Abstract
A strong synergy can result from China–US antimicrobial resistance (AMR) collaborations given similarities and differences between their respective healthcare systems and research infrastructures. The Antibacterial Resistance Leadership Group has employed a model of realistic growth, starting with a feasible, relatively low-resource observational study in a critical priority pathogen. This and other observational studies will provide vital scientific information required for the rational design of future interventional trials. In addition, it provides a mutual, low-risk opportunity for determining the strengths and opportunities of the research collaboration. Issues identified during the observational studies can be addressed prior to the initiation of high-resource interventional studies. Collaborative clinical AMR studies between China and the United States have tremendous potential to decrease AMR rates, improve responsible antibiotic use, and ultimately improve the lives of patients in both countries.
Keywords: antibacterial resistance, China, United States, clinical trials, ARLG
Rising rates of antimicrobial resistance (AMR) are a global phenomenon. The exponential growth of international travel in the last few decades has facilitated the rapid dissemination of a variety of emerging infectious diseases, including those caused by multidrug-resistant bacteria [1–3]. As a global problem, AMR requires a global solution [4].
The healthcare systems of China and the United States share a number of important characteristics. Both countries provide comprehensive healthcare to large, geographically dispersed populations, face antibiotic overuse in both human clinical medicine and agriculture, and have experienced steep increases in bacteria exhibiting resistance to first-line and second-line antibiotics [5, 6].
Building upon a novel public–private partnership, the Antibacterial Resistance Leadership Group (ARLG; www.arlg.org) has established a multidrug-resistant organism (MDRO) network of sites in China and other countries with high rates of MDRO organisms. The goal of this MDRO network is to conduct collaborative clinical research focused on AMR that is relevant to patient care in both China and the United States. In this manuscript, we outline progress to date and outline the vision for the future.
IMPERATIVE FOR CHINA–US AMR COLLABORATIONS
A growing number of examples illustrate the direct microbiological connections between China and the United States, and emphasize the need for collaborative research in AMR between the 2 nations. The first patient with a Klebsiella pneumoniae carbapenemase (KPC)–producing K. pneumoniae was identified in the southeastern United States in 1996 [7]. This event was followed by the emergence of a KPC-2 enzyme producing carbapenem-resistant Enterobacteriaceae (CRE) in the northeastern United States and subsequently in China [8–12]. Currently, KPC-2 is the predominant mechanism of carbapenem resistance in clinical K. pneumoniae and Escherichia coli isolates in China [13] and is a leading enzymatic mechanism in the United States as well [14]. Similarly, the first recognition of the plasmid-mediated colistin resistance gene mcr-1 in China was rapidly followed by global reports of mcr-1–mediated colistin resistance, including in the United States [15, 16].
