We assessed the transmission risk of pulmonary tuberculosis patients whose sputum nucleic acid amplification tests (NAAT) were negative. We estimated that, at minimum, sputum NAAT–negative tuberculosis patients had approximately a 5% risk of transmitting tuberculosis.
Keywords: tuberculosis, diagnosis, transmission, nucleic acid amplification test, infectiousness
Abstract
Background
Among adults with signs and symptoms of pulmonary tuberculosis (TB), recognition of transmissible TB has implications for airborne infection isolation and public health activities. Sputum smear–negative TB patients account for around one-fifth of tuberculosis transmission. The tuberculosis transmission risk of TB patients with negative results on nucleic acid amplification test (NAAT) of respiratory specimens has not been established. We sought to estimate the tuberculosis transmission risk of NAAT-negative TB patients.
Methods
We retrospectively reviewed Maryland TB program data collected from 2004 to 2009, during which time NAAT using the Mycobacterium Tuberculosis Direct Test (MTD) was performed routinely. Patients with sputum Mycobacterium tuberculosis (M.tb) isolates having matching genotypes were assigned to clusters. Transmission sequence was approximated by collection order of individuals’ first culture-positive specimens. Minimum transmission risks of NAAT (MTD)-negative TB patients and of smear-negative TB patients were estimated based on individuals’ positions within clusters.
Results
Among 809 patients with culture-confirmed TB, M.tb genotypes were available for 782 (96.7%). For NAA-negative TB patients, the minimum transmission risk estimate was 5.1% (95% CI 0–11.4). For smear-negative TB patients, the minimum transmission risk estimate was 11.2% (95% CI 7.2–15.3).
Conclusions
Minimum transmission risk of NAAT-negative TB patients was lower than that of smear-negative TB patients. However, transmission risk of NAA-negative TB patients appears to not be negligible.
Historically, airborne isolation decisions and public health management of pulmonary tuberculosis (TB) patients have relied on results of 3 sputum acid-fast bacilli (AFB) smear microscopy exams [1]. Despite longstanding recognition that negative sputum smear results do not exclude TB, limitations of smear microscopy for assessing transmissibility were not apparent until a landmark study by Behr et al in the 1990’s [2]. They applied the then-novel molecular epidemiologic approach of M. tuberculosis (M.tb) strain genotyping to a cohort of San Francisco TB patients, and estimated that smear-negative pulmonary TB patients contributed to at least 17% of TB transmission.
Recently, rapid sputum tests based on amplification of M.tb nucleic acids have been introduced both in the United States and globally. Commercially available nucleic acid amplification tests (NAATs) for TB diagnosis include the Xpert MTB/RIF (Cepheid, Sunnyvale, CA) and the Amplified Mycobacterium tuberculosis Direct test (MTD; Hologic, Inc., San Diego, CA), both of which have sensitivities for pulmonary TB detection that are intermediate between those of smear microscopy and liquid culture [3–5]. Retrospective studies conducted in U.S. hospitals have shown that for diagnostic testing of adults clinically suspected to have TB, the negative predictive value of NAATs on 1 or 2 respiratory specimens is comparable to that of smear microscopy testing of 3 specimens, and use of NAATs reduces airborne isolation duration [6–9]. In 2015, the U.S. Food and Drug Administration expanded the intended use of Xpert MTB/RIF to include testing of 1 or 2 respiratory specimens as an alternative to serial AFB smears, to guide discontinuation of airborne isolation. However, the TB transmission risk of NAAT-negative pulmonary TB patients has not been directly studied and remains unclear.
We sought to estimate the risk of TB transmission from NAAT-negative TB patients in the context of a public health TB program in which NAAT and smear microscopy were routinely performed on respiratory specimens and M.tb isolates were routinely genotyped.
MATERIALS AND METHODS
We conducted a retrospective study of existing medical and laboratory records. The target population was all patients for whom M.tb was isolated from respiratory specimens in Maryland from 1 January 2004 through 1 September 2009, a period during which the relevant tests were performed routinely. Participants were identified through review of Maryland Department of Health (MDH) Reports of Verified Cases of Tuberculosis and laboratory records, which also served as the data sources [10]. This study was approved by the institutional review boards of Johns Hopkins Medical Institutions and the Maryland Department of Health.
