Abstract
Safety concerns persist for long-term pediatric fluoroquinolone use. Seventy children (median age, 2.1 years) treated with levofloxacin 10–20 mg/kg once daily for multidrug-resistant tuberculosis (median observation time, 11.8 months) had few musculoskeletal events, no levofloxacin-attributed serious adverse events, and no Fridericia-corrected QT interval >450 ms. Long-term levofloxacin was safe and well tolerated.
Keywords: levofloxacin, safety, children, MDR-TB, QT prolongation
Levofloxacin is a key component of multidrug-resistant (MDR) tuberculosis (TB) treatment regimens in children, typically for 9–18 months’ duration [1]. Levofloxacin is also used as preventive therapy for MDR-TB in children in some settings for 6 months or longer. Fluoroquinolones cause a destructive arthropathy in juvenile animals, which had traditionally limited their use in children [2]. In addition, the fluoroquinolones may cause the following: Achilles tendon rupture; nausea, vomiting, and diarrhea; central nervous system effects such as hyperactivity, insomnia, hallucinations, and raised intracranial pressure; dysglycemia; and QT interval prolongation [3].
Despite these historical concerns, accumulating data have not demonstrated serious arthropathy, tendinopathy, or other serious safety concerns in children over short durations (7–14 days) [2, 4, 5]. Fluoroquinolones are now recommended by the World Health Organization and others for use in children where there are limited treatment options, including for MDR-TB [6]. However, there is a paucity of data in children on levofloxacin safety and tolerability over long durations and at the higher doses currently used for MDR-TB treatment. Levofloxacin’s QT interval–prolonging effects in children have also not yet been well described; however, this information is needed as levofloxacin is increasingly being combined in treatment regimens with novel TB drugs, which also cause QT interval prolongation [7].
We aimed to characterize the safety and tolerability of levofloxacin in children routinely treated for MDR-TB.
PATIENTS AND METHODS
Study Design, Setting, and Population
We have previously described the design of this prospective observational pharmacokinetics study in Cape Town, South Africa, in detail [8]. In brief, children were included in this study if they were <15 years of age, >5 kg body weight, and routinely treated for MDR-TB with levofloxacin. In this setting, children with MDR-TB are treated with 6–7 drug regimens, which usually contain a fluoroquinolone, amikacin, ethionamide, terizidone, high-dose isoniazid, pyrazinamide, ethambutol, and occasionally para-aminosalicylic acid, linezolid, and clofazimine. Levofloxacin (250-mg tablets) was the recommended fluoroquinolone for children with MDR-TB <8 years of age due to challenges with administering the moxifloxacin 400-mg tablet formulation used in children >8 years of age and adults. Levofloxacin routine dosing in our setting changed from 10–15 mg/kg once daily to 15–20 mg/kg once daily during the study. Children are often hospitalized for 1–6 months at the beginning of MDR-TB treatment and then complete their treatment as outpatients.
Parents or legal guardians provided informed consent. The Health Research Ethics Committees of Stellenbosch University provided study approval (N11/03/059).
Data Collection
Standard clinical and laboratory assessments (alanine aminotransferase [ALT], bilirubin, creatinine, potassium) were done 1–2 monthly throughout treatment. All adverse events were recorded, assessed for attribution to levofloxacin, and graded for severity (National Institute of Allergy and Infectious Diseases, Division of AIDS grading table, version 1.0, August 2009) [9]. Twelve-lead electrocardiograms (ECGs) were performed in triplicate on pharmacokinetic sampling days just prior to the pharmacokinetic blood draws predose and at 2 hours postdose (expected maximum levofloxacin plasma concentration). ECGs were only started later during the study and were interpreted by 1 of 2 pediatric cardiologists; the measured QT interval was corrected using the Fridericia correction (QTcF) and the mean of the triplicate QTcF values was used for analysis. Predose and 2-hour levofloxacin concentrations were obtained according to previously described methods [8]; concentrations below the limit of quantification (BLQ) were assigned a value of zero for this analysis.
Analysis
Demographic and clinical characteristics and QTcF results were summarized using descriptive statistics. The frequency of adverse events was reported by grade for all events, and also for events that were possibly, probably, or definitely related to levofloxacin. Person-time was calculated from the baseline study assessment until the treatment completed or the last available study visit. Event rates were reported per 100 person-years.
