Abstract
While it has been known since the 1940s that men have greater increases in blood pressure (BP) compared with women, there have been intense efforts more recently to increase awareness that women are also at risk for developing hypertension and that cardiovascular diseases (CVDs) are the leading causes of death among both men and women in the United States. With the release of the 2017 Hypertension Clinical Guidelines, 46% of adults in the United States are now classified as hypertensive, and hypertension is the primary modifiable risk factor for the development of CVD. This increase in the prevalence of hypertension is reflected in an increase in prevalence among both men and women across all demographics, although there were greater increases in the prevalence of hypertension among men compared with women. As a result, the well-established gender difference in the prevalence of hypertension is even more pronounced and now extends into the sixth decade of life. The goals of this review are to (i) review the historical clinical trial data and hypertension guidelines from the perspective of both genders and then (ii) review the role of the renin–angiotensin system and T-cell activation in contributing to sex differences in BP control.
Keywords: blood pressure, gender differences, hypertension, immune system, inflammation, renin–angiotensin system, sex differences, T-cells, T-regulatory cells
HYPERTENSION: A BRIEF HISTORY
The leading cause of death in the United States remains heart disease, accounting for 24.4% of all deaths among men and 22.3% of all deaths among women in 2015.1 Hypertension is a complex disorder which involves several organ systems and is the primary modifiable risk factor for heart disease. Hypertension is often termed the “silent killer” because many people with high blood pressure (BP) do not know they have the disease until it has progressed. Uncontrolled high BP leads to numerous complications including, but not limited to, heart attack, stroke, aneurysm, heart failure, renal failure, and dementia. The first goal of this article is to review the historical clinical trial data and hypertension guidelines from the perspective of both genders.
The earliest studies linking uncontrolled hypertension to premature morbidity and mortality were performed in the 1960s, prior to which hypertension was viewed as a natural consequence of the aging process.2 The VA Cooperative Study phase 1 on hypertension was initiated in 1964 and was the first randomized controlled trial on hypertension. The study included 143 male veterans with severe hypertension (diastolic BP (DBP) between 115 and 129 mmHg).2 Participants were randomly assigned to placebo or treatment groups with follow-up at 1.5 years; treatment included reserpine, hydrochlorothiazide, and hydralazine. The primary finding was antihypertensive treatment reduced cardiovascular(CV)/morbid events and overall mortality: 4 deaths and 27 morbid events with placebo compared with 0 deaths and 2 morbid events with treatment. The VA Cooperative Study phase 2 also started recruiting patients in 1964 and included 380 men with less severe hypertension (DBP between 90 and 114) with follow-up of ~4 years.3 Consistent with phase 1 results, patients receiving antihypertensive medications exhibited significant decreases in mortality and morbid events: 19 deaths and 56 morbid events with placebo compared with 8 deaths and 22 morbid events with treatment. It is now well established that there is a direct relationship between BP and CV disease (CVD) where every 20 mm Hg increase in systolic BP (SBP) or 10 mmHg increase in DBP doubles the chances of developing CVD.4
We have made tremendous strides in our understanding of hypertension since the 1960s and developed a clinical arsenal to treat high BP. As a result, clinical trials turned to the question of which treatment is the most effective. Treatment of Mild Hypertension Study (TOMHS) was published in 1993 and included men and women, aged 45–69 years, with DBP less than 100 mmHg. Results indicated that a diuretic, β blocker, α1 adrenergic blocker, calcium channel blocker (CCB), and angiotensin-converting enzyme (ACE) inhibitor reduced SBP and DBP similarly.5 Antihypertensive and Lipid-Lowering Treatment to prevent Heart Attack Trial (ALLHAT) was the largest clinical trial up to this point and was designed to compare the overall effectiveness of a CCB, ACE inhibitor, and α-adrenergic blocker to the standard of care at the time, a diuretic. The trial was initiated in 1994 and ended in 2002 with 42,000 men and women aged 55 and older enrolled. ALLHAT found that the “newer drugs” were inferior for controlling high BP and lowering the risk of CVD compared with the diuretic. As a result of this trial, diuretics were concluded to be the first choice for treating hypertension.6 Consistent with this finding, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) published in 2003 recommended thiazide-type diuretics as the initial therapy in “most people with hypertension.”7
The majority of hypertensive patients require 2 or more antihypertensive medications to achieve BP control to recommended levels.7–10 Based on JNC 7 guidelines, combination treatments typically included a diuretic, but the effectiveness of additional antihypertensive combinations had not been directly assessed. The Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial was a randomized, double-blind trial including 11,506 men and women with hypertension at high risk for CV events. The trial was designed to compare the effectiveness of an ACE inhibitor combined with a CCB to the same ACE inhibitor combined with a thiazide diuretic.11 The trial was terminated early due to the superior outcomes in the individuals randomized to the ACE inhibitor and CCB combination; the results were published in 2008. In 2014, the JNC 8 guidelines were published, and the recommended first line of treatment for “most people with hypertension” remained a thiazide diuretic, CCB, ACE inhibitor, or angiotensin receptor blocker (ARB).12 These recommendations were based on a wide range of studies indicating similar risk profiles for mortality and outcome occurrences among all of these classes of drugs.
