 6-minute walk distance less than 400 ft, and donor/recipient height ratio less than 1 were significantly associated with decreased 1-year patient and graft survival. Chronic graft rejection was still the major cause of death in the long-term follow-up recipients.
Conclusions
Our findings suggested that lung ReTX recipients have poor long-term survival outcomes. Lung ReTX should only be offered to carefully selected patients.
Lung retransplantation (ReTX) comprises about 5% of total lung transplantations performed throughout the world.1 In the United States, although there are about 1800 lung transplants performed annually, roughly about 90 are considered ReTX. Lung ReTX has become more frequent in recent years because of the substantial increases in case-volume across major centers in the world, as well as the significant improvement in primary lung transplantation survival rates.1 However, with the increasing numbers, ReTX is still a complex surgical therapy with a higher risk of perioperative morbidity and mortality, and continues to pose an increased risk of death compared with primary lung transplantation. According to the recent Organ Procurement and Transplantation Network report, patient survival rates at 1, 3, and 5 years for the period of 2008 to 2015 in the primary lung transplant group were: 87.9%, 70.5% and 56.0%, respectively, whereas for the ReTX population, survival rates were 76.0%, 48.9%, and 33.8%, respectively.2 This decreased survival might help explain the reason why there are only 146 centers worldwide that reported performing at least 1 lung ReTX between the period of 1990 to 2012, and 60% (88 centers) of the centers performed less than 10 lung ReTX during that same period.1,2
In the present analysis, we evaluated characteristics associated with ReTX at our large volume transplant center and the patients' short- and long-term outcomes. The study findings should inform transplant physicians in their decision-making process to determine a more effective ReTX approach.
MATERIALS AND METHODS
Patients
The study retrospectively evaluated all patients receiving a lung transplant at Houston Methodist Hospital J.C. Walter Jr Transplant Center from January 1, 2009, through December 31, 2015. Transplant recipients who received a lung for a third time or multiorgan transplantations were excluded from the study. Demographic and clinical characteristics of included patients were used in the analysis. As this analysis used deidentified retrospective data, ethic approval was not required by the Houston Methodist Hospital's Institutional Review Board.
Statistical Analysis
Baseline characteristics were reported as frequencies and proportions for categorical variables and as median and interquartile range (IQR) for continuous variables. We also conducted a 1-to-1 propensity score matching based on recipient age, donor age, recipient body mass index (BMI), lung allocation score (LAS), diagnosis group and pretransplant location. Differences in baseline characteristics between non-ReTX and ReTX patients were compared using the χ2 or Fisher’s exact tests for categorical variables and Kruskal Wallis test for continuous variables, as appropriate. Patient mortality was estimated using the Kaplan-Meier methodology. Difference in survival between groups was evaluated using the log-rank test. Multivariate Cox proportional-hazards model was conducted to compare the adjusted hazard ratio (HR) for mortality within 1 year between the non-ReTX and ReTX patients and also identify characteristics that are independently associated with the mortality. The model performance was evaluated using the C statistic. All the analyses were performed on Stata version 14.2 (StataCorp LLC, College Station, TX). A P value less than 0.05 was considered statistically significant.
RESULTS
From January 1, 2009, to December 31, 2015, 708 lung transplantations were performed at Houston Methodist Hospital; of whom, 57 were ReTX. Twenty recipients (4 with ReTX) who received multiorgan transplantations or ReTX for a third time and 4 patients with early ReTX less than 30 days were excluded from the analysis. Of 684 lung recipients included in the final analysis, 635 (92.8%) received a primary lung transplant (non-ReTX) and 49 (7.2%) were ReTX (Figure 1). The majority of double lung retransplant procedures were performed on cardiopulmonary bypass.
FIGURE 1.
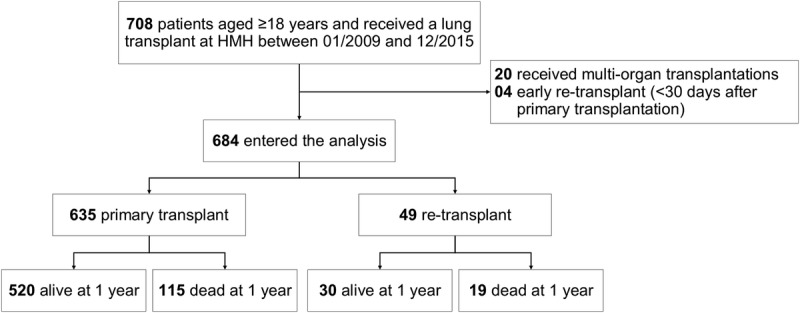
Flowchart of the study population.
