Abstract
Background
Posttransplant hyperglycemia has been associated with increased risks of transplant rejection, infections, length of stay, and mortality.
Methods
To establish a predictive model to identify nondiabetic recipients at risk for developing postliver transplant (LT) hyperglycemia, we performed this secondary, retrospective data analysis of a single-center, prospective, randomized, controlled trial of glycemic control among 107 adult LT recipients in the inpatient period. Hyperglycemia was defined as a posttransplant glucose level greater than 200 mg/dL after initial discharge up to 1 month following surgery. Candidate variables with P less than 0.10 in univariate analyses were used to build a multivariable logistic regression model using forward stepwise selection. The final model chosen was based on statistical significance and additive contribution to the model based on the Bayesian Information Criteria.
Results
Forty-three (40.2%) patients had at least 1 episode of hyperglycemia after transplant after the resolution of the initial postoperative hyperglycemia. Variables selected for inclusion in the model (using model optimization strategies) included length of hospital stay (odds ratio [OR], 0.83; P < 0.001), use of glucose-lowering medications at discharge (OR, 3.76; P = 0.03), donor female sex (OR, 3.18; P = 0.02) and donor white race (OR, 3.62; P = 0.01). The model had good calibration (Hosmer-Lemeshow goodness-of-fit test statistic = 9.74, P = 0.28) and discrimination (C-statistic = 0.78; 95% confidence interval, 0.65-0.81, bias-corrected C-statistic = 0.78).
Conclusions
Shorter hospital stay, use of glucose-lowering medications at discharge, donor female sex and donor white race are important determinants in predicting hyperglycemia in nondiabetic recipients after hospital discharge up to 1 month after liver transplantation.
To date, over 150 000 liver transplants (LT) have been performed in the United States as a lifesaving therapy.1 Hyperglycemia and diabetes mellitus (DM), however, are known sequelae posttransplant and have been associated with increased risks of transplant rejection, high infection rates, increased length of hospital stay,2 and in some cases, early mortality.3
Posttransplant hyperglycemia is a common finding in the postoperative period and is caused by a number of factors, most notably by surgical stress and medications, such as the use of high-dose glucocorticosteroids and calcineurin inhibitors (CNI).4,5 Additionally, posttransplant hyperglycemia can put patients at risk of developing new-onset diabetes after transplantation (NODAT).6-8
Perioperative intensive insulin treatment with better glycemic control has resulted in mixed outcomes in kidney transplant recipients.9-14 In LT recipients, we have recently reported that glycemic control with intensive insulin therapy in the hospital setting, with a glucose goal less than 140 mg/dL versus less than 180 mg/dL significantly reduced post-LT infections.15 However, posttransplant uncontrolled hyperglycemia after discharge from the hospital occurred in many study participants, regardless of treatment assignment, and was difficult to predict.
Hyperglycemia after surgery has been shown previously to result in increased risks for infection and readmission.2,5,10,11,16 Whether treatment of this hyperglycemia following hospital discharge results in better outcomes has not been shown in randomized controlled studies but it is a logical extension from our own study of treatment of immediate post-LT inpatient hyperglycemia15 and other studies in postoperative surgical patients.9,11,17,18 Therefore, identification and treatment of hyperglycemia in this time period would be of benefit. Clearly, patients known to have diabetes prior to transplant can be expected to be hyperglycemic following transplant. However, to our knowledge, no studies have been conducted evaluating donor and recipient characteristics that may put patients not known to have diabetes pretransplant at risk for post-LT hyperglycemia after inpatient discharge. To address this gap, we have developed a novel predictive model utilizing both donor and recipient factors to guide providers in identifying nondiabetic patients at risk for hyperglycemia post discharge up to 1-month post-LT. Such a model may allow clinicians to adequately provide posttransplant follow-up and planning for monitoring and treatment of hyperglycemia postdischarge.