The most recent advance in our understanding of the control and treatment of hypertension comes from the Systolic Blood Pressure Intervention Trial (SPRINT) released in 2015, which found that more aggressive BP control results in improved health outcomes.13 SPRINT was a randomized clinical trial of 9,361 men and women aged 50 years or older. Participants were randomized to an intensive treatment group (SBP target of less than 120 mm Hg) or standard treatment group (SBP target of less than 140 mm Hg). The intensive treatment group had a ~25% greater reduction in CV events compared with those on standard treatment, supporting the benefit of aggressive BP control.13 Intensive BP control also decreased: risk of CV-related death by 43%, risk of heart failure by 38%, and risk of death by 27% compared with standard treatment.
The “Guidelines for the Prevention, Detection, Evaluation, and Management of High BP in Adults” was released in 2017. Based on these guidelines, initial first-line pharmacological therapies for hypertension remain consistent with the JNC 8 recommendations of thiazide diuretics, with CCB, and ACE inhibitors or ARBs as alternatives. The most significant change to our understanding of hypertension, however, was a reduction in the threshold SBP and DBP required to be considered hypertensive. In large part due to the outcomes of SPRINT, the 2017 Hypertension Guidelines redefined hypertension as SBP above 130 mm Hg and DBP above 80 mm Hg.14 As a result, the number of individuals classified as hypertensive dramatically increased. Applying this definition to the National Health and Nutrition Examination Survey (NHANES) data collected between 2011 and 2014, 46% of adults over the age of 20 in the United States have hypertension, which resulted in a 14% increase compared with 32% of hypertensive adults under the previous guidelines.14
Prevalence of hypertension: does gender matter?
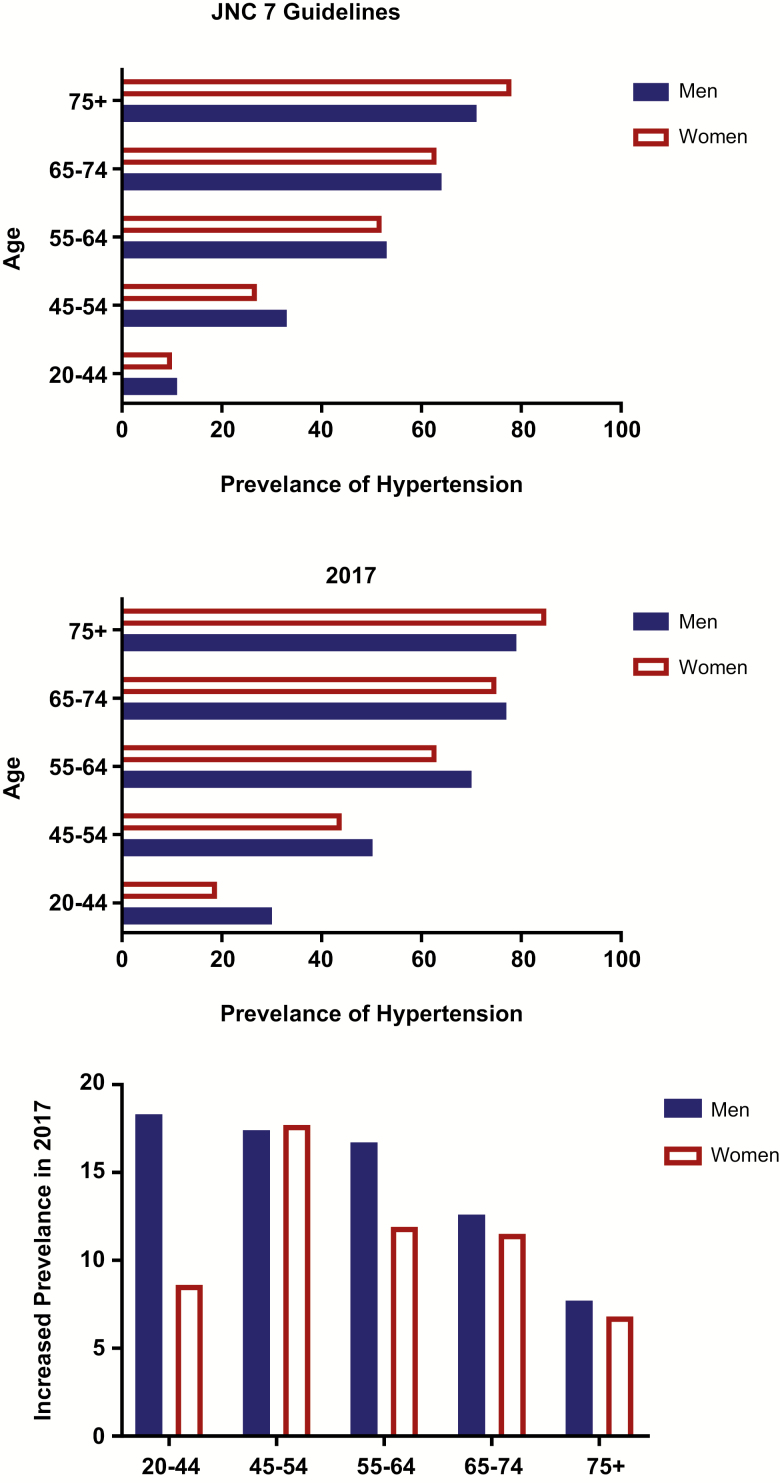
The 2017 Hypertension Guidelines resulted in an immediate and robust increase in the prevalence of hypertension among both men and women in the United States across all age groups. Gender differences in BP were first reported in 1947 in university students where men had consistently higher BPs compared with women.15 It became well established that men had a higher prevalence of hypertension compared with women prior to the onset of menopause, after which women displayed a more rapid increase in the prevalence of hypertension relative to men such that hypertension rates in women eventually exceed that seen in men. This is reflected in the prevalence of hypertension as defined by the JNC 7/8 Guidelines using the NHANES BP data from 2011–2014. Using these definitions of hypertension, 34% of adults over the age of 20 in the United States were defined as hypertensive, and this included 34.5% of men (40.8 million males) and 33.4% of women (44.9 million females). As a result, women accounted for ~51% of the hypertensive population. When examined by age, more men had hypertension from ages 45–54, the prevalence of hypertension was comparable between the genders from ages 20 to 44 and from 55 until 74, but by age 75, more women were hypertensive (Figure 1).