Multivariable linear regression was done to characterize the association between the change in QTcF with the change in levofloxacin concentration, controlling for sex, human immunodeficiency virus (HIV) status, and age. The standard errors were adjusted to account for 1 patient with 2 sets of levofloxacin concentration and ECG data from different days. Stata/SE 14.0 software was used to analyze the data [10].
RESULTS
Seventy children (median age, 2.1 years [range, 0.4–7.3 years]) were included in the safety analysis; 38 (54%) were male and 12 (17%) were HIV infected (see Supplementary Table 1). These children were observed for a total duration of 68.5 person-years (median, 11.6 months [interquartile range, 9.2–14.7 months]). Table 1 shows all adverse events and those at least possibly related to levofloxacin. There were no grade 4 or any serious adverse events attributed to levofloxacin, and no adverse event resulted in permanent levofloxacin discontinuation.
Table 1.
Adverse Events in Children (N = 70) Treated for Multidrug-resistant Tuberculosis With Levofloxacin
| Adverse Event | All Adverse Events | Adverse Effects Possibly, Probably, or Definitely Attributed to Levofloxacin |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients With Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total No. of Events | Event Rate (per 100 PY) | No. of Patients With Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total No. of Events | Event Rate (per 100 PY) | |
| Arthralgia | 3 | 3 | 0 | 0 | 0 | 3 | 4.4 | 2 | 2 | 0 | 0 | 0 | 2 | 2.9 |
| Arthritis | 0 | 0 | 0 | 0 | 0 | 0 | … | 0 | 0 | 0 | 0 | 0 | 0 | … |
| Pain other than trauma | 11 | 11 | 0 | 0 | 0 | 11 | 16.1 | 4 | 4 | 0 | 0 | 0 | 4 | 5.8 |
| Headache | 4 | 4 | 0 | 1 | 0 | 5 | 7.3 | 2 | 1 | 0 | 1 | 0 | 2 | 2.9 |
| Neurosensory alteration | 1 | 1 | 0 | 0 | 0 | 1 | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 | … |
| Insomnia | 1 | 0 | 1 | 0 | 0 | 1 | 1.5 | 1 | 0 | 1 | 0 | 0 | 1 | 1.5 |
| Fatigue/malaise | 1 | 1 | 0 | 0 | 0 | 1 | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 | … |
| Nausea | 12 | 13 | 0 | 0 | 0 | 13 | 19.0 | 8 | 9 | 0 | 0 | 0 | 9 | 13.1 |
| Vomiting | 19 | 23 | 1 | 0 | 0 | 24 | 35.1 | 14 | 16 | 0 | 0 | 0 | 16 | 23.4 |
| Anorexia | 11 | 8 | 5 | 0 | 0 | 13 | 19.0 | 7 | 4 | 3 | 0 | 0 | 7 | 10.2 |
| Cutaneous reaction | 12 | 8 | 6 | 0 | 0 | 14 | 20.4 | 7 | 3 | 4 | 0 | 0 | 7 | 10.2 |
| Pruritus | 13 | 16 | 1 | 0 | 0 | 17 | 24.8 | 7 | 7 | 1 | 0 | 0 | 8 | 11.7 |
| ALT elevation | 22 | 17 | 3 | 2 | 5 | 27 | 39.4 | 16 | 16 | 2 | 0 | 0 | 18 | 26.3 |
| Bilirubin elevation | 0 | 0 | 0 | 0 | 0 | 0 | … | 0 | 0 | 0 | 0 | 0 | 0 | … |
Total person-time of observation = 68.5 years.
Abbreviations: ALT, alanine aminotransferase; PY, person-years.