Demographic, clinical, and diagnostic characteristics of both cohorts (all patients and propensity score matched patients) are shown in Table 1. Compared with non-ReTX patients, ReTX patients were likely to be younger, female, diabetic, a smoker and chronic steroid user, and have a smaller BMI, lower estimated glomerular fraction rate (eGFR) and a shorter 6-minute walk distance. There was no significant difference in the proportion of recipients with primary graft dysfunction (PGD) between the non-ReTX and ReTX patients. There were no significant difference in the days on postoperative ventilation support between primary lung transplant and ReTX groups (median days, 1 [1, 3] vs 1 [1, 4], P = 0.16) (Table 1). Specific primary diagnoses in the 4 diagnostic groups are presented in Table 2. The majority of ReTX recipients (31/49, 63.3%) were diagnosed with Bronchiolitis obliterans syndrome (BOS) (Table 2). A total of 16/49 (32.7%) ReTX recipients were admitted to the ICU prior to ReTX, all required ventilation support and none required postoperative extracorporeal membrane oxygenation (ECMO) support (data not shown). In the ReTX cohort, there was no positive crossmatch and 2 recipients had been treated for acute rejection episodes (data not shown).
TABLE 1.
Baseline characteristics of lung transplant recipients at Houston Methodist Hospital, 2009-2015
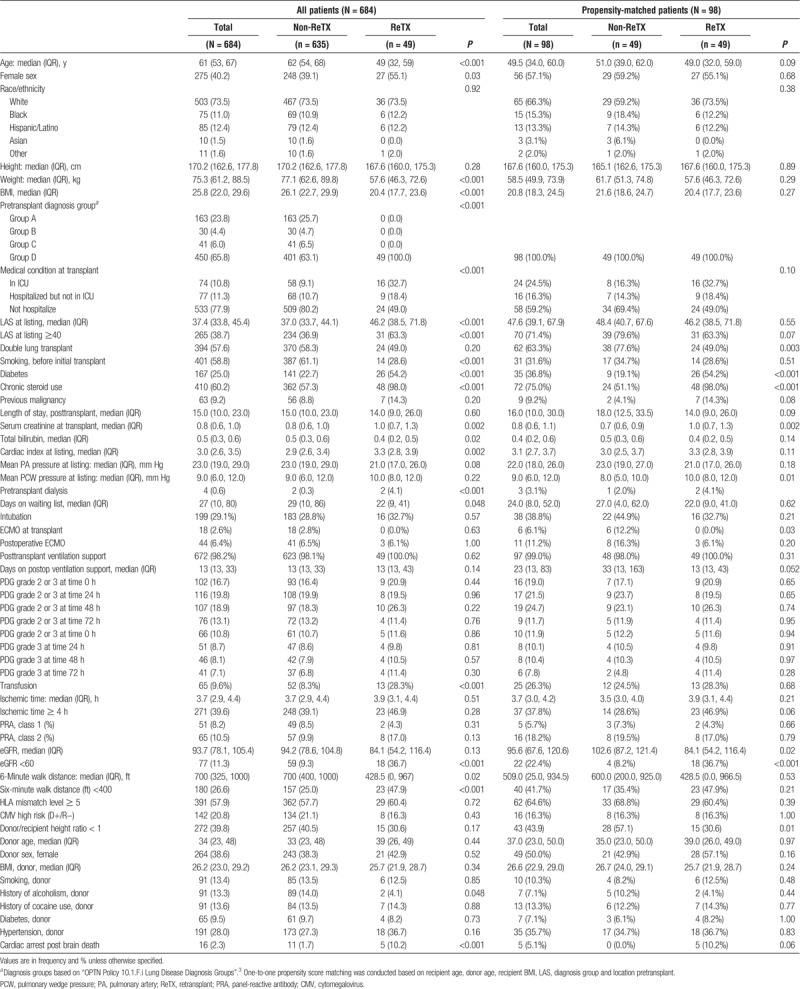
TABLE 2.
