Supplemental digital content is available in the text.
Abstract
Background
The risk of mortality and graft loss is higher in kidney transplant recipients with reduced estimated glomerular filtration rate (eGFR) and albuminuria. It is unclear whether these markers are also associated with cardiovascular events.
Methods
We examined linked healthcare databases in Alberta, Canada to identify kidney transplant recipients between 2002 and 2013 who had at least 1 outpatient serum creatinine and albuminuria measurement at 1-year posttransplant. We determined the relationship between categories of eGFR and albuminuria and the risk of subsequent cardiovascular events.
Results
Among 1069 eligible kidney transplant recipients, the median age was 52 years, 37% were female, and 52% had eGFR ≥60 mL/min per 1.73 m2. Over a median follow-up of 6 years, the adjusted rate of all-cause mortality and cardiovascular events was 2.7-fold higher for recipients with eGFR 15-29 mL/min per 1.73 m2 and heavy albuminuria compared to recipients with eGFR ≥60 mL/min per 1.73 m2 and normal albuminuria (rate ratio, 2.7; 95% confidence interval, 1.3-5.7). Similarly, recipients with heavy albuminuria had a threefold increased risk of all-cause mortality and heart failure compared with recipients with eGFR ≥60 mL/min per 1.73 m2 and normal albuminuria.
Conclusions
These findings suggest that eGFR and albuminuria should be used together to determine the risk of cardiovascular outcomes in transplant recipients.
The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease includes albuminuria as part of the classification system for chronic kidney disease (CKD).1,2 Albuminuria as a marker of kidney damage may also reflect systemic vascular disease burden. In the general population, the risk of cardiovascular outcomes (cardiovascular death, coronary artery disease, heart failure, ischemic stroke) at a given level of estimated glomerular filtration rate (eGFR) increases with higher levels of albuminuria.3-5
Only a few studies have reported the combined effects of lower eGFR and higher albuminuria on clinical outcomes in kidney transplant recipients.6-8 Similar to the general population, the risk of death and kidney failure (return to dialysis or retransplantation) appears to increase with reduced eGFR and heavier albuminuria.6-8 In our cohort of 900 kidney transplant recipients from Alberta, Canada, the risk of all-cause mortality was eightfold higher for recipients with eGFR 15 to 29 mL/min per 1.73 m2 and heavy albuminuria compared with eGFR greater than 60 mL/min per 1.73 m2 and normal albuminuria.9 Similarly, the risk of death-censored graft loss was almost 50 times higher. The impact of renal function and degree of albuminuria on the risk of cardiovascular events in kidney transplant recipients is unknown. To address this knowledge gap, we examined associations of eGFR and albuminuria with cardiovascular outcomes among a large cohort of kidney transplant recipients in a Canadian province.
MATERIALS AND METHODS
Design and Setting
We conducted a population-based, retrospective cohort study using linked healthcare databases within the Alberta Kidney Disease Network (AKDN) that incorporates data from Alberta Health, the provincial health ministry.10 Over 99% of Alberta residents are registered with Alberta Health and have universal access to hospital care and physician services. This study followed guidelines for the reporting of observational studies (Table S1 SDC, http://links.lww.com/TXD/A134) and the protocol was approved by the research ethics boards at the University of Alberta and the University of Calgary, with a waiver of patient consent granted.
Data Sources
We ascertained baseline patient characteristics, covariate information, and outcome data from the AKDN records (Table S2 SDC, http://links.lww.com/TXD/A135). We identified kidney transplant recipients using the Northern and Southern Alberta Renal Program databases, which provide care to all patients treated with chronic dialysis or kidney transplant in the province. The Alberta Health database contains information on demographic data, vital statistics, and diagnostic and procedural information for inpatient and outpatient physician services. We linked these data sources to a provincial laboratory repository via unique, encoded, patient identifiers held by the AKDN. These databases have been previously used for research on health outcomes and services.9,11-14
Population
We included all adult kidney transplant recipients (≥18 years), who received their first kidney-only transplant between May 1, 2002 and March 31, 2013 in Alberta (Figure S1 SDC, http://links.lww.com/TXD/A130). We excluded pediatric recipients (<18 years) and those who had received a previous organ transplant or a simultaneous multiorgan transplant (eg, kidney-pancreas). Eligible recipients had at least 1 outpatient serum creatinine measurement and at least 1 outpatient measurement of albuminuria at approximately 1-year after transplantation. We chose to classify kidney function at this time to ensure stability of renal function and immunosuppression regimen and because this metric has been shown to be predictive of other clinical outcomes, such as mortality.9,15-22 Thus, to be included in the study, recipients must have survived at least 1 year with a functioning graft. We excluded transplant recipients who had graft failure (death or return to dialysis) in the first year posttransplant or whose eGFR was less than 15 mL/min per 1.73 m2. The index date was 1-year posttransplant, and this served as the start date for follow-up.