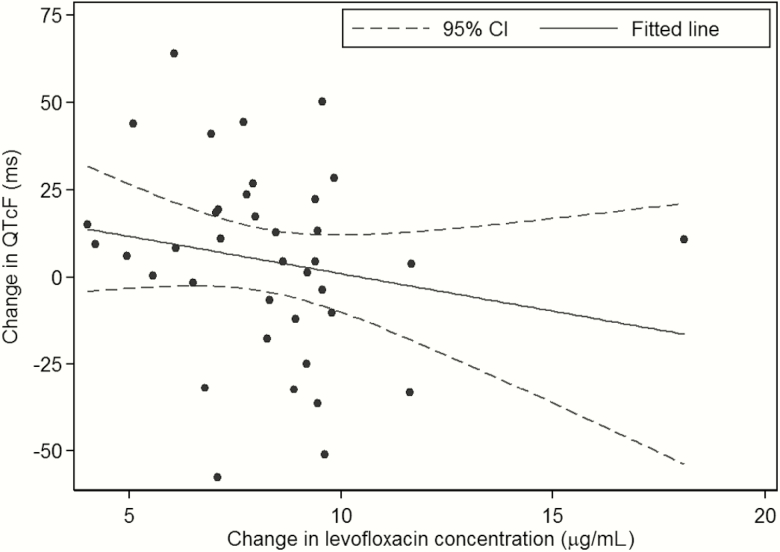
ECG results were available in 41 children (median age, 2.1 years [range, 0.2–4.8 years]); 20 (49%) were male and 10 (24%) were HIV infected. All HIV-infected children were on antiretroviral therapy; 9 were on lopinavir/ritonavir with 2 nucleoside reverse transcriptase inhibitors (NRTIs) and 1 was on efavirenz with 2 NRTIs. Three patients had 1 ECG at 2 hours only and 2 patients had 1 ECG at both 0 and 2 hours. There were 38 predose (0 hour) ECGs, and 41 two-hour ECGs, with 37 children contributing 38 paired results. The mean QTcF was 359 ms (standard deviation [SD], 21.0 ms) at 0 hours and 365.4 ms (SD, 26.6 ms) at 2 hours; no QTcF was >450 ms. The mean change in QTcF from the 0-hour to the 2-hour reading was 4.7 ms (SD, 27.3 ms). Five (13%) had a change in QTcF of 30 to <60 ms from the 0-hour to 2-hour readings, and 1 (3%) had a change >60 ms. For the children with paired ECG results, the mean levofloxacin concentration predose was 0.33 μg/mL (SD, 0.61) and at 2 hours was 8.57 μg/mL (SD, 2.55); 11 (28.9%) predose concentrations were BLQ. Figure 1 shows the change in QTcF vs change in levofloxacin concentrations from 0 to 2 hours. In multivariable linear regression, only age (P = .028) was significantly associated with change in QTcF from 0 to 2 hours, with every 1-year increase in age associated with a 7.36-ms increase in QTcF change (Supplementary Table 2). The 1 patient treated with clofazimine, known to prolong the QT interval, had a QTcF change of 44 ms.
Figure 1.
Change in QT interval with Fridericia correction vs change in levofloxacin concentration in children treated for multidrug-resistant tuberculosis.
Abbreviations: CI, confidence interval; QTcF, QT interval with Fridericia correction.
DISCUSSION
In this cohort of children with MDR-TB, long-term levofloxacin treatment was safe and well tolerated. The few musculoskeletal complaints (pain, arthralgia) were mild and self-limited. Mild musculoskeletal complaints and those in young children may have been underestimated; however, it is unlikely that more severe events were missed, such as those having objective signs of arthritis or those resulting in gait abnormalities or failure to bear weight. This should be reassuring to clinicians and TB programs, some of whom are still hesitant to treat children affected by TB with fluoroquinolones.
Hyperactivity and sleep disturbances have been well described in children treated with fluoroquinolones [11]; however, we observed few such events. These may be underestimated due to children being admitted early in their treatment to the TB hospital without their caregivers, which may have obscured reported changes in behavior and sleep patterns.
The most common events overall were nonspecific, such as rash, nausea, vomiting, and ALT elevation. These likely represent overestimates of the rate of these events due to levofloxacin; more likely these were due to other medications such as isoniazid, pyrazinamide (ALT elevation), and ethionamide (nausea, vomiting). We erred on the side of attributing these events at least possibly to levofloxacin, unless there was strong evidence of the relationship with another medication. The poor palatability of levofloxacin formulation, especially when crushed, may have contributed to some of the nausea and vomiting.
No child had a QTcF >450 ms, and few had a change >30 ms from predose to 2 hours. We did not observe a relationship between QTcF and levofloxacin concentration. Fluoroquinolone-associated QT prolongation is mediated through dose-dependent inhibition of cardiac potassium channels that varies by agent, with moxifloxacin and gatifloxacin having a more potent effect than levofloxcin, ciprofloxacin, and ofloxacin [12]. In previous adult studies, 1000 mg levofloxacin resulted in a mean change in QTc of 3.5–4.8 ms compared with placebo [13], and doses as high as 1500 mg in adults had a minimal impact on corrected QT interval [14]. This is consistent with our findings. The association of older age with QTcF change in our cohort needs further evaluation. A limitation to our study is that these children did not have true pretreatment QTcF values for comparison, as all had already been on levofloxacin for at least 1 week at the time ECGs were completed. However, the predose concentrations were generally low, including many that were BLQ, so the change in QTcF from predose remains a useful evaluation. These data therefore also provide support for using levofloxacin in combination with other QT interval–prolonging TB medications such as clofazimine, bedaquiline, and delamanid. It also establishes a baseline for QT intervals in children treated with levofloxacin-containing MDR-TB regimens for interpreting cardiac safety results of ongoing pediatric bedaquiline and delamanid trials.