Detailed primary diagnoses in pretransplant diagnosis groups
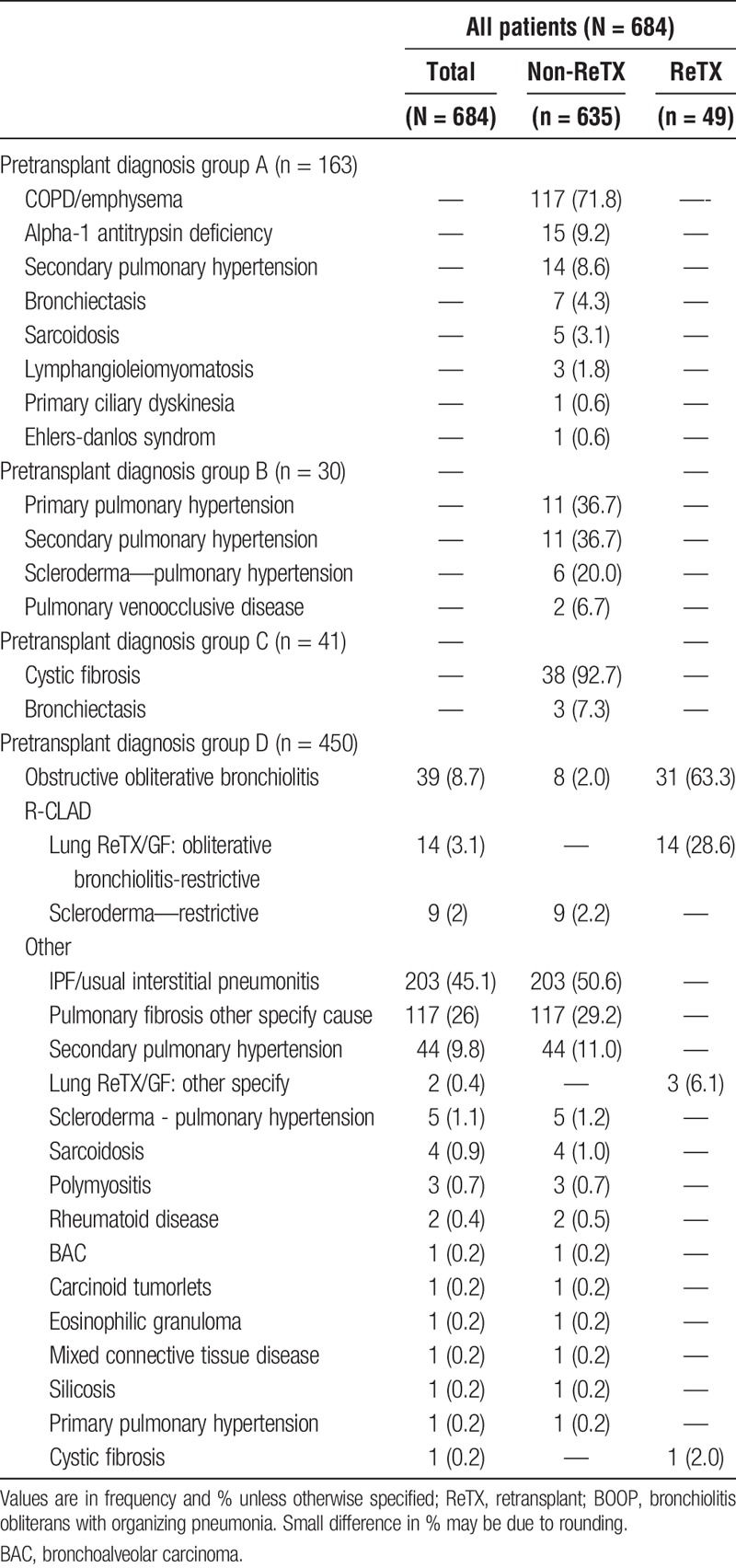
Idiopathic pulmonary fibrosis (IPF), and chronic obstructive pulmonary disease (COPD) were the major indications for primary lung transplantation. Bronchiolitis obliterans syndrome and restrictive chronic lung allograft dysfunction (R-CLAD) were the major indications of late lung ReTX. In our cohort, BOS and R-CLAD were the most common diagnoses for ReTX in 45/49 (91.8%) of recipients. We also found ReTX recipients had increased rates of high blood pressure, diabetes mellitus, renal dysfunction, pancreatic insufficiency, and pulmonary hypertension. Nearly all of our ReTX recipients had 1 of the above comorbidities (data not shown).
