Chikungunya was recently introduced into the Americas. Relatively little data exist about the introduction of chikungunya into naive populations. This study examines differences in transmission intensity and clinical severity between 2 epidemics of chikungunya in a single cohort in Nicaragua.
Keywords: chikungunya, Nicaragua, cohort study, attack rate, epidemic
Abstract
Background
Chikungunya, an arboviral disease, caused massive epidemics in Central and South America in 2014–2016. In a prospective pediatric cohort study, we examined the introduction of chikungunya in a naive population and investigated transmission and clinical characteristics.
Methods
Children presenting to the study health center with a chikungunya-like illness or undifferentiated fever were tested for chikungunya virus (CHIKV) infection by reverse transcriptase-polymerase chain reaction (RT-PCR) and serological assays. Inapparent CHIKV infections in the intervening year were determined by seroconversion in healthy blood samples collected annually.
Results
A total of 4353 children participated in the cohort study from March 2014 to February 2016 during the 2 epidemic waves of chikungunya. A total of 539 cases of chikungunya were documented, for an incidence rate of 80.2 cases per 1000 person-years (95% confidence interval [CI]: 73.7, 87.2); and a total of 893 CHIKV infections were documented, for an incidence rate of 137.1 infections per 1000 person-years (95% CI: 128.4, 146.4). The seroprevalence of anti-CHIKV antibodies increased linearly with age, with seroprevalence of >45% in 14-year-old children at the end of Epidemic 2. Symptom presentation varied between the epidemics, with Epidemic 2 exhibiting both a higher symptomatic-to-inapparent ratio (1:1.20 in Epidemic 1 vs. 1:0.65 in Epidemic 2) and more severe clinical presentation among cases. The mean reproduction number was also greater in Epidemic 2 than in Epidemic 1.
Conclusions
The intensity of transmission and severity of clinical presentation varied between the 2 epidemics, with higher transmission intensity associated with greater disease severity.
Chikungunya is an arboviral disease whose primary vectors are Aedes aegypti and Aedes albopictus, the same mosquitoes that transmit the dengue (DENV) and Zika viruses. In late 2013, chikungunya virus (CHIKV) was introduced into the Caribbean and rapidly spread throughout the region into Central and South America, causing massive epidemics [1]. In Nicaragua, imported cases were reported starting in July 2014, followed by detection of autochthonous transmission in September 2014 [2]. The country then experienced 2 epidemic waves, one in each year: September 2014–February 2015 and July 2015–February 2016.
The rapid spread of CHIKV throughout the region in populations that were previously naive presented a unique opportunity to study the disease and transmission characteristics. A number of studies have reported the introduction of chikungunya into naive, or presumed naive, populations, with many studies reporting high attack rates or seropositivity in single or multiple epidemic waves [3–5]. There are 3 main genotypes of CHIKV existing: Asian, East/Central/South African (ECSA), and West African [6, 7]. The 2013–2015 epidemic in the Americas was due primarily to the introduction of the Asian lineage of CHIKV, although there were a few reports of ECSA genotype viruses in Brazil [8–11].
Chikungunya is an acute disease characterized by high fever, arthralgia, rash, headache, and myalgia. Although chikungunya is not typically life-threatening, it does cause chronic joint pain that may last for weeks to years after the acute symptoms resolve [12]. Chikungunya has a relatively low rate of asymptomatic or pauci-symptomatic infections, with reports varying from 4–28% of infections [13–16]. Fewer data are available on chikungunya in children, but those data indicate that children typically have milder disease symptoms [17] and higher rates of asymptomatic infection than adults [13, 18]. However, atypical, severe manifestations do occur, predominately in those under 2 years of age and in perinatal infections [19–24].