Measurement of Kidney Function and Albuminuria
The eGFR at 1-year posttransplant was estimated using the Chronic Kidney Disease-Epidemiology Collaboration equation.23 Because data on race were not available, recipients were assumed to be non-African American. Misclassification of eGFR was expected to be minimal because less than 2% of the Alberta population are black. Baseline kidney function (index eGFR) was estimated using all outpatient serum creatinine measurements taken within a 3-month look-forward period of the creatinine measurement closest to the 1-year posttransplant date (index creatinine) (Figure S2, http://links.lww.com/TXD/A131). The index eGFR was calculated as the mean of these measurements within the 3-month period.10 Index eGFR was categorized based on the 2012 KDIGO stages of CKD as 60 or higher, 45 to 59, 30 to 44, and 15 to 29 mL/min per 1.73 m2.1
Albuminuria was ascertained from outpatient, random, spot urine measurements of albumin-creatinine ratio (ACR), protein-creatinine ratio (PCR), or urine dipstick and categorized based on the KDIGO definition as normal (A1: ACR <30 mg/g, PCR <15 mg/mmol, or dipstick negative), mild (A2: ACR 30-300 mg/g, PCR 15-100 mg/mmol, or dipstick trace or 1+) or heavy (A3: ACR >300 mg/g, PCR >100 mg/mmol, dipstick ≥2+).1,10,24,25 Albumin-creatinine ratio was the primary measure of albuminuria, and if unavailable, was supplemented with PCR measurements. When both ACR and PCR were unavailable, dipstick urinalysis was used. All outpatient ACR or PCR measurements or urine dipsticks in the 3-month periods before and after the index creatinine value were used to establish baseline albuminuria. For recipients with multiple albuminuria measurements within the 3 months of the index creatinine value, the median value was calculated.
Baseline Characteristics
Baseline demographic data, including age and sex, were determined from the Alberta Health administrative data files. Indigenous race was retrieved from the First Nations status in the registry file. It was not possible to identify other race/ethnic groups, although more than 85% of the Alberta population is white. The presence of 1 or more diagnostic codes in the 3 years before the index date was used for identification of comorbidities according to validated International Classification of Diseases, Ninth Revision, Clinical Modification and International Statistical Classification of Diseases, Tenth Revision coding algorithms applied to physician claims and hospitalization data.26,27 Hypertension and diabetes mellitus were identified from hospital discharge records and physician claims based on validated algorithms.28,29 Data were complete except for income quintile (0.3% missing) and residence location (3.3% missing), which were imputed based on the previous year's values.
Outcomes
Recipients were followed up from the first posttransplant anniversary (index date) until death, emigration from the province, end of study (March 31, 2015), or outcome of interest. The primary outcome was a composite of all-cause mortality and cardiovascular event (defined as a hospitalization for myocardial infarction or ischemic stroke or a procedural code for percutaneous coronary intervention [PCI] or coronary artery bypass graft surgery [CABG]). Additional outcomes included death-censored cardiovascular event, a composite of all-cause mortality and heart failure, and lastly, death-censored heart failure.
Statistical Analyses
Poisson regression with sandwich estimator was used to evaluate the association between the baseline factors and each outcome of interest, with rates expressed per 1000 person-years.30 The 95% confidence interval (CI) was estimated using bootstrap techniques. If the primary assumption that variance equals the mean was not met, a negative binomial model or a generalized Poisson model was used. We calculated unadjusted rates for each of the outcomes by level of eGFR and albuminuria. We then calculated fully adjusted event rates for each outcome, adjusting for the sociodemographic variables and comorbidities listed in Table 1. Two-way interactions between eGFR and albuminuria were assessed for all clinical outcomes. Lastly, we calculated incidence rate ratios using the recipients with eGFR 60 mL/min per 1.73 m2 or higher and normal albuminuria as the referent group.