Mortality
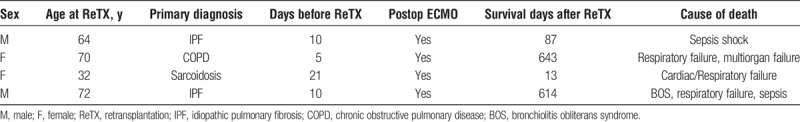
Patient mortality at 3 and 6 months, 1, 2 and 3 years are presented in Table 3. During the 6-year study period, 29/49 (59.2%) ReTX patients died, of whom 19 (38.8%) died within the first year. The difference in mortality between non-ReTX and ReTX groups became significant after 6 months and 1 year posttransplant in the cohort of all patients and the propensity score matched cohort, respectively (Table 3). ReTX recipients had significantly lower survival than non-ReTX patients (P < 0.001, Figure 2). Causes of death in the first year after ReTX were presented in Table 4. Pneumonia/septic shock and respiratory failure were the major causes of death in the first 30 and 90 days after ReTX. Three recipients expired from septic shock in the first 90 days after ReTX. One patient with a left single lung retransplant developed severe aspiration pneumonitis and acute pneumonia, received ECMO support, and finally expired due to multi organ failure (Table 4). In the 4 ReTX recipients, who had severe PGD needing ECMO support with the primary transplant and were excluded from the main analyses, 2 (50%) had graft function failed within 90 days after retransplant (Table 5). One ReTX recipient developed dehiscence of the bronchial anastomosis, multidrug-resistant pseudomonas pneumonia with abscesses, acute renal failure, hepatic insufficiency and GI bleeding, and died 45 days later. Another recipient also developed Pseudomonas pneumonia after ReTX, and eventually developed complete dehiscence of the anastomosis. Though the dehiscence was repaired successfully, the recipient died within 90 days due to other medical issues. In these 2 cases no significant correlation could be identified with either ischemic interval, type of surgical wrap, preoperative or postoperative steroid therapy, or rejection episode (data not shown).
TABLE 3.
Mortality, nonretransplant versus lung retransplant patients
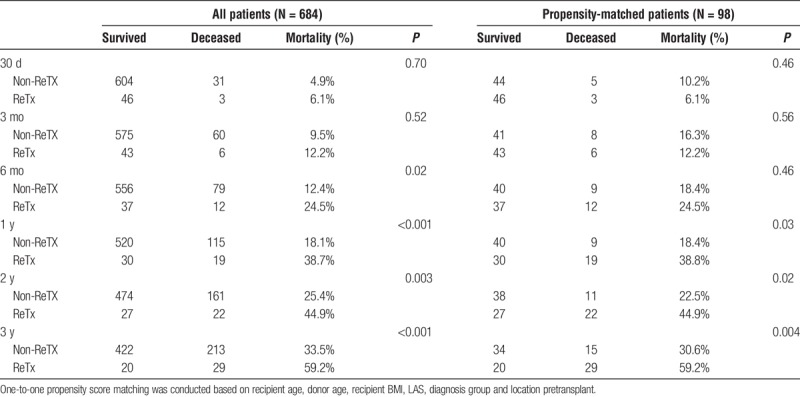
FIGURE 2.
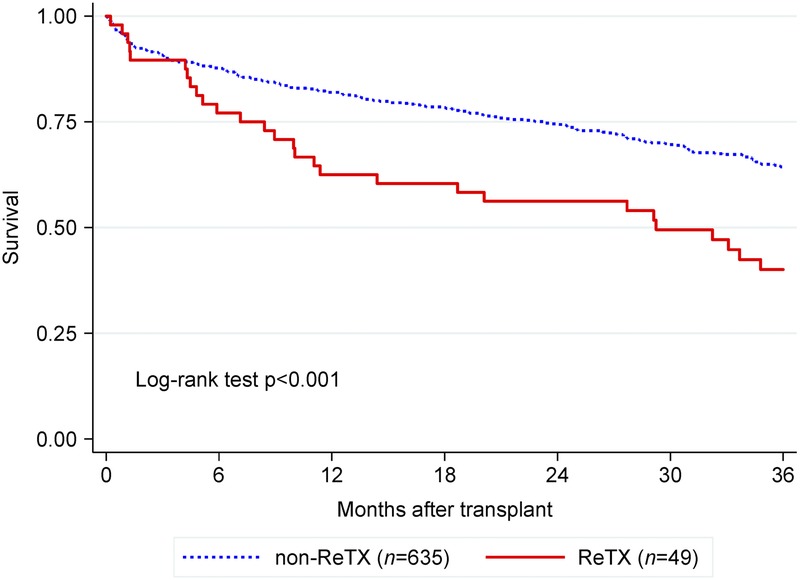
Kaplan-Meier 3-year survival curves in lung transplant recipients at Houston Methodist Hospital, 2009-2015.
TABLE 4.