Established in 2004, the Pediatric Dengue Cohort Study (PDCS) is an ongoing prospective community-based study of DENV infection in children aged 2 to 14 years in Managua, Nicaragua. The cohort was extended to include chikungunya in 2014 [25]. Here we report on the 2 epidemic waves of chikungunya in the PDCS and investigate differences between the 2 epidemic seasons following the virgin-soil introduction of CHIKV into Nicaragua.
MATERIALS AND METHODS
Ethics Statement
This study was approved by the Institutional Review Boards at the University of California, Berkeley; the Nicaraguan Ministry of Health; and the University of Michigan. For each participant, written consent was obtained from a parent or guardian; additionally, verbal assent was obtained from children aged 6 years and older.
Study Population and Follow-up
The PDCS is an ongoing prospective cohort study of children 2 to 14 years of age in Managua, Nicaragua. A detailed description of the study design, methods, and population has been published previously [26]. Briefly, the cohort consists of ~3700 active participants who reside in the catchment area of the study health center, the Health Center Sócrates Flores Vivas (HCSFV). Throughout the year, children are enrolled when they reach 2 years of age and are withdrawn when they reach the age of 15. At enrollment, families agree to bring their children to the HCSFV at the first sign of illness, where they are provided with free medical care through study physicians. Data on approximately 80 variables are recorded systematically at each visit. This analysis uses data collected between 1 March 2014 and 29 February 2016. During this period, annual blood samples were collected in March and April of 2014, 2015, and 2016. In addition, for a subset of new enrollees, annual samples taken prior to enrollment were available from an ongoing, overlapping influenza cohort study [27].
Clinical and Laboratory Definitions
Suspected chikungunya cases were defined as those that presented with either (1) undifferentiated fever (ie, fever without a defined focus), or (2) fever or feverishness and ≥2 of the following: headache, muscle ache, joint pain, retro-orbital pain, rash, hemorrhagic manifestations, or leucopenia [28, 29]. Acute (1–5 days post-onset of symptoms) and convalescent (14–21 days) blood samples were collected from participants meeting either of these conditions.
Suspected cases were considered laboratory-confirmed acute chikungunya cases if (1) the acute sample tested positive for CHIKV by real-time RT-PCR [30, 31], (2) seroconversion was detected by immunoglobulin M enzyme-linked immunosorbent assay (IgM ELISA) [32] in paired acute and convalescent samples, or (3) seroconversion or a ≥4-fold increase in titers was detected by Inhibition ELISA [32, 33] in paired samples.
Participants whose paired annual samples demonstrated seroconversion by CHIKV Inhibition ELISA [32, 33], but who were not documented as an acute chikungunya case during the intervening year were considered to have experienced an inapparent CHIKV infection. To our knowledge, no other alphaviruses circulated in the cohort during the study period.
Analysis
Follow-up time was calculated as the amount of time between the start of the study period or enrollment and the end of the study or study withdrawal. For those lost to follow-up, person-years were calculated as the time between enrollment and the last contact with study personnel, plus one-half the time between the last contact and the date recorded as lost to follow-up. Chikungunya cases and CHIKV infections were excluded from contributing person-time following illness or infection, respectively. Analyses of CHIKV infections was limited to participants who contributed a blood sample at the beginning and end of the study year (i.e., March–April of 2014–2015 and/or March–April of 2015–2016). Since the exact timing of CHIKV infection could not always be ascertained, persons who experienced a CHIKV infection in a given year contributed person-time for that entire year. A Poisson distribution was used to calculate 95% confidence intervals (CIs) for the incidence rates. For case incidence analyses, participant age was defined on a weekly basis. For infection analyses, participant age was defined as the age at the start of the annual sample collection. The subset of children with serum samples obtained through the influenza cohort were not under surveillance for symptomatic CHIKV infection prior to their enrollment in the PDCS, and thus were excluded from analyses of cases among infections for periods that did not have paired serum samples obtained through the PDCS. Environmental data was obtained from the Managua Airport Weather Station (USAF-WBAN:787410). Population density was calculated by dividing the total population per neighborhood by the area. Neighborhood population estimates were obtained from the Ministry of Health. Wilcoxon ranksum tests were used to examine differences in neighborhood population density of cases and infections by year. Statistical analyses were performed in STATA, version 12 (StataCorp LP, College Station, TX).