Mycobacteriology Laboratory Tests Conducted Routinely During the Study Period
The MDH mycobacteriology laboratory routinely performed AFB smear microscopy, mycobacterial culture, and MTD on respiratory specimens from patients undergoing evaluation for pulmonary TB [11].
AFB Smear and Culture
Respiratory specimens were digested, decontaminated, and centrifuged. The resuspended sediment was inoculated into liquid (Bactec 12B or Bactec MGIT; Becton, Dickinson and Company, Franklin Lakes, NJ) and solid media (Lowenstein Jensen slant) for a 6- and 8-week incubation period, respectively, and used for fluorescence smear microscopy. Smears were graded according to published guidelines [12]. A smear was considered positive if at least 1 AFB was observed by fluorochome among 10 fields at 250x magnification (reported as 1+). The M.tb complex was identified in positive cultures by conventional methods [12].
NAAT
MTD was performed for all smear-positive specimens and for all smear-negative specimens in which the MTD box on the MDH laboratory request form was checked. MTD status for each clinical respiratory specimen was based on 2 test runs: the first using an undiluted concentrated specimen, and the second using a 1:10 dilution of the concentrated specimen [13]. If either test run was positive, the overall MTD result for that specimen was positive.
M.tb Genotyping
M.tb culture isolates were routinely submitted to the National TB Genotyping Service [14]. Genotyping results were a combined analysis of (1) spacer oligonucleotide sequences in the direct repeat region of the M.tb genome (spoligotype) and (2) variable number tandem repeats of mycobacteria-interspersed sequences in 12 loci of the M.tb genome [15]. The combined result was assigned a polymerase chain reaction type used to group TB patients into genotypic clusters [14, 16].
Classification of Patients
TB patients with at least 1 respiratory culture positive for M.tb were included in the study. In the primary analysis, patients were considered smear-negative if no AFB was detected from 3 or more respiratory specimens. Patients were considered NAAT-negative if all MTD tests performed from 1 or more respiratory specimens were negative for M.tb. Only MTD-negative or smear-negative respiratory specimens collected within 7 days of treatment initiation were considered. If at least 1 AFB smear or MTD result was positive, a patient was considered smear-positive or NAAT-positive, respectively. In a secondary analysis, patients were stratified by their combined test results into 3 groups: smear-positive/NAAT-positive, smear-negative/NAAT-positive, and smear-negative/NAAT-negative. Since Xpert MTB/RIF device labelling allows for 1 or 2 sputum NAATs to inform airborne isolation decisions, we also considered the transmission risks associated with only the first NAAT result (NAAT1), and, alternatively, with only the first 2 NAAT results (NAAT2).
Tests, Clusters, and Transmission Events
“Test” refers to smear, NAAT, or the combined smear and NAAT result for the respective analysis. To approximate transmission events, patients in each cluster were ordered chronologically by the collection date of their first culture-positive sputum [2]. The first patient in each cluster was considered to be the index case; clusters whose index case had an unknown test status were excluded from the respective analysis. Clusters composed of only a single case (due to other case[s] in the cluster not being captured within the study period) were excluded from the analysis, since transmission link(s) could not be ascertained.