Figure 1.
Prevalence of hypertension among men and women across adulthood. Data are adapted from the US Centers for Disease Control and Prevention/National Center for Health Statistics, National Health and Nutrition Examination Survey, 2011–2014 based on the definition of hypertension as 140/90 mm Hg according to the JNC guidelines (top) and the 2014 Hypertension guidelines using 130/80 mm Hg as hypertensive (middle). The bottom panel is the calculated increase in the prevalence of hypertension based on the newer guidelines. Abbreviation: JNC, Joint National Committee.
Interestingly, when the 2017 Hypertension Guidelines are applied to this same BP data set, the gender difference in the prevalence of hypertension was exacerbated. There is now a more pronounced gender difference where men have a much greater prevalence of hypertension from 20 until 65 years of age where the gap between the genders is narrowed, yet more women over age 75 remain hypertensive. This is driven by much greater increases in the prevalence of hypertension among men when compared with women, suggesting a historically larger number of men with prehypertension (as defined in JNC 7 as SBP 120–139 or DBP 80–89). Indeed, when the prevalence of individuals with prehypertension was examined in 10,380 people from the NHANES data from 1999 to 2006, 36.3% of otherwise healthy adults were prehypertensive and men had a much higher prevalence of prehypertension compared with women (45% vs. 27%).16 This finding is further supported by a recent meta-analysis of 250,741 individuals (120,605 men and 130,136 women) from 13 countries17 where the overall prevalence of prehypertension was 36%, and the pooled prevalence of prehypertension was 40% among men vs. 33% among women.17 Regardless, these data re-establish the notion that young women (20–44) are relatively protected from developing hypertension compared with their male counterparts. It should not be ignored, however, that even at young ages (20–44), 20% of women are hypertensive, and this number is only expected to increase. As a result, gaining better understanding of BP control among women should remain a priority.
Hypertension from her point of view
Based on established gender differences in numerous aspects of CV physiology and pharmacology as discussed elsewhere,18–20 it seems appropriate to ask whether the conclusions drawn in the clinical studies above are equally applicable to both men and women. All of the clinical trial data discussed above were collected in either males alone (VA Studies) or in both sexes; however, the data were not presented separated by sex in the initial publications of the clinical trial results. As a result, it is difficult to draw sex-specific conclusions regarding the impact of (i) hypertension on CV outcomes in women and (ii) the relative effectiveness of antihypertensive agents in controlling BP and reducing CVD risk in women. Furthermore, it is relevant to note that in the clinical trials discussed above that included both men and women, the average age of the participants was 67 (ALLHAT) to 68 (ACCOMPLISH, SPRINT) years of age. At this age, the majority of both men and women are hypertensive, and women are beginning to overtake men in the prevalence of hypertension, yet in the trials that included women, they represented less than 50% of the participants. In addition, BP control rates in women are lowest over the age of 60.21 Therefore, understanding how women respond to antihypertensive medications is critically important.
ALLHAT was the first clinical trial to compare the effects of nondiuretic-based antihypertensive therapy among women; 47% of participants in ALLHAT were women.22 A subgroup analysis examined mortality in 17,411 men and 15,393 women and morbidity in 9,537 men and 12,086 women. When the data were examined separately by sex, no differences in CVD outcomes by sex were detected, thereby supporting the use of diuretics as the first-line defense for both genders in the treatment of hypertension. Although 40% of enrolled participants in ACCOMPLISH and 38% of enrolled participants on TOMHS were women, the BP data have not been analyzed separately by gender as far as we are aware. A review of the literature up to 2006 identified 59 randomized controlled studies treating mild to moderate hypertension in women for at least 6 months of duration.23 From the review of this literature, the authors concluded that “evidence from trials of antihypertensive treatment benefits specific to women is weak, but in studies where the analysis was adjusted for gender, the results appear to be similar for women and men.” However, it should be noted that none of these studies were designed or powered to specifically assess treatment effectiveness between the sexes.