Temporal changes in the transmission potential of infectious diseases are monitored via the effective reproduction number, which is defined as the average number of secondary cases per primary case at calendar time t [34]. Here, we estimated during the early phases of the 2 epidemics of chikungunya using the Generalized-Growth Model (GGM) [35], which characterizes the epidemic growth via 2 parameters: the growth rate (r) and the scaling of growth parameter (p) [36]. The GGM is flexible and can reproduce a range of growth dynamics, from constant incidence (p = 0) to exponential growth (p = 1) [35]. Based on the incidence at calendar time , denoted by , and the discretized probability distribution of the generation interval, denoted by , can be estimated using the renewal equation [34]
The denominator represents the total number of cases that contribute (as primary cases) to generating the number of new cases (as secondary cases) at calendar time [34].
RESULTS
From 1 March 2014 through 29 February 2016, 4353 children participated in the PDCS. Sex and age distributions were similar in both years (Table 1). A total of 2327 children participated for the entire study period. During this period, 2026 children entered or left the cohort: 1098 children were newly enrolled, 543 aged out of the cohort, 303 were withdrawn or lost to follow-up, and 82 both enrolled and were withdrawn or lost to follow-up. Blood samples collected in March and April 2014 from all cohort participants were negative for CHIKV antibodies, indicating that the entire population was CHIKV-naive at that time [33].
Table 1.
Participant Characteristics by Year, Managua, Nicaragua, 2014–2016
| 2014–2015 | 2015–2016 | |
|---|---|---|
| (n = 3837) | (n = 3856) | |
| By sex | ||
| Female | 1929 (50.3) | 1929 (50.0) |
| Male | 1908 (49.7) | 1927 (50.0) |
| By age (years) | ||
| 2–5 | 1355 (35.3) | 1419 (36.8) |
| 6–8 | 866 (22.6) | 894 (23.2) |
| 9–14 | 1616 (42.1) | 1543 (40.0) |
Incidence of Chikungunya Disease
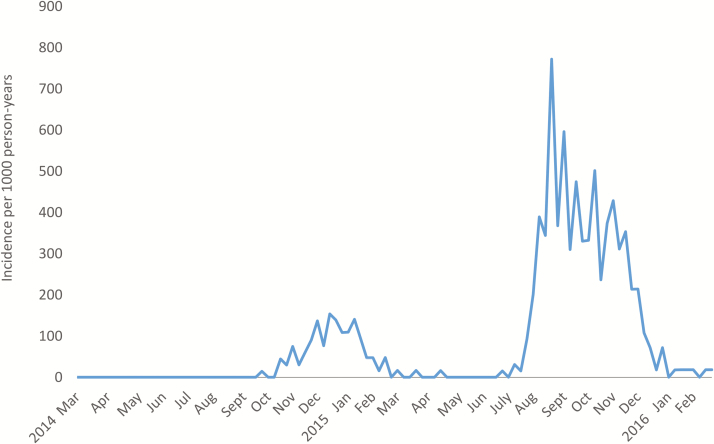
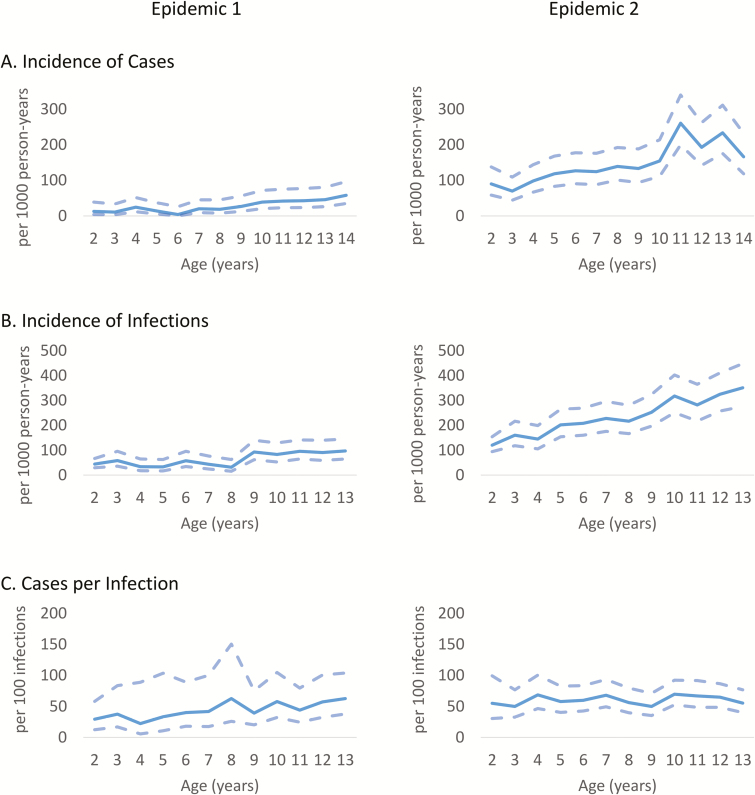