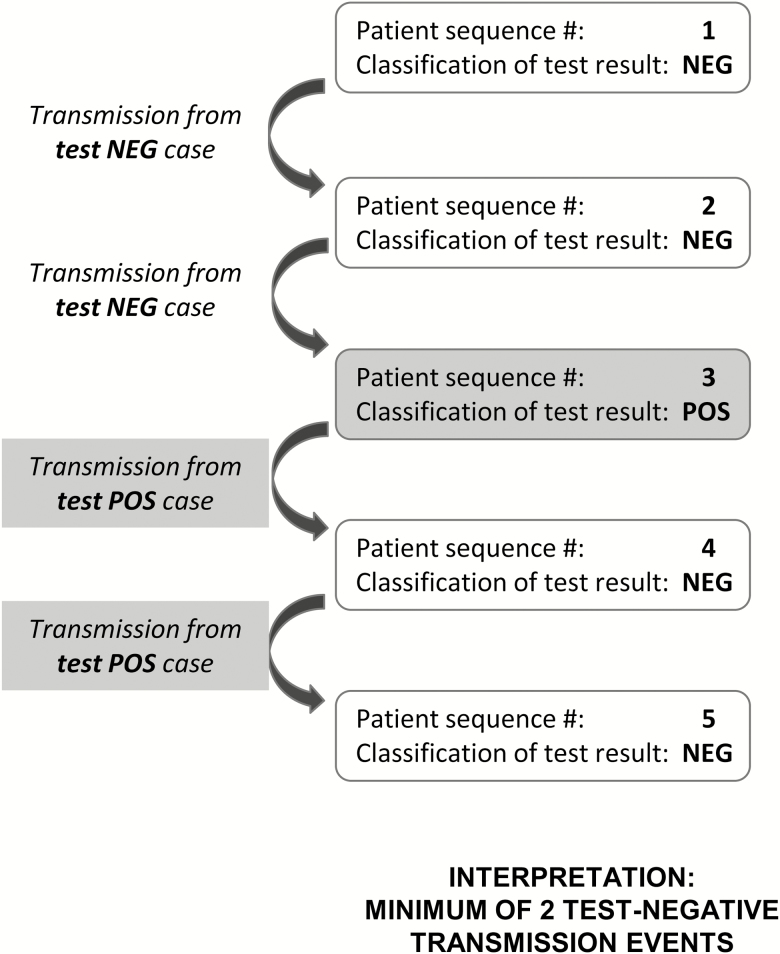
To approximate minimum transmission events from test-negative TB patients, the following assumptions were made about transmission within a cluster (Figure 1): (1) secondary cases occurring after either a test-positive or test-unknown patient’s collection date were attributed to test-positive transmission, and (2) secondary cases following only test-negative cases (no test-positive or test-unknown cases earlier in cluster) were attributed to test-negative transmission.
Figure 1.
Estimation of minimum transmission events from test-negative patients in a hypothetical cluster.
The primary epidemiologic outcome was the minimum transmission risk of a test-negative case, with NAAT and smear considered separately. Minimum transmission risk by combined smear and NAAT status was a secondary outcome. Minimum transmission risk was calculated as [(minimum number of transmission events attributed to patients with the test status of interest)/(total number of cases with the test status of interest)] x 100. We additionally considered our data from several perspectives (secondary outcomes). The minimum percentage of secondary cases infected by a test-negative patient (% of TB transmission) was calculated as (minimum number of transmission events attributed to patients with the test status of interest)/(total number of secondary cases)] x 100. Any remaining secondary cases not due to test-negative transmission were attributed to test-positive transmission, and we estimated the minimum relative transmission risk of test-negative to test-positive TB cases as (minimum transmission risk associated with negative test status for the test of interest)/(transmission risk associated with positive test status for the test of interest).
As data are not independent (and thus chi-square methods do not apply), confidence intervals were computed using the bootstrap procedure by randomly sampling clusters with replacement. For each estimate, 9999 bootstrapped datasets were generated, and the 5th and 95th percentiles were used to estimate 95% confidence intervals.
Sensitivity Analysis to Assess Impact of Potential Biases
To assess the impact of chronologic misclassification, in which a case in a cluster actually infected the preceding case but was diagnosed later, we reversed the order of all consecutive case pairs presenting within 30, 60, 90, 120, and 180 days of each other and repeated the analyses at each time interval. Additionally, we considered potential bias from any unidentified source cases among patients who did not have a TB genotype, or due to exclusion of cases diagnosed outside of the study period and/or the state of Maryland. To study the impact of unidentified source cases, we successively removed cases in 2004, 2005, and 2006 and repeated the analyses at the end of each year. We also randomly censored 10, 20, 30, 40, and 50% of cases from the dataset 9999 times, and computed the 50th percentile when repeating the analyses at each level.
RESULTS
In total, 809 patients with culture-confirmed TB were reported in Maryland during the study period. Genotyping results were available for 782 (97%) of them. Among the 782 patients with genotype results, 393 had no genotypic match and were thus not clustered (singletons; Figure 1). The remaining 389 patients were dispersed among 158 unique genotypic clusters. Of these 158 clusters, 75 clusters had only 1 case identified during the study period (ie, 1 or more cases with a matching M.tb polymerase chain reaction type occurred before or after, but not during, the study period). As transmission links could not be identified, these 75 cases were excluded from the analysis. The remaining 83 multi-member clusters consisted of 231 secondary cases. Characteristics of the 707 patients in the main analysis population (393 singletons and 314 clustered cases) are shown in Table 1. All but 3 of the 46 NAAT-negative cases were smear-negative; only 1 of these 3 NAAT-negative, smear-positive cases had the smear-positive (1+) and MTD-negative result from the same sputum.