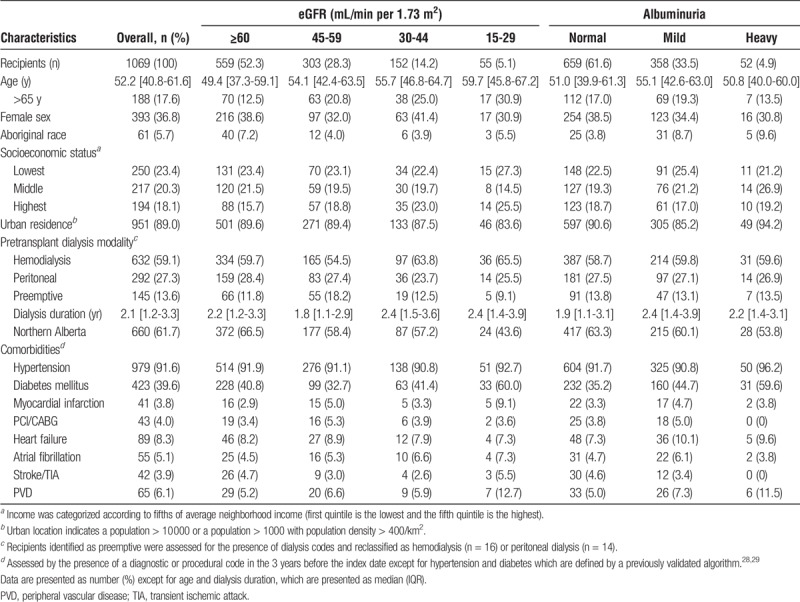
TABLE 1.
Demographic and clinical characteristics of recipients at 1 year posttransplant by level of kidney function and albuminuria
For the primary analysis, we included all patients with at least 1 albuminuria measurement based on ACR, PCR, or urine dipstick. In sensitivity analyses, we included measurements based on ACR and PCR alone (not including urine dipstick). In all analyses, we tested for linear trends across categories of eGFR and albuminuria. The variables used to calculate the tests for trend in eGFR and ACR or PCR were defined by the median values of these parameters in each category. The variable used to calculate the test for trend in dipstick albuminuria were defined by values of 1, 2, and 3 for normal, mild, and heavy albuminuria, respectively. Statistical analyses were performed using Statistical Analysis Software STATA version 13.1 (STATA Corporation, College Station, TX). A P value less than 0.05 was used to define statistical significance.
RESULTS
Among 1387 kidney transplant recipients, 1069 were alive with graft function at the first posttransplant anniversary and had at least 1 outpatient serum creatinine and albuminuria measurement at the 1-year posttransplant date (Figure S1 SDC, http://links.lww.com/TXD/A130). Baseline characteristics of the recipients at their index date are shown in Table 1, according to level of eGFR and albuminuria. At 1-year posttransplant, 52.3% of the recipients had an eGFR of 60 mL/min per 1.73 m2 or greater and 5.1% had an eGFR less than 30 mL/min per 1.73 m2. The median age of the recipients was 52.2 years (interquartile range [IQR], 40.8-61.6) and 17.6% were older than 65 years. The median age increased across declining levels of eGFR (49.4 years for eGFR ≥60 mL/min per 1.73 m2 vs 59.7 years for eGFR <30 mL/min per 1.73 m2). Less than half of the recipients were women (38.6%).
At 1 year, 61.6% of recipients had normal levels of albuminuria as measured by ACR, PCR, or urine dipstick. Compared with these recipients, the 725 recipients in the sensitivity analysis whose albuminuria was measured by ACR or PCR had higher proportions of mild (43.3% vs 33.5%) or heavy albuminuria (5.5% vs 4.9%) (P < 0.01) (Table S3 SDC, http://links.lww.com/TXD/A136).