METHODS
The present study is a secondary, retrospective data analysis based on a single-center, prospective, randomized, controlled trial of glycemic control (140 mg/dL vs 180 mg/dL targets) among LT recipients in the inpatient period. The complete methodology and results of this inpatient trial have been published previously.15 As part of the study, all participants were given a glucose meter and strips, regardless of group assignment. All participants were asked to check home glucose levels 2 to 4 times per day up to 30 days post-LT and data were reported to our study team, along with any additional laboratory glucose measurements taken during the outpatient period. Participants were placed on glucose-lowering medications based on their treatment goal, that is, glucose targets 140 mg/dL versus 180 mg/dL. Patient education and diabetes discharge regimens were standardized between both groups and information on this can be found in the primary study.15 If a patient was readmitted within 30 days post LT, the study team also collected the inpatient glucose measurements.
The clinical trial included adults older than 18 years who underwent LT between April 2009 and December 2014 at Northwestern Medicine and who participated in a randomized control trial of glycemic control. All participants gave written, informed consent under guidelines established by the Northwestern University Institutional Review Board (protocol TU00005806).15 The clinical trial enrolled 164 patients. For the current analysis, we excluded 49 participants who had known pretransplant diabetes. From the 115 participants left, we additionally excluded 8 participants: 3 who died before discharge from the hospital, 2 who had a hospital stay of greater than 30 days and 3 who had missing glucose measurements after discharge. The final sample included 107 nondiabetic LT recipients.
Postdischarge glucose levels from both home glucose meter testing and hospital laboratory values were included. Hyperglycemia for this analysis was defined as a post-LT glucose measurement greater than 200 mg/dL following discharge up to 1 month following LT. The recipient factors obtained via chart review for model inclusion were age, sex, race, body mass index (BMI), DM status before transplant, transplant type (liver, liver/kidney), model of end-stage liver disease (MELD) score, liver disease etiology, length of hospital stay (days), and use of glucose-lowering medications at discharge. Additional donor factors were gathered including, age, sex, race, donor risk index, DM status, donor organ quality (standard criteria, expanded criteria, or CDC high risk donor), donor source (living donor, donation after cardiac death, other), cold ischemic time, warm ischemic time, transplant network location, and principal cause of donor death. Standard immunosuppression at our center during the study time period included induction with steroids alone followed by early CNI initiation. For patients with renal injury, mycophenolate was added at the discretion of the treating physician with the goal of reducing target CNI levels as a renal protective strategy.
Statistical Analysis
The study population was described using mean and standard deviation for continuous variables and proportions for categorical variables. Recipient and donor characteristics between patients with and without hyperglycemia event following discharge up to 1 month of LT were assessed as appropriate using Student t test with unequal variance or Mann-Whitney U test for continuous variables, and χ2 or Fisher exact test for categorical variables, respectively. Six candidate variables with P values less than 0.10 were used to build a multivariable logistic regression model using forward stepwise selection. Covariates were selected for the final model based on statistical significance and additive contribution to the model based on the Bayesian Information Criteria. Missing data that were categorized as “unknown” were excluded from analysis. For internal validation, the bootstrap method was used to account for the generalizability error, and bias-corrected 95% confidence intervals were calculated based on 1000 resamples with replacement. The discrimination of the model was estimated using the C-statistic. The calibration, a measure of the goodness of model fit, was assessed by comparing the observed and predicted number of events in deciles of predicted risk, as calculated by the Hosmer and Lemeshow goodness-of-fit statistic. A P value less than 0.05 was considered as a statistical significance. All analyses were performed using Stata15.0 for Windows (StataCorp LLC, College Station, TX).
RESULTS
The baseline characteristics of the 107 liver transplant recipients and liver transplant donors included in the study are shown in Table 1. The recipients were predominantly white, male, middle-aged and overweight. Of the recipients, 43 (40.2%)had at least 1 hyperglycemia episode after hospital discharge and up to 1 month after LT. The main liver disease etiology was hepatitis C and the majority of recipients (81 total, 31 in the hyperglycemia (>200 mg/dL) group, 50 in the nonhyperglycemia (≤200 mg/dL) group) were discharged from the hospital without any glucose-lowering medication prescriptions. Forty-seven (21.5%) recipients were readmitted to the hospital at least once in the first 30 days post discharge. Donors were predominantly male (59.8%) and white (55.1%), and the principal causes of death were spontaneous intracranial hemorrhage (28.7%) followed by anoxia/hypoxia (25.7%).