The causes of the death in the first year after lung retransplant
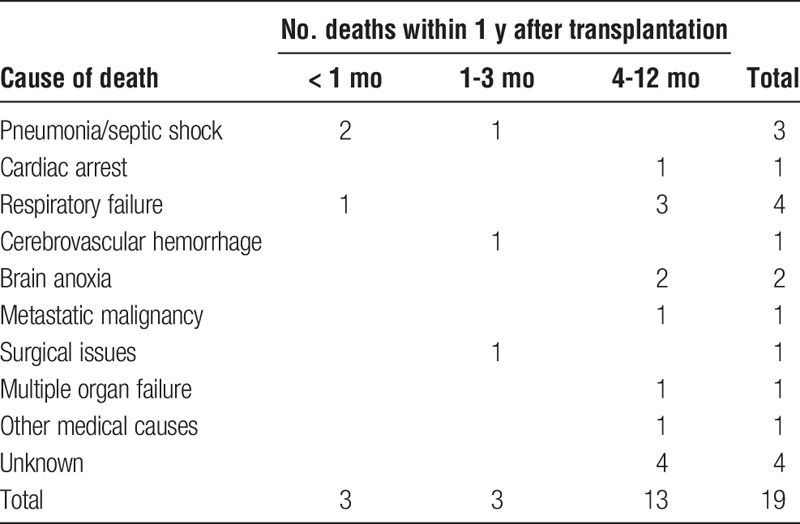
TABLE 5.
Early graft failure due to severe PGD (not included in the main analysis on 684 patients)
The Risk Factors of Mortality
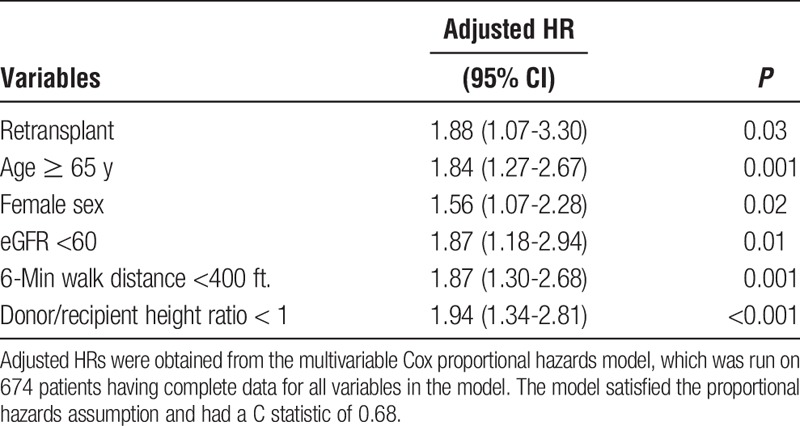
Results of Cox proportional hazards models were presented in Table 6. Compared with non-ReTX patients, ReTX recipients had nearly 2 times the risk for death within 1 year posttransplant. Older age, female sex, hospitalization 15 days or longer, cardiac index at listing, eGFR less than 60 mL/min per 1.73 m2, 6-minute walk distance less than 400 ft, and donor/recipient height ratio less than 1 were independently associated with a higher risk for 1-year mortality in the entire lung transplant cohort. Ages 65 years or older remained significant in ReTX recipients. The Cox proportional hazards models that were run in all patients had a moderate C statistic of 0.68 (Table 6). The stratification analysis by age groups in the retransplant cohort indicated that the number of death within 1 year after retransplant was 8 (38%) of 21 patients 50 years or older (median age of the retransplant cohort) versus 11 (39%) of 28 patients (P = 0.93) younger than 50 years. Meanwhile, 3 (60%) of 5 patients 65 years or older died within 1 year versus 16 (36%) of 44 patients (P = 0.30) younger than 65 years (data not shown). Of 49 ReTX patients, 43 (88%) received a single lung ReTX on the different side or double lung ReTX. Only 6 (12%) received a single lung ReTX on the same side of the primary lung transplantation. There was no significant difference in the mortality within 5 years after transplant by the side the single lung ReTX occurred (30/43, 69.8% vs 4/6, 66.7%, P = 0.99, data not shown).
TABLE 6.