Adjusted Likelihood of Clinical Outcomes by Level of eGFR and Albuminuria
After median follow-up of 6.0 years (IQR, 3.4-8.5 years), 13.5% (n = 144) recipients died, and 9.5% (n = 102) initiated dialysis. The unadjusted rate of all-cause mortality and cardiovascular events significantly increased as kidney function declined (P = 0.002) but this pattern was no longer significant after adjustment for recipient factors (P = 0.37) (Figure 1A). For eGFR 45 to 59 mL/min per 1.73 m2 and 15 to 29 mL/min per 1.73 m2, the adjusted incidence rates increased with worsening albuminuria (P = 0.03 and P = 0.001, respectively). Recipients with eGFR of 15 to 29 mL/min per 1.73 m2 and heavy albuminuria had an almost threefold increased risk of all-cause mortality and cardiovascular events compared to recipients with eGFR of ≥60 mL/min per 1.73 m2 and normal albuminuria (rate ratio, 2.7; 95% CI, 1.3-5.7) (Figure 2A). There did not appear to be any significant association between eGFR, albuminuria, and death-censored cardiovascular events; however, the number of events was lower (Figures 1A and 2B).
FIGURE 1.
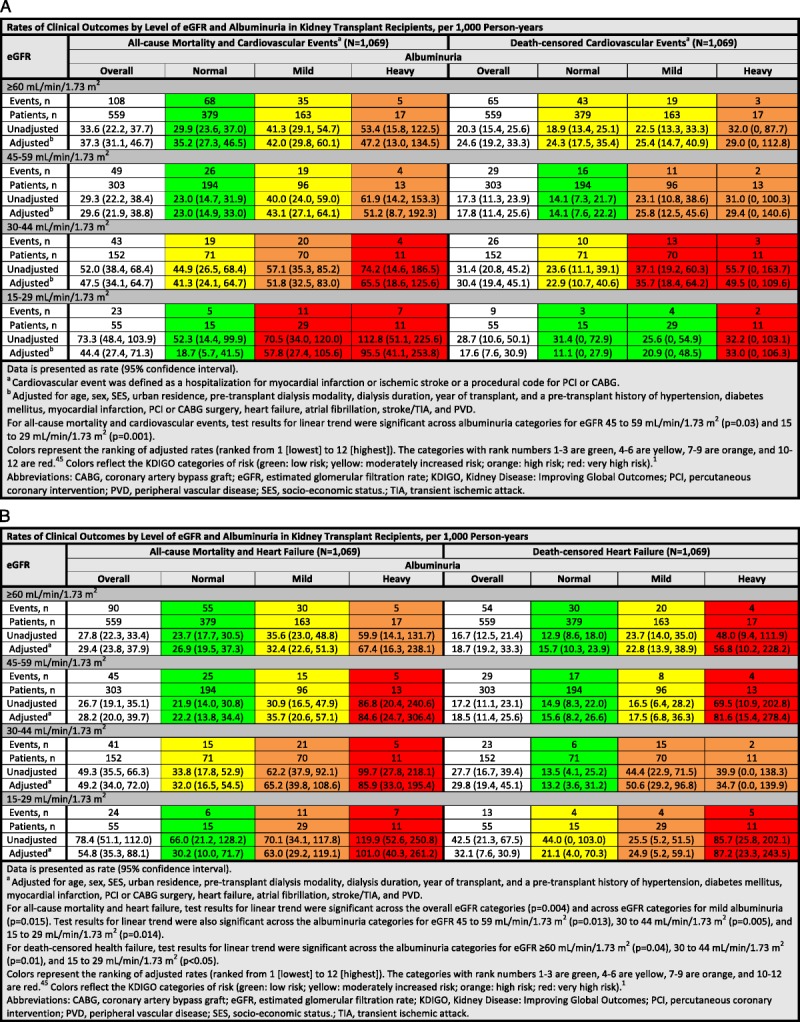
A and B, Rates of clinical outcomes by level of eGFR and albuminuria in kidney transplant recipients, per 1000 person-years.
FIGURE 2.