Based on the far-reaching implications for SPRINT in defining a “safe” level for BP control, it may not be surprising that the implications for SPRINT to women have been considered. Despite women accounting for approximately half of the hypertensive population, only 36% of enrolled participants in SPRINT were women13; potential reasons behind this low enrollment and the implications are discussed elsewhere.24 Although SPRINT planned to determine optimal BP management in both sexes, there were only 77 women compared with 166 men included in the intensive treatment group and 89 women compared with the 230 men in the standard treatment group. The incidence of primary outcomes and all-cause mortality was lower in women when compared with men, regardless of treatment, suggesting that women were lower risk when enrolled in the study.24 Moreover, the study was terminated early due to significantly better treatment outcomes in men receiving the intensive treatment. As a result, conclusions drawn from SPRINT are primarily based on the results among the men, and inadequacy of power in the female cohort prevents drawing strong conclusions about intensive treatment effectiveness in women. In response to these shortcomings from the female perspective, several studies have made attempts to analyze the SPRINT data to investigate both potential sex differences between the standard and intensive care groups and the relative effectiveness of more intensive BP control among women. It should be noted though that the relatively low numbers of women included in the trial prevent strong conclusions regarding women to be drawn.
Foy et al. reported in a gender-based analysis that women in SPRINT had significantly higher SBP than the men, although they tended to be healthier with lower CV risk at enrollment.25 Among both genders, the intensive treatment groups had lower SBP than those randomized to standard treatment 6 months into the study, and SBP in the intensive groups remained lower throughout the study. Moreover, treatment resulted in similar BP values between men and women at the 3-year assessment in both treatment groups, suggesting comparable effectiveness in the ability to lower BP although significance was not reached in the women. The effect of treatment on primary CVD outcomes was also consistent between men and women, supporting a cardioprotective role of more intensive BP control among both genders. In addition, there were no differences in overall serious adverse events by randomized group either in women or in men, and there was not a gender by treatment–group interaction. It was therefore concluded that the intensive treatment was effective in both men and women over 3 years of follow-up.
In a separate study by Ochoa-Jimene et al., the patient-level data from SPRINT were analyzed to determine whether BP treatment effects differed by gender in participants less than 60 or over 6026 in propensity score-matched populations. To account for the difference in baseline CV risk among men and women in SPRINT, propensity score matching was used to take into account this potentially confounding variable that can bias an outcome to control for baseline differences. When the effectiveness of standard vs. intensive therapy was compared among all participants, intensive treatment was clearly beneficial in regards to the primary outcome among men (P < 0.0001). While the intensive treatment also tended to be more beneficial among women, this did not reach statistical significance (P = 0.206). When participants were then stratified by age, the benefits of the intensive treatment did not reach significance in either men (P = 0.09) or women (P = 0.43) less than 60 years old, although after 30 months of treatment, men in the intensive treatment group had a pronounced improvement in primary outcomes. When men and women 60 and over were examined, the intensive treatment was significantly better than standard treatment only in men (P = 0.003). Although significance was again not achieved in women, the older women in the intensive treatment group had consistently lower probability of primary outcomes relative to those in the standard treatment group. Indeed, among all groups, there was a shift toward improved primary outcomes with intensive treatment compared with standard treatment; the differences were in the degree of shift.
The authors in this later study suggested that the lack of a significant difference between the standard and intensive treatment groups among women may suggest that men experience a greater CV benefit from a given decrease in BP compared with women. However, when absolute and relative risks associated with conventional and ambulatory BP measurements and CV complications were examined in men (n = 4,960) and women (n = 4,397), women exhibited a greater risk of CV events with increasing BP and had a higher proportion of potentially preventable events compared with men, despite having a lower overall CV risk.27 In an additional study designed to derive diagnostic thresholds for the awake and asleep SBP and DBP means based upon CVD outcome in men vs. women, men again exhibited greater CVD event rates than women, yet the relationship between ambulatory BP and CVD risk remained steeper in women than men.28 The authors concluded that the outcome-based reference thresholds for men were 135/85mm Hg for the awake and 120/70mm Hg for the asleep SBP/DBP means compared with threshold values of 125/80mm Hg for the awake and 110/65mm Hg for the asleep SBP/DBP for women. Together, these findings suggest that women would be expected to actually have greater CV benefits from more intensive decreases in BP, yet this does not appear to be supported by the results of SPRINT where men clearly benefitted more from the intensive treatment relative to women.
Regardless, the data question the current guidelines recommending the same level of BP control in men and women and underscores the importance of continued research to better understand the impact of more aggressive BP control in hypertensive women across the lifespan. The average age of the women included in the studies directly comparing the impact of BP on CVD outcomes was ~53, yet this is not the age with the highest prevalence of hypertension among women, and therefore, these are not women most at risk for increased CV morbidity and mortality. There have been no randomized controlled trials powered to assess CV morbidity and BP outcomes in hypertension specifically in women, and as a result, we still do not know enough about the protective effects of lowering BP in at-risk women.