Table 1.
Characteristics by Test Status for Patients in the Main Analysis Population*
| NAAT (-) | NAAT (+) | NAAT (u) | Smear (-) | Smear (+) | Smear (u) | |
|---|---|---|---|---|---|---|
| (n = 46) | (n = 382) | (n = 279) | (n = 166) | (n = 393) | (n = 148) | |
| Age, mean (SD) | 34.0 (18.5) | 37.0 (18.5) | 41.0 (19.6) | 39.0 (18.1) | 39.0 (18.6) | 39.5 (20.9) |
| Male, n (%) | 17 (37%)b,c | 233 (61%)c | 180 (65%)b | 91 (55%) | 246 (63%) | 93 (63%) |
| Ethnic origin, n (%) | ||||||
| Caucasian | 2 (4%) | 29 (8%) | 27 (10%) | 9 (5%) | 35 (9%) | 14 (9%) |
| Hispanic | 7 (15%) | 90 (24%) | 45 (16%) | 22 (13%)c | 90 (23%)c | 30 (20%) |
| Black | 26 (57%) | 180 (47%) | 129 (46%) | 72 (43%) | 184 (47%) | 79 (53%) |
| Asian/other | 11 (24%) | 83 (22%) | 78 (28%) | 63 (38%)b,c | 84 (21%)c | 25 (17%)b |
| Born in United States, n (%) | 12 (26%) | 103 (27%) | 94 (34%) | 35 (21%)b | 118 (30%) | 56 (38%)b |
| HIV-positive, n (%) | 3 (7%) | 47 (12%) | 44 (16%) | 22 (13%) | 41 (10%) | 31 (21%) |
| Lung cavitation on chest imaging, n (%) | 7 (15%)c | 183 (48%)c | 85 (30%) | 19 (11%)b,c | 222 (56%)c | 34 (23%)b |
| Days to treatment initiation, mean (SD) | 14 (17)c | 3 (7)c | 9 (17) | 14 (17)b,c | 2 (8)c | 7 (14)b |
| Environment, n (%) | ||||||
| Long-term care facility | 1 (2%) | 4 (1%) | 12 (4%) | 1 (1%)b | 3 (1%) | 13 (9%)b |
| Homeless | 1 (2%) | 16 (4%) | 21 (8%) | 10 (6%) | 20 (5%) | 8 (5%) |
| Health worker | 4 (9%) | 13 (3%) | 11 (4%) | 8 (5%) | 14 (4%) | 6 (4%) |
| Correct. facility | 0 | 2 (1%) | 6 (2%) | 6 (4%) | 2 (1%) | 0 |
| # Specimens tested,a n (%) | ||||||
| 0 | 0 | 0 | 279 (100%) | 0 | 0 | 97 (66%) |
| 1 | 6 (13%) | 211 (55%) | 0 | 0 | 151 (38%) | 31 (21%) |
| 2 | 30 (65%) | 142 (37%) | 0 | 0 | 121 (31%) | 19 (13%) |
| 3 | 10 (22%) | 28 (7%) | 0 | 156 (94%) | 118 (30%) | 1 (1%) |
| 4 or more | 0 | 1 (0%) | 0 | 10 (6%) | 3 (1%) | 0 |
| Smear status | ||||||
| Negative | 36 (78%) | 46 (12%) | 84 (30%) | 166 (100%) | ||
| Positive | 3 (7%) | 309 (81%) | 81 (29%) | 393 (100%) | ||
| Unknown | 7 (15%) | 27 (7%) | 114 (41%) | 148 (100%) | ||
*N = 707.
Abbreviations: (-), negative; (+), positive; HIV, human immunodeficiency virus; NAAT, nucleic acid amplification test; SD, standard definition; (u), unknown.
aExcludes test-negative specimens collected after 7 days of TB treatment and test-indeterminate specimens.
b P < .01 for comparison between test-unknown and test-negative patients.
c P < .01 for comparison between test-positive and test-negative patients.