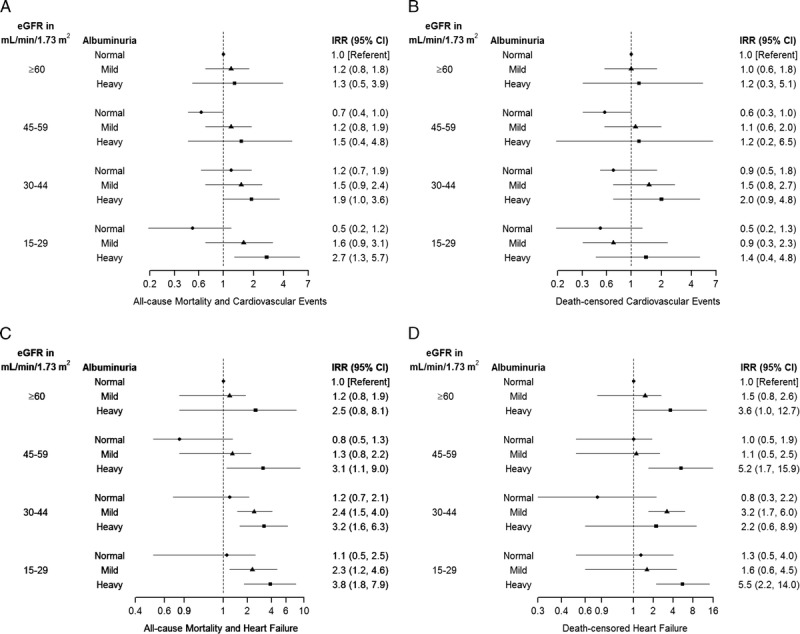
Adjusted Incidence rate ratios by Level of eGFR and Albuminuria in Kidney Transplant Recipients. All values ≥1, including point estimates and confidence limits, are plotted on log scale. All values <1 are plotted on −(1/3*x) scale for outcomes of all-cause mortality and cardiovascular events and death-censored cardiovascular events, and are plotted on −(1/x) scale for outcomes of all-cause mortality and heart failure and death-censored heart failure. IRR, incidence rate ratio.
The rate of all-cause mortality and heart failure increased as kidney function declined. The adjusted incidence rate for eGFR ≥60, 45 to 59, 30 to 44, and 15 to 29 mL/min per 1.73 m2 was 29.4, 28.2, 49.2, and 54.8 per 1000 person-years, respectively (P = 0.004) (Figure 1B). Except for eGFR ≥60 mL/min per 1.73 m2, the adjusted incidence rates across the other eGFR categories significantly increased with worsening albuminuria (P < 0.02). For these recipients, the risk of all-cause mortality and heart failure was threefold higher for recipients with heavy albuminuria compared to recipients with eGFR ≥60 mL/min per 1.73 m2 and normal albuminuria (rate ratio range, 3.1-3.8) (Figure 2C).
Similarly, the unadjusted rate of death-censored heart failure increased as kidney function declined (P = 0.006) but, again, the adjusted rate did not reach statistical significance (P = 0.06). Except for eGFR 45 to 59 mL/min per 1.73 m2, the adjusted incidence rates across the other eGFR categories significantly increased with worsening albuminuria (P < 0.05). The risk of death-censored heart failure was sixfold higher in recipients with eGFR 15 to 29 mL/min per 1.73 m2 compared with recipients with normal graft function and albuminuria (rate ratio 5.5, 95% CI 2.2-14.0) (Figure 2D). The analyses for the subgroup of recipients (n = 725) whose albuminuria was measured by ACR or PCR alone (not by urine dipstick) are presented in Figure S3, SDC (http://links.lww.com/TXD/A132 and http://links.lww.com/TXD/A133). The patterns were similar, although the smaller number of events led to wider CIs.
DISCUSSION
In this population-based study of 1069 kidney transplant recipients, we found that the risk of all-cause mortality and cardiovascular events significantly increased with worsening albuminuria for certain eGFR categories (45 to 59 and 15 to 29 mL/min per 1.73 m2). The association was stronger for all-cause mortality and heart failure, where the adjusted incidence rate increased with worsening albuminuria for the lower eGFR categories. The risk of this composite outcome was threefold higher for recipients with heavy albuminuria compared with recipients with an eGFR of 60 mL/min per 1.73 m2 or higher and normal albuminuria.