Mechanisms of BP control: linking the RAS to inflammation
With 46% of the population now defined as hypertensive and the prevalence of hypertension expected to increase, there is more interest than even in better understanding the molecular mechanisms regulating BP control in both sexes. Drugs targeting the renin–angiotensin–aldosterone system are widely used in the treatment of hypertension, in large part through the efforts and advances made by John Laragh.29,30 Dr Laragh discovered the central role of renin in contributing to increases in BP and the associated end-organ damage in hypertension.31–33 This work provided the foundation and rationale for the development of 2 classes of antihypertensive drugs that remain a first-line defense for the treatment of hypertension, ACE inhibitors, and ARBs.
While both hypertensive men and women are routinely treated with ACE inhibitors and ARBs, there is an expansive literature describing sex and gender differences in responses to both the activation and inhibition of the renin–angiotensin system (RAS) that have recently been reviewed elsewhere.18,34–36 In general, males have greater expression levels and physiological responses to activation of the classical RAS (Ang II, angiotensin II type 1 receptor (AT1R), ACE), while females have greater expression and physiological responses to activation of the nonclassical RAS (Ang (1–7), angiotensin II type 2 receptor (AT2R), Mas receptor, ACE2). Our goal with the remainder of this review is to assess the basic science literature describing sex differences in the RAS and how these differences may ultimately underlie sex differences in BP control.
As defined by The Institute of Medicine Committee on Understanding the Biology of Sex and Gender Differences,37 “sex” refers to classification based on reproductive organs and functions assigned by the chromosomal complement, whereas “gender” includes the individual’s self-representation and presentation as male or female based on socially constructed characteristics. Therefore, clinical studies above referred to gender differences where participants self-reported their gender, while basic science studies will refer to sex differences based on phenotype alone.
There are sex differences in the RAS in hypertension
Angiotensinogen is synthesized in the liver, and renin converts angiotensinogen into angiotensin I (Ang I).38 ACE 1 further cleaves Ang I to Ang II, which can then bind to 1 of 2 receptors: AT1R or AT2R. When bound to AT1R, Ang II constricts blood vessels, increases sodium reabsorption, and leads to increases in BP. In contrast, AT2R-bound Ang II triggers vasodilation and natriuresis. Angiotensin (1–7) is a vasodilatory peptide of the RAS which binds to the Mas receptor to increase nitric oxide (NO) production, trigger vasodilation, and induce natriuresis.39
Work from our group and others have shown that male spontaneously hypertensive rats (SHR) have greater AT1R mRNA and protein expression in the vasculature and kidney, while females have greater AT2R expression.40,41 Female SHR also have greater renal Ang (1–7) levels compared with males, although renal Ang II levels are comparable between the sexes.41 Sex differences in the expression levels of RAS components in hypertension are paralleled by sex differences in CV responses. Greater AT1R expression in male SHR vs. greater AT2R expression in females translates into enhanced constriction to Ang II in isolated aorta and mesenteric microvessels from males.40 Hypertensive male SHR are also more sensitive to chronic and acute Ang II-induced increases in BP,41,42 and greater increases in BP with chronic Ang II infusion in male SHR are mediated, in part, by greater Ang II-induced increases in oxidative stress. In contrast, enhanced Ang (1–7) production in females attenuates Ang II-induced increases in BP,41,43 and increased Ang (1–7) in female SHR during treatment with an ARB contributes to the BP-lowering effect of the ARB only in females.44
Role of the kidney and immune cells in sex differences in Ang II hypertension
The kidney is known to play a central role in the control of BP, in particular Ang II-induced increases in BP. This was elegantly shown using a kidney cross-transplantation strategy to separate the actions of systemic and renal AT1AR.45 The authors found that renal AT1AR were primarily responsible for mediating the hypertensive actions of Ang II in male mice. Interestingly, the kidney is also central to mediating sex differences in the BP response to Ang II. Using cross-sex renal transplantation studies in C57BL/6 mice, it was found that Ang II-induced increases in BP in male mice were attenuated following transplant of a female, but not a male, kidney, while females receiving a male kidney exhibited a more robust increase in BP.46 The enhanced BP response to Ang II in the female mouse receiving a male kidney was associated with an increase in pro-inflammatory cytokines. Based on the growing body of literature linking immune cell activation to the development of Ang II hypertension, it is tempting to speculate that sex differences in inflammation and the immune system may underlie sex differences in Ang II hypertension.
Adaptive immune cells, more specifically T cells, are well established to contribute to experimental hypertension in males (see recent reviews).47–49 In a seminal study by Guzik et al.,50 it was found that RAG-1−/− male mice which lack T and B cells have a blunted increase in BP to Ang II that is restored after adoptive transfer of T cells. Later studies that included both male and female RAG-1−/− mice yielded a number of interesting findings. First, the sex difference in the BP response to Ang II observed in wild-type mice is absent in Rag1−/− mice,51,52 suggesting a critical role for T cells in mediating sex differences in the BP response to Ang II. Second, adoptive transfer of CD3+ T cells from a male donor only restores Ang II hypertensive responses to male recipients; female RAG1−/− receiving T cells from a male donor do not display increases in BP.51 Third, adoptive transfer of T cells from a female donor into a male RAG-1−/− mouse failed to restore Ang II-induced hypertension.52 These data suggest that sex of both the donor and recipient is critical determinant of the BP response to Ang II.