PROGRESS TO DATE OF ARLG-SUPPORTED AMR COLLABORATIONS BETWEEN CHINA AND THE UNITED STATES
China’s Involvement in the Consortium on Resistance Against Carbapenems in Klebsiella and Enterobacteriaceae
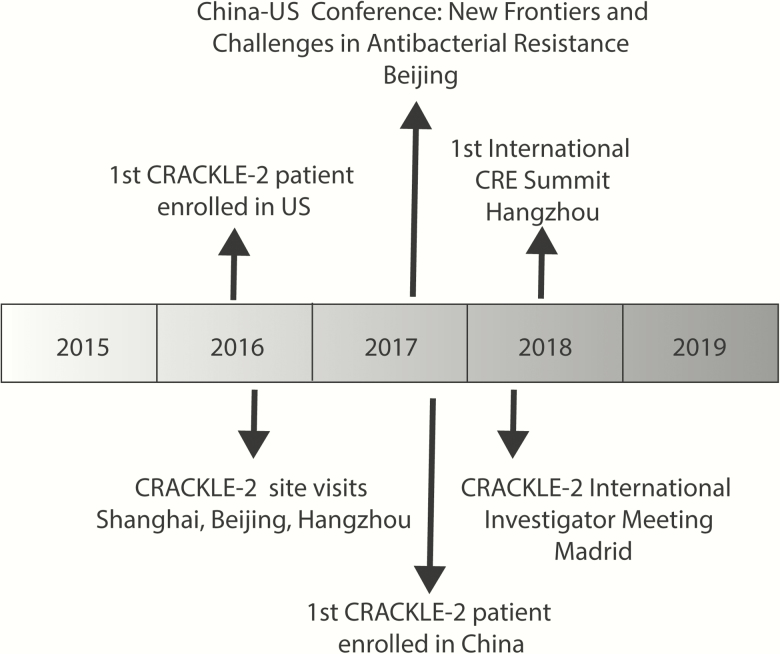
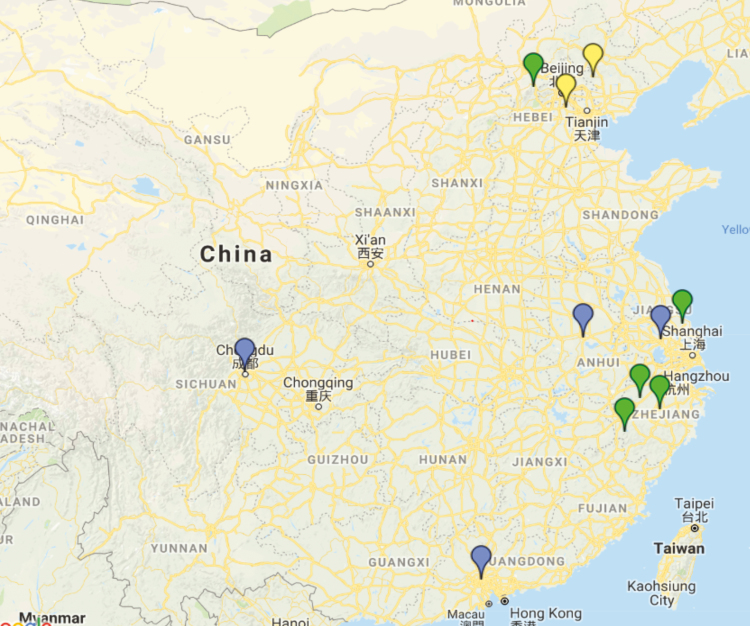
The Consortium on Resistance Against Carbapenems in Klebsiella and Enterobacteriaceae (CRACKLE) is an ARLG-funded multicenter, prospective, observational study of hospitalized patients with CRE [14, 17, 18]. In 2016, CRACKLE-2 began enrollment in 91 US hospitals in 17 states. In the same year, a US delegation that included representation from the National Institute of Allergy and Infectious Diseases (NIAID) of the US National Institutes of Health (NIH), the ARLG, GlaxoSmithKline (GSK), and prominent Chinese investigators in the field of AMR met in China to select CRACKLE-2 study sites in China (Figure 1). Professor Minggui Wang of Huashan Hospital in Shanghai was identified as the coordinating center principal investigator for CRACKLE-China, and extensive site visits were conducted to hospitals in Beijing, Shanghai, and Hangzhou. A formal site selection tool was developed and employed. A total of 7 hospitals were selected to join the MDRO network in the initial round. Two more rounds of site visits in China were undertaken by the ARLG team in September 2017 and May 2018, to expand the number of MDRO network sites in China. Currently, 5 hospitals are enrolling patients in the CRACKLE study, 2 sites are awaiting activation (Figure 2), and several more sites are under consideration. To date, >100 hospitalized patients with CRE have been included from China.
Figure 1.
Timeline of National Institute of Infectious Diseases Antibacterial Resistance Leadership Group–supported China–US antimicrobial resistance research collaboration. Abbreviations: CRACKLE, Consortium on Resistance Against Carbapenems in Klebsiella and Enterobacteriaceae; CRE, carbapenem-resistant Enterobacteriaceae.
Figure 2.
Current and prospective Consortium on Resistance Against Carbapenems in Klebsiella and Enterobacteriaceae (CRACKLE) study sites in China, including currently active sites (green), sites awaiting activation (yellow), and sites under consideration (blue).
In addition to site visits, 2 important meetings have facilitated the China–US collaborations. In September 2017, the Chinese Academy of Medical Sciences and NIAID co-sponsored a workshop on New Frontiers and Challenges in Antibacterial Resistance Research in Beijing. This workshop brought together government representatives, academic researchers, and biomedical companies from both countries to discuss the current state of knowledge, scientific and regulatory challenges, research resources, and potential opportunities for partnerships to advance understanding and encourage development of new medical countermeasures to address antibacterial resistance. In May 2018, the first International CRE Summit was held in Hangzhou. At this meeting, AMR experts from China, the United States, and other countries gathered to consider recent developments in the epidemiology, treatment, and prevention of carbapenem resistance, and to explore further opportunities for international collaborations.