Cardiovascular disease remains the leading cause of death in kidney transplant recipients, accounting for 30% of deaths with a functioning graft.31 Although the risk of cardiovascular death is significantly lower with transplantation compared to dialysis, the risk remains higher than the general population.32 In a retrospective study of 4954 kidney transplant recipients from Ontario, Canada, we found that the 3-year cumulative incidence of death and major cardiovascular event (myocardial infarction, PCI, CABG, or ischemic stroke) was 9.0% (3.2 events per 100 person-years), and this was higher than the age- and sex-matched general population (2.6%, 0.89 events per 100 person-years).33 For these reasons, the 2009 KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients suggests that recipients be considered to be at the highest risk for cardiovascular disease.34 Cardiovascular events in kidney transplant recipients may be high due to the combination of traditional cardiovascular risk factors, such as hypertension and diabetes, and renal-specific cardiovascular risk factors, such as exposure to dialysis and immunosuppressive medications.35,36 Our results likely underestimate the burden of vascular disease in this patient population, as our composite outcome did not include diagnoses such as arrhythmias, unstable angina, or peripheral vascular disease.
Our results expand on our previous findings that the combined assessment of graft function and albuminuria at 1 year posttransplantation was associated with the risks of all-cause mortality and graft loss.9 Only a few other studies have examined the interactive effects of graft function and albuminuria on clinical outcomes; however, none of these prior studies have assessed cardiovascular outcomes, including heart failure.6-8
Lentine et al37 reported that the 3-year incidence of de novo heart failure in 27 011 kidney transplant recipients from the United States from 1995 to 2001 was 18%. Risk factors for heart failure included older age, female sex, and pretransplant comorbidities, such as diabetes mellitus and myocardial infarction. Heart failure was associated with an almost threefold higher risk of death and death-censored graft failure. In a Canadian population from 1969 to 1999, Rigatto et al38 reported that the incidence of de novo heart failure in 638 kidney transplant recipients was 1.3 per 100 patient-years and was associated with a 1.8-fold increased the risk of death posttransplantation. Graft function was not independently associated with heart failure although it was a strong univariate predictor. Neither the American nor Canadian study assessed the combined impact of graft function and albuminuria on the development of posttransplant heart failure. In our study, 11% of recipients had heart failure over a median follow-up of 6 years. Recipients with an eGFR of 15 to 29 mL/min per 1.73 m2 and heavy albuminuria had an approximately sixfold higher risk of death-censored heart failure compared with recipients with eGFR ≥60 mL/min per 1.73 m2 with normal albuminuria.
In the nontransplant population, the risk of cardiovascular events at a given level of eGFR increases with worsening albuminuria. In a retrospective study of 1.5 million patients in Alberta, Canada, Bello et al3 reported that after a median follow-up of 35 months, the rate of heart failure, peripheral vascular disease, and cerebral vascular accident or transient ischemic attack all increased with lower eGFR and heavier albuminuria. Similar results were found in larger, collaborative meta-analyses of the general population, high-risk population, and CKD population.4,5,39
We assessed albuminuria at 1-year posttransplantation and its association with cardiovascular events. Albuminuria from the native kidneys typically declines within the first 3 weeks after transplantation, due to the reduction in glomerular filtration.40 Thus, albuminuria detected at 1 year likely represents allograft pathology, and prior studies have shown this to be a predictor of long-term graft outcomes.41,42 Currently, it is unclear whether or not interventions aimed at improving posttransplant albuminuria lead to improved clinical outcomes. In a randomized controlled trial of 213 kidney transplant recipients with albuminuria (≥0.2 g/d), Knoll et al43 did not show a reduction in the composite outcome of doubling of serum creatinine, end-stage renal disease, or death for ramipril compared with placebo. Due to the number of recipients enrolled in the study, there may have been insufficient power to detect a treatment effect.44
Our study population represents a large Canadian cohort of recipients (>1000) followed up over a 13-year period. The serum creatinine measurements in our study have been standardized across provincial laboratories, reducing interlaboratory variation in measurements. In addition to this, we allowed for multiple measurements of albuminuria, reducing the risk of misclassification. Our outcomes were based on diagnostic and procedural codes that have been shown to have good validity in previous validation studies (Table S2 SDC, http://links.lww.com/TXD/A135).26,45-47 There are, however, limitations worth noting. Given that we included recipients with a functioning graft and blood and urine tests at 1 year, there is a risk of survival and selection bias. Recipients eligible for study inclusion may have differed from recipients who experienced graft failure, died, or were lost to follow-up within a year of transplant. We based graft function on serum creatinine with GFR estimation rather than cystatin C, because this was not available in our data sets. We also lacked data on certain baseline characteristics (eg, smoking, blood pressure measurements, body mass index), cardiovascular and immunosuppressive medications (eg, renin-angiotensin system inhibitors, sirolimus), and cardiovascular investigation results done as part of the transplant assessment (eg, echocardiography, coronary angiogram). We were able to incorporate other important comorbidities associated with cardiovascular events, such as hypertension and diabetes mellitus. Unfortunately, we were not able to accurately determine the cause of death, including cardiovascular death, in our data sets. Lastly, our results include kidney transplant recipients from 1 large Canadian province, and it is unclear whether these results are generalizable to other recipients, particularly those of non-Caucasian race.