During 2014–2015, CHIKV was introduced into the study population [25, 33]. The first epidemic wave (95 cases) was followed by a second, larger wave in 2015–2016 (444 cases; Figure 1). In 2014–2015, all cases were confirmed by RT-PCR, while in 2015–2016, 438 cases (98.6%) were confirmed by RT-PCR and the rest by serology. The overall incidence of chikungunya disease in the cohort was 81.6 cases per 1000 person-years (95% CI: 75.0, 88.8; Epidemics 1 and 2), with 27.3 cases per 1000 person-years (95% CI: 22.2, 33.3) in 2014–2015 (Epidemic 1) and 142.2 cases per 1000 person-years (95% CI: 129.6, 156.1) in 2015–2016 (Epidemic 2). Incidence of chikungunya disease was significantly associated with age (Figure 2A and Supplementary Table 1).
Figure 1.
Incidence of chikungunya cases in the cohort by study year and month, March 2014–February 2016.
Figure 2.
Age effect on case incidence, infection incidence, and the number of symptomatic cases per infection.
Incidence of Chikungunya Virus Infections and Seroprevalence
Of the 4353 children who participated in the cohort, 1040 provided 1 pair of annual serum samples and 2783 provided 2 pairs, including those obtained through the influenza cohort study, representing 6513 person-years. In total, there were 893 CHIKV infections, yielding an overall incidence of 137.4 CHIKV infections per 1000 person-years (95% CI: 128.7, 146.7). Similar to the case incidence, the incidence of infection was significantly higher in Epidemic 2, at 218.1 infections per 1000 person-years (95% CI: 202.4, 235.0), compared to Epidemic 1, at 61.1 infections per 1000 person-years (95% CI: 53.2, 70.0). The incidence of infection increased significantly with age, with children in the youngest age group having one-half the infection rate of children in the oldest age group (Figure 2B and Supplementary Table 2). However, the relationship between infection and age differed in the 2 epidemics. In Epidemic 1, the incidence of infection increased in a step fashion at age 9, whereas in Epidemic 2, there was a linear relationship between age and infection incidence (R2 = 0.94).