TABLE 1.
Demographic and clinical characteristics of liver transplant nondiabetic recipients and donors from April 2009 to December 2014 at Northwestern Medicine
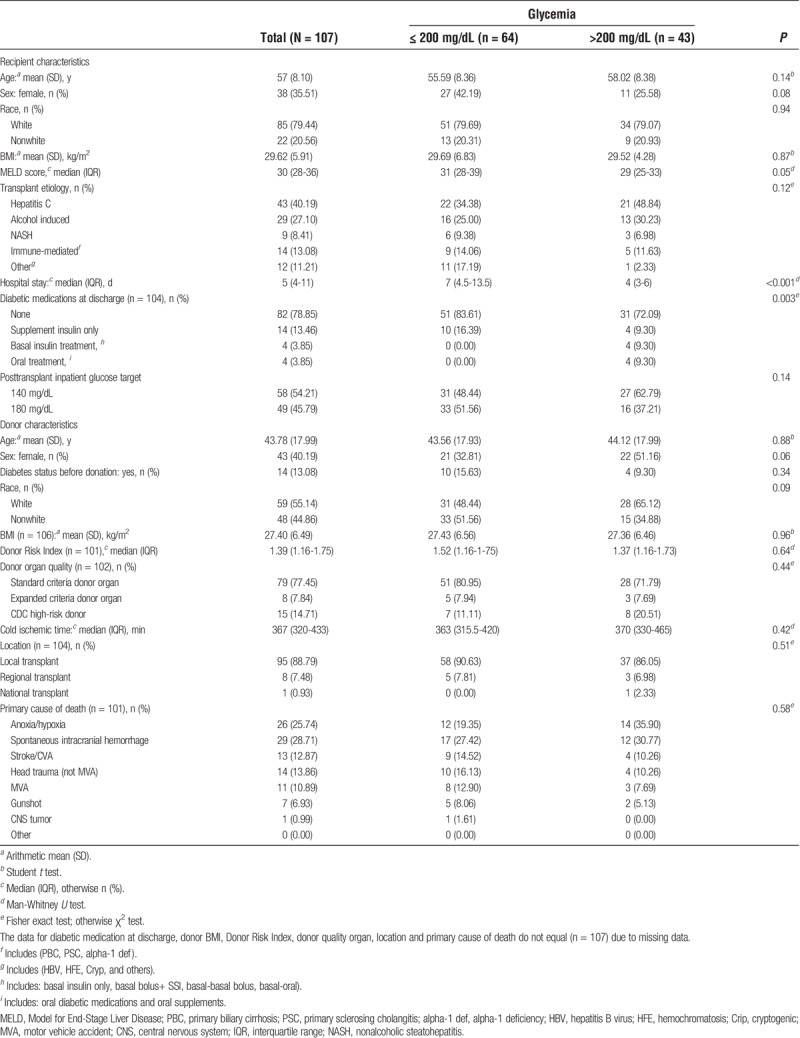
Overall, compared with the 64 patients without post-LT hyperglycemia, the 43 patients with post-LT hyperglycemia were significantly more likely to be male, had lower MELD scores, had shorter hospital stays, and were more likely to be discharged on insulin (Table 1).
The regression coefficients, odds ratio [ORs] and 95% confidence intervals for all risk factors in the final multivariate model are summarized in Table 2. Variables selected for model inclusion included length of hospital stay, use of glucose-lowering medications at discharge, donor sex and donor race. The model demonstrated good discrimination (C statistic, 0.78; 95% confidence interval [CI], 0.65-0.81) and bias-corrected C statistic, 0.78 (95% CI, 0.7965-0.81). The Hosmer-Lemeshow goodness-of-fit test statistic was 9.74(P = 0.28), which indicated that the model had good calibration with no significant difference between the predicted and observed probabilities (Figure 1). As a sensitivity analysis, the analysis was redone including the treatment group in the multivariable analysis and the resulting C-statistic of 0.78 (95% CI, 0.69-0.86), bias-corrected C-statistic of 0.78, which is similar to the C-statistic of the predictive model without the intervention arm (see above).