Transmission Risk of NAAT-Negative TB Cases
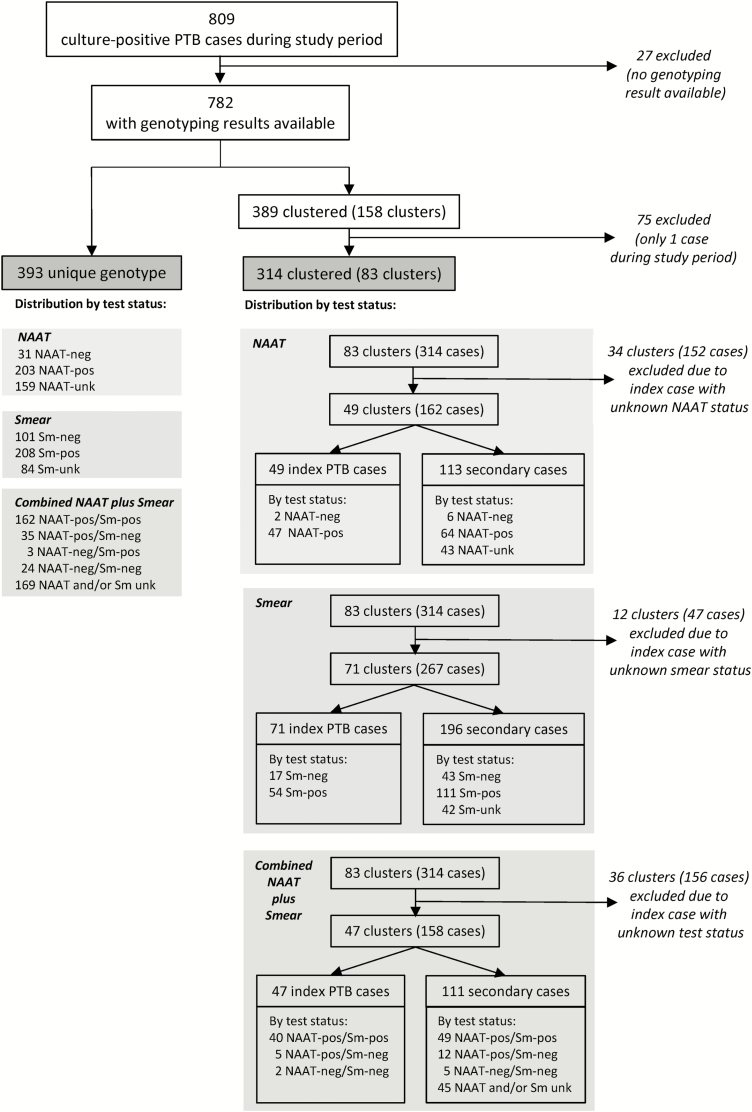
Among the 83 clusters, 34 (41.0%) clusters had a NAAT-unknown index case and were excluded from the NAAT analysis (Figure 2). The remaining 49 clusters consisted of 2 NAAT-negative and 47 NAAT-positive index cases and 113 secondary cases. Therefore, estimation of NAAT-negative transmission risk was based on 39 NAAT-negative cases (17 singletons, 2 index cases, and 10 secondary cases). The 2 NAAT-negative index cases, who were both smear-negative, included a 42-year-old U.S.-born African-American man of unknown HIV status and a 26-year-old Asian-born, HIV-negative woman. While neither index case had unique epidemiologic risk factors noted, both were started on treatment approximately 1 month after collection of their first culture-positive sputum.
Figure 2.
Analysis flowchart of clustered and non-clustered patients, test status, and transmission events. Italics denote patients and/or clusters that were excluded from respective analyses. Abbreviations: NAAT, nucleic acid amplification test; PTB, pulmonary tuberculosis.
At minimum, there were 2 NAAT-negative transmission events from the 2 NAAT-negative index cases, representing 1.8% of the recorded TB transmissions (Table 2). The minimum transmission risk of NAAT-negative TB was 5.1% (95% CI 0–11.4). Attributing the remaining 111 secondary cases to transmission from NAAT-positive cases resulted in an estimated NAAT-positive TB transmission risk of 35.4% (111/314; 95% CI 26.5–43.2). Therefore, the minimum relative transmission risk of NAAT-negative to NAAT-positive TB was 0.14 (95% CI 0–0.35).