Integration of China Sites Into CRACKLE-2
The goal of CRACKLE-2 is to collect and analyze clinical and microbiological data on consecutive hospitalized patients with CRE, in a standardized manner between all sites in various participating global regions. To this end, CRACKLE-2 uses web-based data entry into a centralized clinical database. Clinical variables are collected in an identical way in Chinese and US hospitals, using an English-language platform. Written electronic clinical report form instructions are provided in various languages of participating centers, including in Chinese. A translation of the study protocol is also provided. A key feature of CRACKLE-2 is its goal to provide planning data, trial site infrastructure, and investigator experience in anticipation of future, more complex, clinical trials as a part of the MDRO Network. Therefore, Good Clinical Practice training is required of and provided to all CRACKLE-2 investigators worldwide. To facilitate training of site coordinators and to obtain input from site investigators, an international CRACKLE-2 Investigator Meeting was held in Madrid in the spring of 2018. Oversight of data entry is crucially important for the integrity of any clinical study, and GSK is using its robust research infrastructure in China to provide training data review for CRACKLE-China.
Role of GSK
GSK has been active in the discovery, development, manufacturing, and commercialization of antibiotics for >70 years. Some of the ways GSK is addressing AMR include conserving current antibiotics through supporting their appropriate use and stewardship and encouraging the development of new antibiotics and AMR-relevant vaccines [19]. GSK was recently recognized in the Access to Medicines Foundation’s first AMR benchmark, which assessed the response to AMR from 30 pharmaceutical, generics, and biotech companies [20]. In 2016, GSK established the Institute for Infectious Diseases and Public Health in Beijing to help tackle issues of public health significance in China such as human immunodeficiency virus, tuberculosis, hepatitis, and AMR [21]. In 2017, GSK entered into a nonmonetary research collaboration agreement with Duke University to utilize its local clinical development expertise to support the review, selection, training, and quality assurance of the hospital sites in China. GSK local clinical experts supported both the Duke team and Chinese hospital sites for rapid and high-quality initiation of the study.
CHALLENGES AND SOLUTIONS
Operational challenges included site selection, executing contractual agreements, flowing down NIH regulations to study sites, standardizing laboratory and data procedures, interfacing study compliance needs with local regulatory requirements, and navigating complex bilingual communications through widely spread time zones. Leaders from the ARLG, NIAID, GSK, and Fudan University collectively decided to establish Huashan Hospital as the coordinating center for CRACKLE-2 in China. In addition, the ARLG operations leadership identified Dr Peidi Gu, whose clinical training originated in China, as Dr Wang’s operational partner. GSK China supports Huashan hospital and functions as the ARLG’s training partner. The bacterial phenotypic and genotypic characterization will be performed at the central research laboratory at the Institute for Antibiotics of Fudan University in China. This requires standardization of the microbiological and molecular approach to isolate analysis between China and the United States, which is currently ongoing.
FUTURE DIRECTIONS IN ARLG-SUPPORTED AMR RESEARCH INTERACTIONS BETWEEN THE UNITED STATES AND CHINA
Many AMR threats are shared between China and the United States. The ARLG has aligned its priorities with the World Health Organization ranking and will address these threats with a variety of study designs in which US and China study sites will participate (Table 1). CRACKLE is the first ARLG-funded study in China to establish China-US collaborations in AMR. The direct aim of CRACKLE is to evaluate differences and similarities between patients with CRE in the 2 countries, as well as other regions of the world. In addition, by building this research infrastructure, an MDRO network will be constructed with sites not only in the United States and China, but also in Central and South America and the Pacific. Studies conducted through this MDRO network will include observational studies, interventional diagnostic studies, and interventional treatment studies.
Table 1.
Topics of Interest and Critical Priority According to the World Health Organization [22]
| Topic | Study Examples |
|---|---|
| CRE | CRACKLE [14] |
| Impact of rapid diagnostics on outcomes in CRE infections | |
| Combination therapy vs monotherapy in CRE bloodstream infections | |
| Carbapenem-resistant Acinetobacter baumannii | Study Network of Acinetobacter as a Pathogen (SNAP) |
| Infection prevention strategy trial to limit spread of CRAb | |
| Antibiotic strategy trial on role of high-dose sulbactam in combination regimens | |
| Carbapenem-resistant Pseudomonas aeruginosa | Prospective Observational Pseudomonas (POP) study |
| RCT on antibody-mediated protection against ventilator-associated pneumonia caused by P. aeruginosa (EVADE) | |
| Diagnostics | Rapid Diagnostics in Categorizing Acute Lung Infections (RADICAL) [23] |
| MASTER Protocol for Multiple Infection Diagnostics (MASTERMIND) [24] |
Abbreviations: CRACKLE, Consortium on Resistance Against Carbapenems in Klebsiella and Enterobacteriaceae; CRAb, carbapenem-resistant Acinetobacter baumannii; CRE, carbapenem-resistant Enterobacteriaceae; RCT, randomized controlled trial.
Notes
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Financial support. This research was funded by the NIH (grant number UM1AI104681).
Supplement sponsorship. This supplement was sponsored by MSD.