TABLE 2.
Predictive model for hyperglycemia after liver transplant
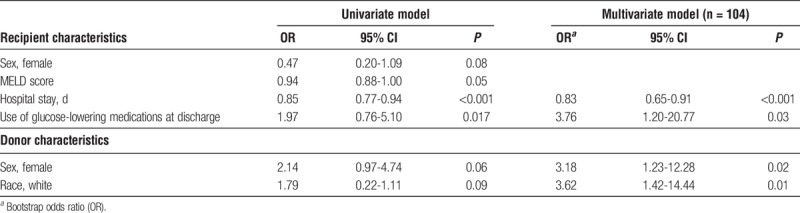
FIGURE 1.
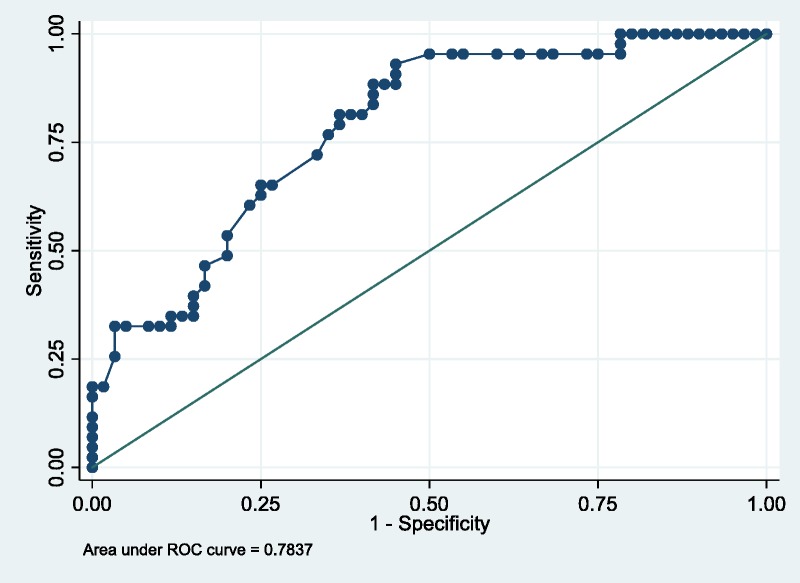
ROC curve. The area under the ROC curve represents the C-statistic. The values of the C-statistic on the figure were corrected for optimism as is detailed in Methods. ROC, receiver operating characteristic curve.
DISCUSSION
This study provides the first liver transplant-specific prognostic model in nondiabetic recipients for the prediction of posttransplant hyperglycemia following hospital discharge after solid organ transplantation with very good model accuracy. We identified 4 significant predictors of early (30-day) hyperglycemia: 2 recipient factors—shorter length of stay and the use of glucose-lowering medications at discharge and 2 donor factors—female gender and white race, among a sample of liver transplant recipients who had already undergone initial correction of immediate postoperative hyperglycemia using standard inpatient insulin drips and subsequent subcutaneous basal/bolus insulin protocols.
Post-LT hyperglycemia is a multifactorial medical problem that starts during LT surgery, where the blood glucose level rises mainly due to stress, glucose-containing intravenous fluid administration, and glycogenolysis from the donor liver.19 Some studies have highlighted the importance of adequate intraoperative glucose monitoring and intraoperative treatment of hyperglycemia due to the association of intraoperative hyperglycemia with increased postoperative infection and mortality rates.3,20 Furthermore, hyperglycemia in the post-LT (reperfusion) phase has been correlated with delay in the functional recovery of the LT graft.21 It is also important to mention that, DM is an independent risk factor for postreperfusion severe hyperglycemia.21
Use of glucose-lowering medications at discharge was also an important risk factor for the occurrence of hyperglycemia after LT. In our study, 22 (21.1%) patients were prescribed insulin or an oral hypoglycemic agent treatment upon discharge, reflecting persistent hyperglycemia at the time of discharge, despite not having a diagnosis of diabetes before transplantation. Our study also showed that a shorter hospital stay increased the risk for post-LT hyperglycemia compared with a longer hospital stay. It may be that patients discharged earlier may not have been well controlled, or still had residual effects from the stress of surgery and/or steroid dosing at discharge.