Based on the central role of the kidney in mediating Ang II hypertension as discussed above, the renal T-cell profile was examined in RAG-1−/− mice assessing the impact of sex of the donor and sex of the T cell on Ang II hypertension. Although Ang II administration did not alter the infiltration of T cells into the kidney of either male or female RAG-1−/− mice following adoptive transfer of T cells from a male donor, consistent with greater increases in BP, males exhibited increases in pro-inflammatory cytokines (interleukin (IL)-2, tumor necrosis factor-α, and monocyte chemoattractant protein 1).51 Neither T cells nor cytokines were increased in females following adoptive transfer of T cells from a male donor and Ang II infusion, although, since the female mice did not display an increase in BP, this finding may not be surprising. Similarly, there were no differences in renal infiltration of T cells in male RAG-1−/− regardless of the sex of the donor T cell for adoptive transfer.52 However, male RAG-1−/− mice that received T cells from a female donor exhibited greater renal mRNA expression of the T regulatory cell (Treg) marker FoxP3 and the anti-inflammatory cytokine IL-10 but also greater expression of the pro-inflammatory markers IL-2, IL-6, IL-17A, IFN-γ, and monocyte chemoattractant protein 1.
Based on the anti-inflammatory and antihypertensive roles of Tregs, we propose that Tregs contribute to the blunted increase in BP with Ang II infusion when females served as the T-cell donor. Adoptive transfer of Tregs blunts Ang II-induced increases in BP and attenuates vascular and renal end-organ injury in male mice.53–59 Our group has examined the renal T-cell profile in hypertensive males and females. Male SHR have greater renal Th17 cell infiltration, while female SHR have greater Tregs.60–63 Greater Tregs in female SHR is dependent on increases in BP with age,61,63 suggesting this increase is a compensatory mechanism that contributes to the lower BP in young adult female SHR compared with males.64 In addition, Ang II-induced increases in BP in male and female Sprague-Dawley rats result in greater increases in Tregs in the females compared with the males.65
CONCLUSIONS
A 2010 meta-analysis examining the representation of women in randomized clinical trials for CVD prevention reported that representation of women in trials for hypertension at that time was 44%, which was increased from inclusion rates of 34% in 2006, yet still below the percentages reflected in the general population.66 Disappointingly, inclusion rates in SPRINT were well below this historical average, and we remain unable to draw strong gender-specific conclusion regarding the impact of BP control on overall CV health in women.
At the same time, however, there is an increase in the numbers of basic science studies that incorporate both sexes, and we are slowly advancing our understanding of the mechanisms underlying hypertension in both sexes. Recent studies examining immune cell activation and the role of T cells in BP control have the potential to (i) provide key insight into how persistent overactivation of the RAS leads to hypertension and (ii) explain the basis underlying sex differences in BP control where activation of the RAS preferentially increases Tregs in the female which contributes to the delayed increase in BP. More clinical and basic science studies are needed however to better understand BP control in women and determine optimal treatment. The last “new” class of antihypertensive medications to become a first-line treatment was ARBs and that was decades ago. It is tempting to speculate on the potential of immunotherapy to selectively increase Tregs to lower BP and protect against end-organ damage, particularly among women.
ACKNOWLEDGMENT
This work was funded by the National Institutes of Health (R0 HL127091 and P01HL134604 to J.C.S.) the American Heart Association (17EIA33410565 to J.C.S).
DISCLOSURE
The authors declared no conflicts of interest.
REFERENCES
- 1. Arias E, Heron M, Xu J.. United States life tables. Natl Vital Stat Rep 2013; 66:64. [PubMed] [Google Scholar]
- 2. Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967; 202:1028–1034. [PubMed] [Google Scholar]
- 3. Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects morbidity of treatment on hypertension: II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA 1970; 213:1143–1152. [PubMed] [Google Scholar]
- 4. McConnell KJ. 2017 ACC/AHA guidelines for BP management in adult patients. Pharm Today 2018; 24:57–73. [Google Scholar]
- 5. Neaton JD, Grimm RH Jr, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, Cutler JA, Flack JM, Schoenberger JA, McDonald R. Treatment of mild hypertension study. Final results. Treatment of mild hypertension study research group. JAMA 1993; 270:713–724. [PubMed] [Google Scholar]
- 6. The ALLHAT officers and coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 7. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 8. Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT Jr, Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM; ALLHAT Collaborative Research Group . Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich) 2002; 4:393–404. [DOI] [PubMed] [Google Scholar]
- 9. Black HR, Elliott WJ, Neaton JD, Grandits G, Grambsch P, Grimm RH Jr, Hansson L, Lacoucière Y, Muller J, Sleight P, Weber MA, White WB, Williams G, Wittes J, Zanchetti A, Fakouhi TD, Anders RJ. Baseline characteristics and early blood pressure control in the CONVINCE trial. Hypertension 2001; 37:12–18. [DOI] [PubMed] [Google Scholar]