Potential conflicts of interest. D. v. D. has received personal fees from Astellas, Achaogen, T2 Biosystems, Shionogi, Allergan, NeuMedicine, Roche, and Merck, and grants from NIH. V. G. F. served as Chair of the V710 Scientific Advisory Committee (Merck); has received grant support from Basilea, Cerexa/Actavis, Pfizer, Advanced Liquid Logics, NIH, MedImmune, Cubist/Merck, Karius, Contrafect, Regeneron, and Genentech; has NIH Small Business Technology Transfer/Small Business Innovation Research grants pending with Affinergy, Locus, and Medical Surface, Inc; has been a paid consultant for Achaogen, Astellas, Arsanis, Affinergy, Basilea, Bayer, Cerexa, Contrafect, Cubist, Debiopharm, Durata, Grifols, Genentech, MedImmune, Merck, The Medicines Co, Pfizer, Novartis, Novadigm, Theravance, xBiotech, and Regeneron; has received honoraria from Theravance and Green Cross; and has a patent pending in sepsis diagnostics. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017; 8:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Global action plan on antimicrobial resistance 2017. Available at: http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/. Accessed 25 May 2018.
- 3. Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis 2013; 13:1057–98. [DOI] [PubMed] [Google Scholar]
- 5. Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect 2016; 22(Suppl 1):S9–14. [DOI] [PubMed] [Google Scholar]
- 6. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the centers for disease control and prevention, 2011–2014. Infect Control Hosp Epidemiol 2016; 37:1288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med 2005; 165:1430–5. [DOI] [PubMed] [Google Scholar]
- 9. Wei ZQ, Du XX, Yu YS, Shen P, Chen YG, Li LJ. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother 2007; 51:763–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang R, Zhou HW, Cai JC, Chen GX. Plasmid-mediated carbapenem-hydrolysing beta-lactamase KPC-2 in carbapenem-resistant Serratia marcescens isolates from Hangzhou, China. J Antimicrob Chemother 2007; 59:574–6. [DOI] [PubMed] [Google Scholar]
- 11. Chen S, Hu F, Xu X, et al. High prevalence of KPC-2-type carbapenemase coupled with CTX-M-type extended-spectrum beta-lactamases in carbapenem-resistant Klebsiella pneumoniae in a teaching hospital in China. Antimicrob Agents Chemother 2011; 55:2493–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abdallah M, Olafisoye O, Cortes C, et al. Rise and fall of KPC-producing Klebsiella pneumoniae in New York City. J Antimicrob Chemother 2016; 71:2945–8. [DOI] [PubMed] [Google Scholar]
- 13. Zhang R, Liu L, Zhou H, et al. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine 2017; 19:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Duin D, Perez F, Rudin SD, et al. Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother 2014; 58:4035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 2016; 16:161–8. [DOI] [PubMed] [Google Scholar]
- 16. Mediavilla JR, Patrawalla A, Chen L, et al. Colistin- and carbapenem-resistant Escherichia coli harboring mcr-1 and blaNDM-5, causing a complicated urinary tract infection in a patient from the United States. mBio 2016; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Duin D, Cober E, Richter SS, et al. Impact of therapy and strain type on outcomes in urinary tract infections caused by carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother 2015; 70:1203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Duin D, Lok JJ, Earley M, et al. Colistin vs. ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. GlaxoSmithKline. GSK public policy positions [press release]. 2016. Available at: https://www.gsk.com/media/2942/incentivising-antibacterial-research.pdf. Accessed 25 May 2018. [Google Scholar]
- 20. Access to Medicines Foundation. GSK AMR benchmark report Available at: https://amrbenchmarkorg/report_card/glaxosmithkline-plc 2018. Accessed 25 May 2018.
- 21. GlaxoSmithKline. GSK Institute for Infectious Diseases and Public Health to partner with Tsinghua University to tackle global public health challenges [press release]. 2016. Available at: http://www.gsk-china.com/en-gb/media/press-releases/2016/gsk-institute-for-infectious-diseases-to-partner-with-tsinghua-university-to-tackle-global-public-health-challenges/. Accessed 25 May 2018.
- 22. Tacconelli E, Carrara E, Savoldi A, et al. ; WHO Pathogens Priority List Working Group Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18:318–27. [DOI] [PubMed] [Google Scholar]
- 23. Tsalik EL, Henao R, Nichols M, et al. Host gene expression classifiers diagnose acute respiratory illness etiology. Sci Transl Med 2016; 8:322ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel R, Tsalik EL, Petzold E, Fowler VG Jr, Klausner JD, Evans S; Antibacterial Resistance Leadership Group (ARLG) MASTERMIND: bringing microbial diagnostics to the clinic. Clin Infect Dis 2017; 64:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]