Table 2.
Estimates of % of Tuberculosis Transmission, Transmission Risk, and Relative Transmission Risk by Test Status of Interest
| Minimum % of TB Transmissiona | Minimum Transmission Risk%b |
Minimum Relative Transmission Riskc | |
|---|---|---|---|
| 95% CI | 95% CI | 95% CI | |
| n/n | n/n | n/n | |
| NAAT-negative | 1.8 | 5.1 | 0.14 |
| (0.0, 4.3) | (0.0, 11.4) | (0.00, 0.35) | |
| 2/113 | 2/39 | 5.1/35.4 | |
| Smear-negative | 9.2 | 11.2 | 0.23 |
| (5.7, 14.0) | (7.2, 15.3) | (0.15, 0.36) | |
| 18/196 | 18/161 | 11.2/48.5 | |
| NAAT-positive/smear-positive | 46.9 | 20.7 | Not applicable |
| (41.3, 61.2) | (17.2, 26.8) | ||
| 52/111 | 52/251 | ||
| NAAT-positive/smear-negative/ | 5.4 | 11.5 | Not applicable |
| (0.9, 7.0) | (0.3, 18.4) | ||
| 6/111 | 6/52 | ||
| NAAT-negative/smear-negative | 1.8 | 6.5 | Not applicable |
| (0, 4.3) | (0, 14.3) | ||
| 2/111 | 2/31 |
Abbreviations: CI, confidence interval; NAAT, nucleic acid amplification test; TB, tuberculosis.
aDefined as [(minimum number of transmission events attributed to patients with the test status of interest)/(total number of secondary cases)] × 100.
bDefined as [(minimum number of transmission events attributed to patients with the test status of interest)/(total number of cases with the test status of interest)] × 100.
cDefined as (minimum transmission risk associated with negative test status for the test of interest)/(transmission risk associated with positive test status for the test of interest).
When considering only the first 1 or the first 2 NAAT results, estimated minimum transmission risks were similar to overall NAAT-negative transmission risk estimates (Supplementary Table 1). Notably, 10 (22%) of the 46 NAAT-negative cases had more than 2 respiratory specimens tested by NAAT (Table 1); these 10 NAAT-negative cases led to a minimum of 0 transmission events.
Transmission Risk of Smear-negative TB Cases
Among the 83 clusters, 12 (14.4%) clusters had a smear-unknown index case and were excluded from the smear analysis (Figure 2). The remaining 71 clusters consisted of 17 smear-negative and 54 smear-positive index cases, and 196 secondary cases. Therefore, estimation of smear-negative transmission risk was based on 161 smear-negative cases (101 singletons, 17 index, and 43 secondary cases).
At minimum there were 18 smear-negative transmission events from the 17 smear-negative index cases, accounting for 9.2% of TB transmission (Table 2). Minimum transmission risk due to smear-negative TB was 11.2% (95% CI 7.2–15.3). Attributing the remaining 178 secondary cases to smear-positive transmission resulted in an estimated smear-positive TB transmission risk of 47.7% (178/373; 95% CI 36.5–58.6). The minimum relative smear-negative to smear-positive TB transmission risk was 0.23 (95% CI 0.15–0.36).
Transmission Risk Based on Combined Smear and NAAT Status
Among the 83 clusters, 47 (57%) had determinate results for both smear and NAAT. Among the 47 clusters and all singletons, there were 251 NAAT-positive/smear-positive cases, 52 NAAT-positive/smear-negative cases, and 31 NAAT-negative/smear-negative cases (Figure 2). For each group, the minimum% of TB transmission and transmission risk are shown in Table 2.
Sensitivity Analyses
Chronologic Misclassification
Reversing consecutive cases with different test status and diagnosed temporally close to each other led to a minimum transmission risk estimate that was within 0–3% of baseline estimates (Supplementary Figure 1).
Unidentified Source-case Bias
Exclusions of cases in 2004, 2005, 2006 and random censoring of cases led to fluctuations in estimates at each level. However estimates of minimum NAAT-negative transmission risk did not reach 0 and remained lower than respective estimates of minimum smear-negative transmission risk (Supplementary Figures 2 and 3).