- 10. Webster R, Salam A, de Silva HA, Selak V, Stepien S, Rajapakse S, Amarasekara S, Amarasena N, Billot L, de Silva AP, Fernando M, Guggilla R, Jan S, Jayawardena J, Maulik PK, Mendis S, Mendis S, Munasinghe J, Naik N, Prabhakaran D, Ranasinghe G, Thom S, Tisserra N, Senaratne V, Wijekoon S, Wijeyasingam S, Rodgers A, Patel A; TRIUMPH Study Group . Fixed low-dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: a randomized clinical trial. JAMA 2018; 320:566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ; ACCOMPLISH Trial Investigators . Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 12. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 13. The SPRINT research group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 15. Boynton RE, Todd RL. Blood pressure readings of 75,258 university students. Arch Intern Med (Chic) 1947; 80:454–462. [DOI] [PubMed] [Google Scholar]
- 16. Gupta AK, McGlone M, Greenway FL, Johnson WD. Prehypertension in disease-free adults: a marker for an adverse cardiometabolic risk profile. Hypertens Res 2010; 33:905–910. [DOI] [PubMed] [Google Scholar]
- 17. Guo X, Zou L, Zhang X, Li J, Zheng L, Sun Z, Hu J, Wong ND, Sun Y. Prehypertension: a meta-analysis of the epidemiology, risk factors, and predictors of progression. Tex Heart Inst J 2011; 38:643–652. [PMC free article] [PubMed] [Google Scholar]
- 18. Gillis EE, Sullivan JC. Sex differences in hypertension: recent advances. Hypertension 2016; 68:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Putignano D, Bruzzese D, Orlando V, Fiorentino D, Tettamanti A, Menditto E. Differences in drug use between men and women: an Italian cross sectional study. BMC Womens Health 2017; 17:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cui C, Huang C, Liu K, Xu G, Yang J, Zhou Y, Feng Y, Kararigas G, Geng B, Cui Q. Large-scale in silico identification of drugs exerting sex-specific effects in the heart. J Transl Med 2018; 16:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoon SS, Fryar CD, Carrol MD. Hypertension prevalence and control among adults: United States, 2011-2014. NCHS Data Brief 2015; 220:1–8. [PubMed] [Google Scholar]
- 22. Oparil S, Davis BR, Cushman WC, Ford CE, Furberg CD, Habib GB, Haywood J, Margolis K, Probstfield JL, Whelton PK, and Wright JT Jr, for the ALLHAT Collaborative Research Group . Mortality and morbidity during and after ALLHAT: results by gender. Hypertension 2013; 61:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ljungman C, Mortensen L, Kahan T, Manhem K. Treatment of mild to moderate hypertension by gender perspective: a systematic review. J Womens Health (Larchmt) 2009; 18:1049–1062. [DOI] [PubMed] [Google Scholar]
- 24. Wenger NK, Ferdinand KC, Bairey Merz CN, Walsh MN, Gulati M, Pepine CJ; American College of Cardiology Cardiovascular Disease in Women Committee . Women, hypertension, and the Systolic Blood Pressure Intervention Trial. Am J Med 2016; 129:1030–1036. [DOI] [PubMed] [Google Scholar]
- 25. Foy CG, Lovato LC, Vitolins MZ, Bates JT, Campbell R, Cushman WC, Glasser SP, Gillespie A, Kostis WJ, Krousel-Wood M, Muhlestein JB, Oparil S, Osei K, Pisoni R, Segal MS, Wiggers A, Johnson KC; SPRINT Study Research Group . Gender, blood pressure, and cardiovascular and renal outcomes in adults with hypertension from the Systolic Blood Pressure Intervention Trial. J Hypertens 2018; 36:904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ochoa-Jimenez R, Viquez-Beita K, Daluwatte C, Zusterzeel R. Sex differences of patients with systemic hypertension (From the analysis of the Systolic Blood Pressure Intervention Trial [SPRINT]). Am J Cardiol 2018; 122:985–993. [DOI] [PubMed] [Google Scholar]
- 27. Boggia J, Thijs L, Hansen TW, Li Y, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Olszanecka A, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Maestre G, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA; International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes Investigators . Ambulatory blood pressure monitoring in 9357 subjects from 11 populations highlights missed opportunities for cardiovascular prevention in women. Hypertension 2011; 57:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hermida RC, Ayala DE, Mojón A, Fontao MJ, Chayán L, Fernández JR. Differences between men and women in ambulatory blood pressure thresholds for diagnosis of hypertension based on cardiovascular outcomes. Chronobiol Int 2013; 30:221–232. [DOI] [PubMed] [Google Scholar]
- 29. Sealey JE. John H. Laragh, MD: clinician-scientist. Am J Hypertens 2014; 27:1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sealey-Laragh J. John H. laragh MD: the man. Am J Hypertens 2015; 28:955–957. [DOI] [PubMed] [Google Scholar]
- 31. Case DB, Atlas SA, Laragh JH, Sealey JE, Sullivan PA, McKinstry DN. Clinical experience with blockade of the renin-angiotensin-aldosterone system by an oral converting-enzyme inhibitor (SQ 14,225, captopril) in hypertensive patients. Prog Cardiovasc Dis 1978; 21:195–206. [DOI] [PubMed] [Google Scholar]
- 32. Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, Bard RH, Bühler FR. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med 1972; 286:441–449. [DOI] [PubMed] [Google Scholar]
- 33. Case DB, Wallace JM, Keim HJ, Weber MA, Sealey JE, Laragh JH. Possible role of renin in hypertension as suggested by renin-sodium profiling and inhibition of converting enzyme. N Engl J Med 1977; 296:641–646. [DOI] [PubMed] [Google Scholar]