A limitation of our study is the lack of children >8 years of age who may have a different adverse event profile, would likely be able to report subjective symptoms better, and may have different QT effects; they should be included in future studies.
In summary, levofloxacin, at doses up to 20 mg/kg once daily, was safe and well tolerated and should remain a mainstay of pediatric MDR-TB treatment.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge the children and their caregivers who participated in the study; the Desmond Tutu TB Centre research team who assisted with implementation of the study; and the clinical teams at the Brooklyn Chest Hospital, Brewelskloof Hospital, and Tygerberg Hospital C3A Clinic for their support. We also acknowledge our colleague Professor P. L. van der Merwe, in memoriam, who contributed to this work.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A. C. H. (South African Research Chairs Initiative Chair in Paediatric Tuberculosis) and H. S. S. also received funding from the South Africa National Research Foundation.
Financial support. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (award number R01HD069169 to A. C. H.).
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis: 2016 update. Available at: http://www.who.int/entity/tb/areas-of-work/drug-resistant-tb/MDRTBguidelines2016.pdf. Accessed 30 May 2016. [PubMed] [Google Scholar]
- 2. Burkhardt JE, Walterspiel JN, Schaad UB. Quinolone arthropathy in animals versus children. Clin Infect Dis 1997; 25:1196–204. [DOI] [PubMed] [Google Scholar]
- 3. Thee S, Garcia-Prats AJ, Donald PR, Hesseling AC, Schaaf HS. Fluoroquinolones for the treatment of tuberculosis in children. Tuberculosis (Edinb) 2015; 95:229–45. [DOI] [PubMed] [Google Scholar]
- 4. Noel GJ, Bradley JS, Kauffman RE, et al. . Comparative safety profile of levofloxacin in 2523 children with a focus on four specific musculoskeletal disorders. Pediatr Infect Dis J 2007; 26:879–91. [DOI] [PubMed] [Google Scholar]
- 5. Yee CL, Duffy C, Gerbino PG, Stryker S, Noel GJ. Tendon or joint disorders in children after treatment with fluoroquinolones or azithromycin. Pediatr Infect Dis J 2002; 21:525–9. [DOI] [PubMed] [Google Scholar]
- 6. Committee on Infectious Diseases. The use of systemic fluoroquinolones. Pediatrics 2006; 118:1287–92. [DOI] [PubMed] [Google Scholar]
- 7. Harausz E, Cox H, Rich M, Mitnick CD, Zimetbaum P, Furin J. QTc prolongation and treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2015; 19:385–91. [DOI] [PubMed] [Google Scholar]
- 8. Denti P, Garcia-Prats AJ, Draper HR, et al. . Levofloxacin population pharmacokinetics in South African children treated for multidrug-resistant tuberculosis. Antimicrob Agents Chemother 2018; 62. doi: 10.1128/AAC.01521-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS table for grading the severity of adult and pediatric adverse events, version 1.0. Available at: http://rsc.tech-res.com/docs/default-source/safety/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf. Accessed 5 May 2015. [Google Scholar]
- 10.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015.
- 11. Upton C. Sleep disturbance in children treated with ofloxacin. BMJ 1994; 309:1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang J, Wang L, Chen XL, Triggle DJ, Rampe D. Interactions of a series of fluoroquinolone antibacterial drugs with the human cardiac K+ channel HERG. Mol Pharmacol 2001; 59:122–6. [DOI] [PubMed] [Google Scholar]
- 13. Noel GJ, Natarajan J, Chien S, Hunt TL, Goodman DB, Abels R. Effects of three fluoroquinolones on QT interval in healthy adults after single doses. Clin Pharmacol Ther 2003; 73:292–303. [DOI] [PubMed] [Google Scholar]
- 14. Noel GJ, Goodman DB, Chien S, Solanki B, Padmanabhan M, Natarajan J. Measuring the effects of supratherapeutic doses of levofloxacin on healthy volunteers using four methods of QT correction and periodic and continuous ECG recordings. J Clin Pharmacol 2004; 44:464–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.