As noted in our study, etiology of liver disease was not associated with post-LT hyperglycemia (P = 0.12). However, it is important to mention that hepatitis C virus (HCV) infection has been associated with increased insulin resistance, similar to type 2 DM,22 and may have viral or immune-mediated deleterious effects on the pancreatic beta cell function.23,24 HCV infection has also been noted to be a risk factor for new onset DM after transplantation (NODAT).25 Consistent with national transplant trends, HCV infection was the most common indication for transplant among recipients in our study. Other known risk factors for hyperglycemia, such as age, steatohepatitis and severity of illness (MELD score), were not found to be significant in the multivariate model.
Donor characteristics, such as Donor Risk Index, a marker of allograft quality, and donor BMI, a surrogate marker for allograft steatosis, did not demonstrate an association with posttransplant hyperglycemia. However, donor female sex increased the risk for hyperglycemia after LT. Many,26-28 but not all,29 studies have shown that donor female sex when the recipient is male is related to decreased graft survival for liver, heart and kidney. It has been hypothesized that moving a “female” allograft to an estrogen-deprived environment may have an adverse effect on recovery from ischemic-reperfusion injury30 and it has been shown that female liver allografts demonstrate greater oxidative stress than male liver allografts in rats.31 Oxidative stress has been linked to insulin resistance32,33 but whether that is the mechanism for the hyperglycemia in our patients related to a female donor liver is speculative.
The mechanism behind the increased incidence of post-LT hyperglycemia among recipients with white donors also remains unclear. Other studies have shown that race/ethnicity mismatch results in greater risks of graft failure and mortality without a specifically worse outcome with white donors.29 Hence, this finding requires further exploration in future predictive models. Likewise, our study did not find an association between donor diabetes status and posttransplant hyperglycemia. A previous study showed that donor diabetes increased the risk of NODAT when present in deceased but not living liver donors.34
Our study had certain limitations. This research was performed at a single, tertiary, high-transplant-volume hospital with a uniform LT protocol used by experienced endocrinologists, nurses, and hospital staff. Therefore, whether our results can be extrapolated to other institutions and populations remains to be determined. Furthermore, because this is a secondary data analysis from a randomized controlled trial of participants who were consented during listing, but before transplantation, the total number of patients evaluated represented only a small percentage of the total number of patients undergoing liver transplant during this time period. In another study we have done that evaluated the course of those with hyperglycemia but without preexisting diabetes following transplant, 23% had resolution of hyperglycemia within 1 month, 53% had resolution of hyperglycemia between 1 month and 1 year, 18% remained persistently hyperglycemic out to 1 year, and 6% had resolution of hyperglycemia but then later developed biochemical criteria for diabetes (NODAT) before 1 year.35 In addition, prospective evaluations of our model among different transplant institutions will result in its refinement. In a similar manner, future investigations are needed to evaluate the use of the model in other solid organ transplant surgeries.
In conclusion, post-LT hyperglycemia in nondiabetic patients up to 1 month after liver transplantation can be predicted with accuracy using the proposed prognostic model, which includes both recipient and donor factors. Shorter hospital stay, use of glucose-lowering medications at discharge, donor female sex and donor white race, are factors that can be used to guide the physician in the identification of patients at risk for post-LT hyperglycemia. Clearly, this model needs to be validated in other populations. Although routine chemistries are performed during the first month post-LT, glucose levels and trends that might otherwise appear innocuous might be missed if there is no highlighting of patients that might be at higher risk for developing much higher glucose levels, which may lead to infection and readmission.2,5,10,11,16 It is a logical extension from our own study of treatment of immediate post-LT inpatient hyperglycemia15 and other studies in postoperative surgical patients9,11,17,18 that addressing hyperglycemia early in the immediate postdischarge period would have additional benefit, although this has not been proven in randomized controlled trials. Thus, our proposed model may allow an early identification of at risk patients so that preventive interventions (diet, exercise, medications) or intensification of hyperglycemic treatment can be implemented in a timely fashion.