- 34. Vivas L, Dadam FM, Caeiro XE. Sex differences in body fluid homeostasis: sex chromosome complement influences on bradycardic baroreflex response and sodium depletion induced neural activity. Physiol Behav 2015; 152:416–421. [DOI] [PubMed] [Google Scholar]
- 35. Leete J, Gurley S, Layton AT. Modeling sex differences in the renin angiotensin system and the efficacy of antihypertensive therapies. Comput Chem Eng 2018; 112:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sandberg K, Ji H. Sex and the renin angiotensin system: implications for gender differences in the progression of kidney disease. Adv Ren Replace Ther 2003; 10:15–23. [DOI] [PubMed] [Google Scholar]
- 37. Task Force on Women’s Health Issues. Report of the Public Health Service Task Force on Women’s Health Issues. Public Health Rep 1985; 100:73–106. [PMC free article] [PubMed] [Google Scholar]
- 38. Lu H, Cassis LA, Kooi CW, Daugherty A. Structure and functions of angiotensinogen. Hypertens Res 2016; 39:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bérard E, Niel O, Rubio A. Is the renin-angiotensin system actually hypertensive?Pediatr Nephrol 2014; 29:951–960. [DOI] [PubMed] [Google Scholar]
- 40. Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 2004; 62:587–593. [DOI] [PubMed] [Google Scholar]
- 41. Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1-7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 2010; 56:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elmarakby AA, Bhatia K, Crislip R, Sullivan JC. Hemodynamic responses to acute angiotensin II infusion are exacerbated in male versus female spontaneously hypertensive rats. Physiol Rep 2016;4:e12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bhatia K, Elmarakby AA, El-Remessy AB, El-Remessey A, Sullivan JC. Oxidative stress contributes to sex differences in angiotensin II-mediated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2012; 302:R274–R282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zimmerman MA, Harris RA, Sullivan JC. Female spontaneously hypertensive rats are more dependent on ANG (1-7) to mediate effects of low-dose AT1 receptor blockade than males. Am J Physiol Renal Physiol 2014; 306:F1136–F1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 2006; 103:17985–17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang L, Wang X, Qu HY, Jiang S, Zhang J, Fu L, Buggs J, Pang B, Wei J, Liu R. Role of kidneys in sex differences in angiotensin II-induced hypertension. Hypertension 2017; 70:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caillon A, Paradis P, Schiffrin EL. Role of immune cells in hypertension. Br J Pharmacol 2018; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med 2018; 215:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crislip GR, Sullivan JC. T-cell involvement in sex differences in blood pressure control. Clin Sci (Lond) 2016; 130:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 2014; 64:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 2014; 64:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mian MO, Barhoumi T, Briet M, Paradis P, Schiffrin EL. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J Hypertens 2016; 34: 97–108. [DOI] [PubMed] [Google Scholar]
- 54. Fabbiano S, Menacho-Márquez M, Robles-Valero J, Pericacho M, Matesanz-Marín A, García-Macías C, Sevilla MA, Montero MJ, Alarcón B, López-Novoa JM, Martín P, Bustelo XR. Immunosuppression-independent role of regulatory T cells against hypertension-driven renal dysfunctions. Mol Cell Biol 2015; 35:3528–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou Y, Wu W, Lindholt JS, Sukhova GK, Libby P, Yu X, Shi GP. Regulatory T cells in human and angiotensin II-induced mouse abdominal aortic aneurysms. Cardiovasc Res 2015; 107:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T Regulatory lymphocytes prevent angiotensin II–induced hypertension and vascular injury. Hypertension 2011; 57:469–476. [DOI] [PubMed] [Google Scholar]
- 57. Matrougui K, Abd Elmageed Z, Zakaria AE, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol 2011; 178:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 2009; 119:2904–2912. [DOI] [PubMed] [Google Scholar]
- 59. Chen XH, Ruan CC, Ge Q, Ma Y, Xu JZ, Zhang ZB, Lin JR, Chen DR, Zhu DL, Gao PJ. Deficiency of complement C3a and C5a receptors prevents angiotensin ii-induced hypertension via regulatory T cells. Circ Res 2018; 122:970–983. [DOI] [PubMed] [Google Scholar]
- 60. Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol 2012; 303: R359–R367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 2014; 64:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brinson KN, Elmarakby AA, Tipton AJ, Crislip GR, Yamamoto T, Baban B, Sullivan JC. Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. Am J Physiol Regul Integr Comp Physiol 2013; 305:R701–R710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sullivan JC, Gillis EE. Sex and gender differences in hypertensive kidney injury. Am J Physiol Renal Physiol 2017; 313:F1009–F1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2007; 293:R1573–R1579. [DOI] [PubMed] [Google Scholar]
- 65. Zimmerman MA, Baban B, Tipton AJ, O’Connor PM, Sullivan JC. Chronic ANG II infusion induces sex-specific increases in renal T cells in Sprague-Dawley rats. Am J Physiol Renal Physiol 2015; 308:F706–F712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, Dolor RJ, Douglas PS, Mark DB, Newby LK. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 2010; 3:135–142. [DOI] [PubMed] [Google Scholar]