In summary, in a Canadian cohort of over 1000 incident kidney transplant recipients, we found that the combined effects of eGFR and albuminuria at 1 year posttransplant were associated with higher risk of all-cause mortality and cardiovascular events, including heart failure. These results suggest that in kidney transplant recipients, albuminuria provides additional information to eGFR on the prognosis of clinically important outcomes.
Supplementary Material
ACKNOWLEDGMENTS
This study is based, in part, on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta, nor Alberta Health or Alberta Health Services express any opinion in relation to this study. NNL was supported by a KRESCENT New Investigator Award.
Footnotes
Published online 6 September 2018.
The authors declare no funding or conflicts of interest.
N.N.L. conceived of the study and drafted the article. F.Y. performed the statistical analyses and created the figure. All authors interpreted the results, revised the article, and approved the final version of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Kidney Disease. Improving Global Outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 2.Levin A, Stevens PE. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. [DOI] [PubMed] [Google Scholar]
- 3.Bello AK, Hemmelgarn B, Lloyd A, et al. Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol. 2011;6:1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernández D, Pérez G, Marrero D, et al. Early association of low-grade albuminuria and allograft dysfunction predicts renal transplant outcomes. Transplantation. 2012;93:297–303. [DOI] [PubMed] [Google Scholar]
- 7.Bucşa C, Stefan G, Tacu D, et al. Does the KDIGO CKD risk stratification based on GFR and proteinuria predict kidney graft failure? Int Urol Nephrol. 2014;46:1857–1865. [DOI] [PubMed] [Google Scholar]
- 8.White CA, Akbari A, Talreja H, et al. Classification of kidney transplant recipients using a combination of estimated GFR and albuminuria reflects risk. Transplant Direct. 2016;2:e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam NN, Tonelli M, Lentine KL, et al. Albuminuria and posttransplant chronic kidney disease stage predict transplant outcomes. Kidney Int. 2017;92:470–478. [DOI] [PubMed] [Google Scholar]
- 10.Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. [DOI] [PubMed] [Google Scholar]
- 12.Bello A, Padwal R, Lloyd A, et al. Using linked administrative data to study periprocedural mortality in obesity and chronic kidney disease (CKD). Nephrol Dial Transplant. 2013;28(Suppl 4):iv57–iv64. [DOI] [PubMed] [Google Scholar]
- 13.Leung KC, Pannu N, Tan Z, et al. Contrast-associated AKI and use of cardiovascular medications after acute coronary syndrome. Clin J Am Soc Nephrol. 2014;9:1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander RT, Hemmelgarn BR, Wiebe N, et al. Kidney stones and cardiovascular events: a cohort study. Clin J Am Soc Nephrol. 2014;9:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariharan S, McBride MA, Cherikh WS, et al. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62:311–318. [DOI] [PubMed] [Google Scholar]
- 16.Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75:1291–1295. [DOI] [PubMed] [Google Scholar]
- 17.Fellström B, Jardine AG, Soveri I, et al. Renal dysfunction is a strong and independent risk factor for mortality and cardiovascular complications in renal transplantation. Am J Transplant. 2005;5:1986–1991. [DOI] [PubMed] [Google Scholar]
- 18.Kasiske BL, Israni AK, Snyder JJ, et al. The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis. 2011;57:466–475. [DOI] [PubMed] [Google Scholar]
- 19.Lenihan CR, O’Kelly P, Mohan P, et al. MDRD-estimated GFR at one year post-renal transplant is a predictor of long-term graft function. Ren Fail. 2008;30:345–352. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Li H, Huang H, et al. Slope of changes in renal function in the first year post-transplantation and one-yr estimated glomerular filtration rate together predict long-term renal allograft survival. Clin Transplant. 2010;24:862–868. [DOI] [PubMed] [Google Scholar]