Characteristics associated with 1-y survival, Cox proportional hazards models in patients having data available
DISCUSSION
Analysis of our 6-year cohort showed that lung ReTX recipients had good short-term outcomes, especially for 30 and 90 days, where survival rates dropped from 94% to 88%, respectively, which were similar to the survival rate of primary lung transplantation recipients (95% and 91%, respectively). However, mortality declined dramatically after 90 days with the 1-year survival of 61.3% (vs 81.9% in non-ReTX patients, P < 0.001). The 1-year survival of our ReTX is comparable to that of the historic control cohort described by Sommer et al,4 who were not retransplanted following the minimally invasive protocol (63.2%). Notably, Sommer et al suggested that the short- and midterm outcome of the ReTX patients could be substantially improved with the minimally invasive protocol. In fact, their minimally invasive ReTX cohort had a comparable 1-year survival to that of our primary lung transplantation cohort (80.6% vs 81.9%).4 Perioperative bleeding, severe infection, PGD and airway dehiscence were the major causes of early mortality within 90 days after ReTX. Surgical complications in the perioperative period after Re-TX were bleeding and airway complications. In general, lung ReTX recipients carry higher mortality risk because of technically challenging issues including chest wall bleeding due to significant adhesions. Moreover, these patients have a high prevalence of bleeding after the surgery due to coagulopathy due to cardiopulmonary bypass pump runs, or renal insufficiency. Prolonged operation times, increased use of blood products and cardiopulmonary bypass can also be major contributing factors to mortality and morbidity. Severe airway complications are associated with infection, rejection, surgical techniques or persistent ischemia and use of vasoconstrictor agents. Complete dehiscence of the anastomosis can be associated with high morbidity and mortality.5
Surgical anastomotic techniques plus allograft infection, with positive cultures for aggressive microbes such as Pseudomonas have a significant impact on airway complications and can be a substantial treatment challenge. Infection after lung ReTX has a significant impact on early and late mortality in our lung retransplant recipients. There were 3 deaths due to pneumonia and septic shock, which accounted for 16% of the 1-year ReTx mortality. These data clearly elucidate that infection poses a high risk for a poor outcome. Pretransplant immunosuppression may potentially be responsible for the higher rate of serious infection in these patients.6,7 Increasingly potent immunosuppressive agents given for induction and maintenance therapy have dramatically reduced the incidence of rejection of transplanted organs while increasing patients' susceptibility to opportunistic infections. Literature review suggests bacterial infections comprise more than half of all infectious complications after lung transplant.8 Aguilar-Guisado and coworkers reported a cohort study of 85 episodes of pneumonia in 236 lung transplants and identified bacterial pneumonia (82.7%) as being more frequent than fungal (14%) and viral pneumonia (10.4%). The most frequent microorganisms in each etiological group were Pseudomonas aeruginosa (n = 14, 24.6%), cytomegalovirus (CMV; n = 6, 10.4%) and Aspergillus spp. (n = 5, 8.8%).9 The etiology of pneumonia in lung transplantation is varied and includes 4 overlapping sources: donor-derived infections, recipient-derived infections, nosocomial infections, and community-acquired infections.10 A significant number of the early episodes of bacterial pneumonia are derived from donor lungs.8 Although infection and surgical complications account for most of recipient deaths in 90 days in our lung retransplant recipients, infection and chronic rejection accounted for the most retransplant recipient deaths between 90 days to 1 year.11
Our univariate analysis suggests that recipients who require dialysis have a significantly greater increased mortality risk after lung transplant. Of 4 recipients who had pretransplant dialysis, 2 were ReTX candidates and offered ReTX with acceptable renal function (eGFR>60 mL/min per 1.73 m2). Given the small number of recipients receiving pretransplant dialysis (n = 4), this variable was not included in the Cox proportional hazards model. Although pretransplant dialysis has not been well described as a risk factor for poorer survival after lung transplantation, pretransplant eGFR less than 60 mL/min per 1.73 m2, has been recognized as a risk factor for mortality in lung transplant recipients.12 This observation clearly indicates that lung ReTX should be avoided in patients on current dialysis unless concomitant renal transplantation is available as a reasonable therapeutic option, which may reduce the high mortality for this high-risk specific subgroup.
In our cohort, BOS and R-CLAD were the leading causes of death in the ReTX after the first year of lung transplantation.13-16 We had 4 recipients who had respiratory failure due to R-CLAD within the first year after lung ReTX. R-CLAD accounted for about 21% of the total deaths in the first year. Major risk factors related with R-CLAD including allograft acute rejection, CMV infection, human leukocyte antigen (HLA) mismatching, non-CMV infection, and allograft ischemic time.17,18 Further investigations are needed to discern why the development of R-CLAD occurs so early after lung ReTX and quickly leads to graft dysfunction or failure within the first year after ReTX. In addition, some significant differences of the patients' condition at baseline (ReTX patients were more likely to be females, have a lower BMI, and eGFR, have a higher rate of previous steroid use, and history of diabetes compared with non-ReTX patients) may also contribute to the ReTX patients' poor outcomes via the increase of infections and higher levels of immune suppression at or before the transplant.