DISCUSSION
We sought to determine the transmissibility of pulmonary TB patients whose sputum NAATs were negative via a public health TB control program that incorporated mandated case reporting; routine testing of respiratory specimens using smear microscopy, NAAT (MTD), and culture; and routine genotyping of M.tb isolates. Modelling our approach after that used by Behr et al in their analysis of smear-negative TB transmission in San Francisco [2], we found that minimum TB transmission risk was lower for NAAT-negative than for smear-negative pulmonary TB patients, but a minimum of about 5% of NAAT-negative pulmonary TB patients still transmit TB. These findings were consistent when only the first 1 and, alternatively, the first 2 NAAT result(s) were considered. Our estimates were imprecise, as demonstrated by broad confidence intervals. However, several features support the validity of our findings. Trends were consistent across different analytic approaches and robust to sensitivity analyses. When patients were classified using combined NAAT and smear results, the ranking of highest to lowest minimum transmission risk matched the ranking of estimated sputum bacterial burden. Reassuringly, for assessments of transmission from smear-negative patients, our results were comparable to those reported by Behr et al. The estimated smear-negative minimum transmission risk was 11% in our study and 6% in the Behr study; the minimum proportion of secondary cases infected by a smear-negative patient was 9% in our study and 17% in the Behr study; and the estimated minimum smear-negative to smear-positive relative transmission risk was 0.23 in our study and 0.22 in the Behr study [2].
An unequivocal determination of 0 transmission risk from NAAT-negative TB cases would have straightforward implications for infection control and public health practice. Our finding of a low but non-zero transmission risk may be useful nevertheless, particularly when clinical and/or epidemiologic suspicion of pulmonary TB is high. Levels of acceptable transmission risk may differ from setting to setting based on factors such as presence of vulnerable populations sharing the same air, available resources and/or facilities for reducing transmission risk, drug susceptibility status of the TB patient, and institutional tolerance of risk. Additionally, among symptomatic individuals undergoing diagnostic evaluation for TB, TB prevalence influences the negative predictive value of NAATs. In low-prevalence settings such as the United States, the vast majority of NAAT-negative patients do not have TB, and therefore cannot transmit TB; this calculus is relevant for decision-making about discontinuation of airborne isolation for NAAT-negative patients whose diagnosis has not been confirmed. Futhermore, mean time to treatment initiation was longer for NAAT-negative and smear-negative patients compared to NAAT-positive and smear-positive patients (14 versus 2–3 days, respectively), including a 1-month delay to treatment initiation among the 2 NAAT-negative index cases. This observation informs the context of a non-zero NAAT-negative transmission risk and reinforces considerations for prompt treatment of NAAT-negative and smear-negative patients when clinical suspicion for TB is high.
Our retrospective study has important limitations. While study tests were performed routinely during the 6-year study period, relevant test results, especially NAAT, were not available for a considerable proportion of patients; however, even inclusion of these patients under a known test status would not have resulted in 0 transmission risk from NAAT-negative patients. Exclusion of approximately 10% of TB cases known to be members of clusters in which the other member(s) were diagnosed outside the study period may have resulted in underestimates of transmission from unidentified index cases. Additionally, we assumed that collection order of the first culture-positive specimen was a proxy for transmission sequence within a cluster, but transmission may follow a nonlinear sequence and/or differ from the order in which patients were diagnosed. However, sensitivity analyses suggested that unidentified source cases and chronological misclassification had limited impacts on transmission estimates.
Another important limitation of this study is that the NAAT utilized was the MTD test, which mostly has been supplanted by Xpert MTB/RIF. However, for TB case detection, the MTD and Xpert MTB/RIF Version G4 each have similar sensitivities that lie between those of smear and liquid cultures [3]. Given the absence of epidemiologic data on the risk of TB transmission from TB patients whose sputum is determined to be negative by Xpert MTB/RIF G4 testing, extrapolation of MTD-based results may be reasonable. Accordingly, we speculate that negative Xpert MTB/RIF G4 results may not definitively exclude the possibility of transmissible TB. It remains to be seen whether next-generation NAAT tests like Xpert MTB/RIF Ultra are sufficiently sensitive to exclude the possibility of TB transmission from a test-negative TB patient.