Footnotes
Published online 20 September, 2018.
A.W. receives research support from Eli Lilly, serves a consultant for Glytec, and is a trial adjudicator for Lexicon Therapeutics. L.B.V.W. is on the speaker's bureau for Salix Pharmaceuticals outside of the submitted work. Dr. Molitch receives research support from Novartis, NovoNordisk, Bayer, and Johnson and Johnson and receives honoraria for consultations from Novartis, Merck and Pfizer.
A.W. is supported by the American Diabetes Association Junior Faculty Award 1-13-JF-54. L.B.V.W. is supported by the National Institutes of Health's National Center for Advancing Translational Sciences (KL2TR001424) and the National, Heart, Lung and Blood Institute (K23HL136891). L.Z. is supported by the National Institutes of Health grant R21AG049385.
Clinical Trial Notation: ClinicalTrials.gov identifier, NCT01211730.
H.Z., L.B.V.W., M.E.M., and A.W. participated in the research design, the writing of the article, the performance of the research, and in data analysis. T.P., D.H., L.Z., and A.Y. participated in the performance of the research and in data analysis.
REFERENCES
- 1.U.S. Department of Health & Human Services. Organ Procurement and Transplantation Network National Data. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed June 2017. [Google Scholar]
- 2.Wallia A, Parikh ND, Molitch ME, et al. Post-transplant hyperglycemia is associated with increased risk of liver allograft rejection. Transplantation. 2010;89:222–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ammori JB, Sigakis M, Englesbe MJ, et al. Effect of intraoperative hyperglycemia during liver transplantation. J Surg Res. 2007;140:227–233. [DOI] [PubMed] [Google Scholar]
- 4.Chakkera HA, Weil EJ, Castro J, et al. Hyperglycemia during the immediate period after kidney transplantation. Clin J Am Soc Nephrol. 2009;4:853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas MC, Moran J, Mathew TH, et al. Early peri-operative hyperglycaemia and renal allograft rejection in patients without diabetes. BMC Nephrol. 2000;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galindo RJ, Wallia A. Hyperglycemia and diabetes mellitus following organ transplantation. Curr Diab Rep. 2016;16:14. [DOI] [PubMed] [Google Scholar]
- 7.Wallia A, Illuri V, Molitch ME. Diabetes care after transplant: definitions, risk factors, and clinical management. Med Clin N Am. 2016;100:535–550. [DOI] [PubMed] [Google Scholar]
- 8.Chakkera HA, Knowler WC, Devarapalli Y, et al. Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin J Am Soc Nephrol. 2010;5:1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas MC, Mathew TH, Russ GR, et al. Early peri-operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study. Transplantation. 2001;72:1321–1324. [DOI] [PubMed] [Google Scholar]
- 10.Ganji MR, Charkhchian M, Hakemi M, et al. Association of hyperglycemia on allograft function in the early period after renal transplantation. Transplant Proc. 2007;39:852–854. [DOI] [PubMed] [Google Scholar]
- 11.Hosseini MS, Nemati E, Pourfarziani V, et al. Early hyperglycemia after allogenic kidney transplantation: does it induce infections. Ann Transplant. 2007;12:23–26. [PubMed] [Google Scholar]
- 12.Van den Berg TJ, Bogers H, Vriesendorp TM, et al. No apparent impact of increased post-operative blood glucose levels on clinical outcome in kidney transplant recipients. Clin Transplant. 2009;23:256–263. [DOI] [PubMed] [Google Scholar]
- 13.Hermayer KL, Egidi MF, Finch NJ, et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes. J Clin Endocrinol Metab. 2012;97:4399–4406. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez SC, Maaske J, Kim Y, et al. The association between glycemic control and clinical outcomes after kidney transplantation. Endocr Pract. 2014;20:894–900. [DOI] [PubMed] [Google Scholar]