Our study indicates mortality for ReTX is comparable to mortality rates for primary lung transplant recipients in the short term (within 90 days after transplantation), but had poor results at 1 year. These outcomes are similar with national data.2 Although survival after lung ReTX has had definite improvement in recent years,19-21 outcome measures such as mortality are still poorer compared with outcome measures after primary lung transplantation. Moreover, donor organs are scarce and in high demand, thus outcome measures must be weighed in the decision algorithm.
ReTX carries a high risk of operative complications and poorer overall outcomes. Hence, patients who are considered for ReTX must meet strict criteria to ensure the best possible outcomes for our patients. Therefore, our lung retransplant protocol is based on current data and the scarcity of donor organs and was recently revised. Based on these new revised criteria, patients being considered for lung retransplant undergo the same stringent evaluation as the initial lung transplant evaluation. Patients and families are counseled that not all patients who have had a transplant in the past are appropriate candidates for ReTX. The evaluation also includes a psychosocial and economic evaluation to identify any concerns that would hinder our goal to provide the best outcomes and survival for our lung transplant patients. Ages 65 years or older, being female, having posttransplant length of stay 15 days or longer, eGFR less than 60, 6-minute walk distance less than 400 ft, and donor/recipient height ratio less than 1, considered a surrogate for donor-recipient lung size match in our analysis, have been identified by the Cox proportional hazards model as risk factors for having higher risk for 1-year mortality, in which ages 65 years or older remained significant after stratification for ReTX group. Although preoperative ICU stay and mechanical ventilation were included in the initial multivariate model, these 2 variables were not significant and were removed from the final model. The risk factors identified associated with poor posttransplant outcome by our multivariable Cox proportional hazard model has some practical implementations: (1) more careful consultations and extensive evaluations should be done before making a decision regarding lung retransplant for the “high risk” patients, (2) if a lung retransplant is done on a patient having one of the risk factors (such as older than 65 year, female, eGFR<60, 6 minute walk distance <400 and donor/recipient height ratio < 1), the patient would need greater attention and more aggressive medical support measures should be allocated. In addition, certain medical support should be considered to improve the modifiable parameters (such as 6-minute walk distance or carefully choose a matched donor lung size) that would ultimately improve the patient's posttransplant survival. However, given the small number of event in ReTX patients, stratification analysis by non-ReTX versus ReTX for the Cox proportional hazards model was not conducted. Although only 5 (10.2%) patients in our retransplant cohort are 65 years or older, these patients had higher mortality (3/5, 60%), a 24% increase compared with patients younger than 65 years. However, this findings should be interpreted with caution given the small sample size of the retransplant cohort. Although preoperative ICU stay and mechanical ventilation were included in the initial multivariate model, these 2 variables were not significant and were removed from the final model. Improving the 6-minute walk distance, carefully choosing a size-matched donor lung, and more aggressive postop medical supports would be among the approaches to improve patient survival. The implementation of minimally invasive procedures should also be taken into consideration for ReTX patients.4 In our center, 24/49 (49.0%) of patients received bilateral ReTX. Medical and surgical issues drive review board decisions toward offering single versus double ReTX. Single lung ReTX was offered more in older recipients, obstructive CLAD phenotype, and primary single lung transplants with graft failure where the remaining native lung was removed and transplanted. Double lung retransplants were offered for younger patients, restrictive CLAD phenotype, concomitant chronic infections or colonization, sicker patients in ICU with severe graft dysfunction. Surgical considerations predisposing to single lung transplants included presence of diaphragm paralysis, significant pleuroparenchymal chest wall adhesions or loculation and ipsilateral or double lung retransplants were generally avoided. However, there was no significant difference in the outcome between groups of single versus bilateral retransplantation (data not shown).
CONCLUSIONS
Lung retransplantation is a treatment option for highly selected patients. Outcomes of lung retransplantation are good in the short-term, but long-term survival results in lung retransplant recipients are poor. Severe PGD, infections and surgical issues are the main causes of death during the early postoperative (90 days) period. Chronic rejection still is the major cause of death in lung transplantation long-term follow-up. Those individuals who undergo lung retransplantation for chronic rejection have a better survival than those with early graft failure/acute rejection. Lung retransplantation indication should be evaluated more vigorously, especially in patients older than 65 years, female, having eGFR less than 60 mL/min per 1.73 m2 or 6-minute walk distance less than 400 ft. Although some risk factors are not modifiable (such as older age or sex), improving the modifiable parameters (such as 6-minute walk distance or careful choices of a matched donor lung size) would be among the approaches to improve the patient's posttransplant survival.
Footnotes
Published online 25 October, 2018.