- 21.Schnitzler MA, Johnston K, Axelrod D, et al. Associations of renal function at 1-year after kidney transplantation with subsequent return to dialysis, mortality, and healthcare costs. Transplantation. 2011;91:1347–1356. [DOI] [PubMed] [Google Scholar]
- 22.Schnitzler MA, Lentine KL, Gheorghian A, et al. Renal function living, standard criteria deceased and expanded criteria deceased donor kidney transplantation: impact on graft failure and death. Transpl Int. 2012;25:179–191. [DOI] [PubMed] [Google Scholar]
- 23.Shaffi K, Uhlig K, Perrone RD, et al. Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis. 2014;63:1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem. 2009;46(Pt 3):205–217. [DOI] [PubMed] [Google Scholar]
- 25.Tonelli M, Muntner P, Lloyd A, et al. Impact of age on the association between CKD and the risk of future coronary events. Am J Kidney Dis. 2014;64:1–8. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 27.Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–516. [DOI] [PubMed] [Google Scholar]
- 29.Quan H, Khan N, Hemmelgarn BR, et al. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54:1423–1428. [DOI] [PubMed] [Google Scholar]
- 30.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 31.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(Suppl 1):S1–S672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–S119. [DOI] [PubMed] [Google Scholar]
- 33.Lam NN, Kim SJ, Knoll GA, et al. The risk of cardiovascular disease is not increasing over time despite aging higher comorbidity burden of kidney transplant recipients. Transplantation. 2017;101:588–596. [DOI] [PubMed] [Google Scholar]
- 34.KDIGO. Clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. [DOI] [PubMed] [Google Scholar]
- 35.Liefeldt L, Budde K. Risk factors for cardiovascular disease in renal transplant recipients and strategies to minimize risk. Transpl Int. 2010;23:1191–1204. [DOI] [PubMed] [Google Scholar]
- 36.Israni AK, Snyder JJ, Skeans MA, et al. Predicting coronary heart disease after kidney transplantation: patient outcomes in renal transplantation (PORT) study. Am J Transplant. 2010;10:338–353. [DOI] [PubMed] [Google Scholar]
- 37.Lentine KL, Schnitzler MA, Abbott KC, et al. De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. Am J Kidney Dis. 2005;46:720–733. [DOI] [PubMed] [Google Scholar]
- 38.Rigatto C, Parfrey P, Foley R, et al. Congestive heart failure in renal transplant recipients: risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol. 2002;13:1084–1090. [DOI] [PubMed] [Google Scholar]
- 39.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. [DOI] [PubMed] [Google Scholar]
- 40.Myslak M, Amer H, Morales P, et al. Interpreting post-transplant proteinuria in patients with proteinuria pre-transplant. Am J Transplant. 2006;6:1660–1665. [DOI] [PubMed] [Google Scholar]
- 41.Naesens M, Lerut E, Emonds MP, et al. Proteinuria as a noninvasive marker for renal allograft histology and failure: an observational cohort study. J Am Soc Nephrol. 2016;27:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halimi JM, Buchler M, Al-Najjar A, et al. Urinary albumin excretion and the risk of graft loss and death in proteinuric and non-proteinuric renal transplant recipients. Am J Transplant. 2007;7:618–625. [DOI] [PubMed] [Google Scholar]
- 43.Knoll GA, Fergusson D, Chasse M, et al. Ramipril versus placebo in kidney transplant patients with proteinuria: a multicentre, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:318–326. [DOI] [PubMed] [Google Scholar]
- 44.Toto RD. Transplantation: the role of RAAS blockade in kidney transplantation. Nat Rev Nephrol. 2015;12:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quan H, Li B, Duncan Saunders L, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee DS, Stitt A, Wang X, et al. Administrative hospitalization database validation of cardiac procedure codes. Med Care. 2013;51:e22–e26. [DOI] [PubMed] [Google Scholar]
- 47.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144:290–296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