- 15.Wallia A, Schmidt K, Oakes DJ, et al. Glycemic control reduces infections in post-liver transplant patients: results of a prospective, randomized study. J Clin Endocrinol Metab. 2017;102:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77–81. [DOI] [PubMed] [Google Scholar]
- 17.Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352–360. [DOI] [PubMed] [Google Scholar]
- 18.Zerr KJ, Furnary AP, Grunkemeier GL, et al. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63:356–361. [DOI] [PubMed] [Google Scholar]
- 19.Liu LL, Niemann CU. Intraoperative management of liver transplant patients. Transplant Rev. 2011;25:124–129. [DOI] [PubMed] [Google Scholar]
- 20.Park C, Hsu C, Neelakanta G, et al. Severe intraoperative hyperglycemia is independently associated with surgical site infection after liver transplantation. Transplantation. 2009;87:1031–1036. [DOI] [PubMed] [Google Scholar]
- 21.Chung HS, Lee S, Kwon SJ, et al. Perioperative predictors for refractory hyperglycemia during the neohepatic phase of liver transplantation. Transplantation Proc. 2014;46:3474–3480. [DOI] [PubMed] [Google Scholar]
- 22.Kingston ME, Ali MA, Atiyeh M, et al. Diabetes mellitus in chronic active hepatitis and cirrhosis. Gastroenterology. 1984;87:688–694. [PubMed] [Google Scholar]
- 23.Caronia S, Taylor K, Pagliaro L, et al. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–1063. [DOI] [PubMed] [Google Scholar]
- 24.Masini M, Campani D, Boggi U, et al. Hepatitis C virus infection and human pancreatic β-cell dysfunction. Diabetes Care. 2005;28:940–941. [DOI] [PubMed] [Google Scholar]
- 25.Bloom RD, Lake JR. Emerging issues in hepatitis C virus-positive liver and kidney transplant recipients. Am J Transplant. 2006;6:2232–2237. [DOI] [PubMed] [Google Scholar]
- 26.Brooks BK, Levy MF, Jennings LW, et al. Influence of donor and recipient gender on the outcome of liver transplantation. Transplantation. 1996;27:1784–1787. [DOI] [PubMed] [Google Scholar]
- 27.Rustgi VK, Marino G, Halpern MT, et al. Role of gender and race mismatch and graft failure in patients undergoing liver transplantation. Liver Transpl. 2002;8:514–518. [DOI] [PubMed] [Google Scholar]
- 28.Zeier M, Dohler B, Opelz G, et al. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002;13:2570–2576. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y. Impact of donor recipient gender and race mismatch on graft outcomes in patients with end-stage liver disease undergoing liver transplantation. Prog Transplant. 2017;27:39–47. [DOI] [PubMed] [Google Scholar]
- 30.Croome KP, Segal D, Hernandez-Alejandro R, et al. Female donor to male recipient gender discordance results in inferior graft survival: a prospective study of 1,042 liver transplants. J Hepatobiliary Pancreat Sci. 2014;21:269–274. [DOI] [PubMed] [Google Scholar]
- 31.Gasbarrini A, Addolorato G, DiCampli C, et al. Gender affects reperfusion injury in rat liver. Dig Dis Sci. 2001;46:1305–1312. [DOI] [PubMed] [Google Scholar]
- 32.Coope A, Torsoni AS, Velloso LA. Metabolic and inflammatory pathways on the pathogenesis of type 2 diabetes. Eur J Endocrinol. 2016;174:R175–R187. [DOI] [PubMed] [Google Scholar]
- 33.Rehman K, Akash MS. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016;23:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yadav AD, Chang Y-H, Aqel BA, et al. New onset diabetes mellitus in living donor versus deceased donor liver transplant recipients: analysis of the UNOS/OPTN database. J Transplant. 2013;2013:269096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta S, Pollack T, Fulkerson C, et al. Hyperglycemia in the post-transplant period: PTDM vs. NODAT. Presented at the 100th Annual Meeting of The Endocrine Society—ENDO 2018, Chicago, March, 2018. [Google Scholar]