D.R., T.K., E.A.G., D.N., N.S., A.G., E.S., M.L., S.S., B.B. participated in the research design. E.A.G., D.N. participated in data analysis. D.R., T.K., E.A.G., D.N. participated in result interpretation. D.R., E.A.G., D.N. participated in writing of the article. D.R., T.K., A.G., I.A., E.A.G., D.N. reviewed and edited article.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirty-first adult lung and heart-lung transplant report—2014; focus theme: retransplatation. J Heart Lung Transplant. 2014;33:1009–1024. [DOI] [PubMed] [Google Scholar]
- 2.Organ Procurement and Transplantation Network. Lung Kaplan-Meier patient survival rates for transplants performed: 2008-2015. Available at https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed on December 19, 2017.
- 3.Organ Procurement and Transplantation Network - OPTN. Policy 10.1.F.i lung disease diagnosis groups. Available at https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Accessed on March 8, 2018.
- 4.Sommer W, Ius F, Kühn C, et al. Technique and outcomes of less invasive lung retransplantation. Transplantation. 2018;102:530–537. [DOI] [PubMed] [Google Scholar]
- 5.Mahajan AK, Folch E, Khandhar SJ, et al. The diagnosis and management of airway complications following lung transplantation. Chest. 2017;152:627–638. [DOI] [PubMed] [Google Scholar]
- 6.Parada MT, Alba A, Sepúlveda C. Early and late infections in lung transplantation patients. Transplant Proc. 2010;42:333–335. [DOI] [PubMed] [Google Scholar]
- 7.Dowling RD, Zenati M, Yousem SA, et al. Donor-transmitted pneumonia in experimental lung allografts. Successful prevention with donor antibiotic therapy. J Thorac Cardiovasc Surg. 1992;103:767–772. [PubMed] [Google Scholar]
- 8.Yun JH, Lee SO, Jo KW, et al. Infections after lung transplantation: time of occurrence, sites, and microbiologic etiologies. Korean J Intern Med. 2015;30:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aquilar-Guisado M, Givalda J, Ussetti P, et al. Pneumonia after lung transplantation in the Resitra cohort: a multicenter prospective study. Am J Transplant. 2007;7:1989–1996. [DOI] [PubMed] [Google Scholar]
- 10.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. [DOI] [PubMed] [Google Scholar]
- 11.Chan KM, Allen SA. Infectious pulmonary complications in lung transplant recipients. Semin Respir Infect. 2002;17:291–302. [DOI] [PubMed] [Google Scholar]
- 12.Banga A, Mohanka M, Mullins J, et al. Interaction of pre-transplant recipient characteristics and renal function in lung transplant survival. J Heart Lung Transplant. Published online August 12, 2017. DOI:10.1016/j.healun.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Christie JD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report—2010. J Heart Lung Transplant. 2010;29:1104–1118. [DOI] [PubMed] [Google Scholar]
- 14.Heng D, Sharples LD, McNeil K, et al. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17:1255–1263. [PubMed] [Google Scholar]
- 15.Verleden G. Chronic allograft rejection (obliterative bronchiolitis). Semin Respir Crit Care Med. 2001;22:551–557. [DOI] [PubMed] [Google Scholar]
- 16.Ouwens JP, van der Mark TW, Koeter GH, et al. Bronchiolar airflow impairment after lung transplantation: an early and common manifestation. J Heart Lung Transplant. 2002;21:1056–1061. [DOI] [PubMed] [Google Scholar]
- 17.Belperio JA, Lake K, Tazelaar H, et al. Bronchiolitis obliterans syndrome complicating lung or heart-lung transplantation. Semin Respir Crit Care Med. 2003;24:499–530. [DOI] [PubMed] [Google Scholar]
- 18.Hayes D., Jr A review of bronchiolitis obliterans syndrome and therapeutic strategies. J Cardiothorac Surg. 2011;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawut SM, Lederer DJ, Keshavjee S, et al. Outcomes after lung retransplantation in the modern era. Am J Respir Crit Care Med. 2008;177:114–120. [DOI] [PubMed] [Google Scholar]
- 20.Osaki S, Maloney JD, Meyer KC, et al. Redo lung transplantation for acute and chronic lung allograft failure: long-term follow-up in a single center. Eur J Cardiothorac Surg. 2008;34:1191–1197. [DOI] [PubMed] [Google Scholar]
- 21.Strueber M, Fischer S, Gottlieb J, et al. Long-term outcome after pulmonary retransplantation. J Thorac Cardiovasc Surg. 2006;132:407–412. [DOI] [PubMed] [Google Scholar]