Taken together, our findings suggest that the transmission risk posed by sputum NAAT-negative pulmonary TB patients is lower than that posed by sputum smear-negative TB patients. However, the risk of TB transmission from sputum NAAT-negative pulmonary TB patients is likely non-zero. Therefore, clinical judgement is required in the infection control management of sputum NAAT-negative individuals with, or suspected to have, pulmonary TB.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Y. L. X. performed the design conceptualization, data acquisition, and analysis, and wrote the first draft of this manuscript. W. A. C. and R. O. assisted in data acquisition and interpretation. J. E. G. assisted with study design and interpretation of data. M. P. contributed to statistical analysis and review of the manuscript. S. Cohn assisted with database preparation. S. R. C. contributed to interpretation of study data and preparation of the final manuscript. C. E. B. supported the execution of this study. S. E. D. supervised this study, including conceptualization, design, and the final writing.
Acknowledgments. The authors gratefully acknowledge Kush Shankar (University of Southern California) and Lori Dodd, PhD (Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]) for their contribution in considering various analysis methods.
Financial support. This work was supported in part by the National Institutes of Health (K24AI104830) and by the Intramural Research Program of NIAID, NIH (AI001081-08, AI001214-02) .
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5417a1.htm. Accessed 30 December 2005. [Google Scholar]
- 2. Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 1999; 353:444–9. [DOI] [PubMed] [Google Scholar]
- 3. Teo J, Jureen R, Chiang D, Chan D, Lin R. Comparison of two nucleic acid amplification assays, the Xpert MTB/RIF assay and the amplified Mycobacterium tuberculosis direct assay, for detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol 2011; 49:3659–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blakemore R, Story E, Helb D, et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol 2010; 48:2495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergmann JS, Yuoh G, Fish G, Woods GL. Clinical evaluation of the enhanced Gen-Probe amplified Mycobacterium tuberculosis direct test for rapid diagnosis of tuberculosis in prison inmates. J Clin Microbiol 1999; 37:1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaisson LH, Roemer M, Cantu D, et al. Impact of GeneXpert MTB/RIF assay on triage of respiratory isolation rooms for inpatients with presumed tuberculosis: a hypothetical trial. Clin Infect Dis 2014; 59:1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campos M, Quartin A, Mendes E, et al. Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am J Respir Crit Care Med 2008; 178:300–5. [DOI] [PubMed] [Google Scholar]
- 8. Lippincott CK, Miller MB, Popowitch EB, Hanrahan CF, Van Rie A. Xpert MTB/RIF assay shortens airborne isolation for hospitalized patients with presumptive tuberculosis in the United States. Clin Infect Dis 2014; 59:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luetkemeyer AF, Firnhaber C, Kendall MA, et al. ; AIDS Clinical Trials Group A5295 and Tuberculosis Trials Consortium Study 34 Teams Evaluation of Xpert MTB/RIF versus AFB smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin Infect Dis 2016; 62:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. CDC tuberculosis surveillance data training – report of verified case of tuberculosis. Available at: https://www.cdc.gov/tb/programs/rvct/default.htm. Accessed 1 September 2012. [Google Scholar]
- 11. Guerra RL, Hooper NM, Baker JF, et al. Use of the amplified Mycobacterium tuberculosis direct test in a public health laboratory: test performance and impact on clinical care. Chest 2007; 132:946–51. [DOI] [PubMed] [Google Scholar]
- 12. Kent PT, Kubica GP.. Public health mycobacteriology: a guide for the level III laboratory. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control; 1985. [Google Scholar]
- 13. Guerra RL, Baker JF, Alborz R, et al. Specimen dilution improves sensitivity of the amplified Mycobacterium tuberculosis direct test for smear microscopy-positive respiratory specimens. J Clin Microbiol 2008; 46:314–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Center for Disease Control and Prevention. Tuberculosis genotyping. Available at: https://www.cdc.gov/tb/publications/factsheets/statistics/genotyping.htm. Accessed 1 September 2012. [Google Scholar]
- 15. Kamerbeek J, Schouls L, Kolk A, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 1997; 35:907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Tuberculosis Controllers Association/Centers for Disease Control and Prevention Advisory Group on Tuberculosis Genotyping. Guide to the application of genotyping to tuberculosis prevention and control. Atlanta, GA: US Department of Health and Human Services, CDC; June 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.