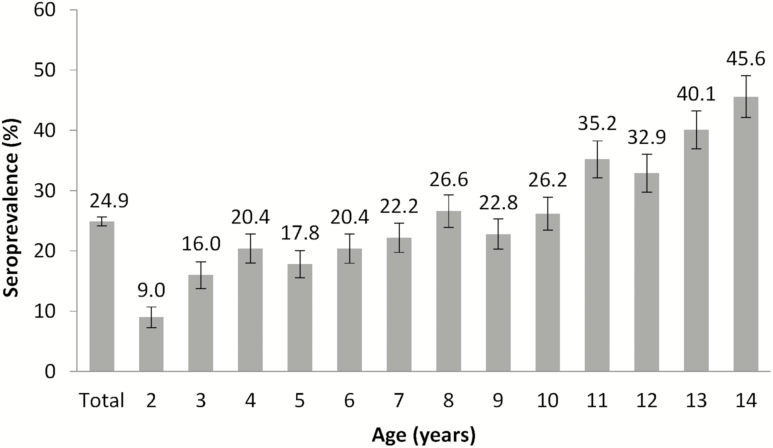
At the end of Epidemic 2, overall seroprevalence of anti-CHIKV antibodies was 24.9% (95% CI: 23.5, 26.4). Seroprevalence increased with age in a linear fashion (Figure 3). Thus, the highest seroprevalence was seen in 14-year-old children (45.6% [95% CI:38.9, 52.5]) and the lowest in 2-year-old children (9.0% [95% CI: 6.1, 13.0]).
Figure 3.
Seroprevalence of anti-chikungunya virus antibodies by age in the cohort in 2016.
Symptomatic Cases among Infections
The rate of symptomatic cases among CHIKV infections was 57.1 cases per 100 infections (95% CI: 52.2, 62.4) for a symptomatic-to-inapparent ratio (S:I ratio) of 1:0.75 (Table 2). The number of chikungunya cases per infection was lower in Epidemic 1, at 45.4 cases per 100 infections (95% CI: 37.0, 55.9), than in Epidemic 2, at 60.6 cases per 100 infections (95% CI: 54.9, 66.9), although the confidence intervals overlapped slightly. Accordingly, the S:I ratio was 1:1.20 in Epidemic 1 and 1:0.65 in Epidemic 2. In a multivariable logistic model, infected children in Epidemic 2 had 1.86 (95% CI: 1.35, 2.57) times the odds of being symptomatic compared to children experiencing an infection in Epidemic 1, when adjusted for age and sex.
Table 2.
Number of Chikungunya Cases per 100 Chikungunya Virus Infections
| All Years (2014–2016) | Epidemic 1 (2014–2015) | Epidemic 2 (2015–2016) | |
|---|---|---|---|
| Overall | 57.1 (52.2, 62.4) | 45.5 (37.0, 55.9) | 60.6 (54.9, 66.9) |
| By sex | |||
| Female | 55.5 (48.8, 63.1) | 42.6 (31.2, 58.0) | 59.2 (51.4, 68.2) |
| Male | 58.6 (51.8, 66.4) | 48.1 (36.4, 63.4) | 62.1 (54.0, 71.3) |
| By age | |||
| 2–5 | 51.5 (42.5, 62.4) | 31.4 (19.2, 51.2) | 58.3 (47.2, 71.8) |
| 6–8 | 58.5 (48.9, 70.0) | 45.7 (28.0, 74.6) | 61.2 (50.5, 74.1) |
| 9–14 | 58.9 (52.2, 66.6) | 51.8 (40.0, 67.0) | 61.4 (53.5, 70.6) |
Severity of Epidemic Waves
To further characterize the differences between the 2 epidemics, the severity of cases was examined. As this is a community-based study with few cases of severe chikungunya, the presence of several systemic symptoms as markers of severity was investigated. In multivariable models, the odds of arthralgia in chikungunya cases in Epidemic 2 was 2.05 (95% CI: 1.12, 3.74) times the odds in chikungunya cases during Epidemic 1, when adjusted for age, sex, and day of presentation (Table 3). Similarly, the adjusted odds of myalgia in chikungunya cases in Epidemic 2 was 2.16 times the odds in chikungunya cases during Epidemic 1. A severity score was constructed by summing the presence of arthralgia, myalgia, measured fever >38°C, and headache. The mean symptom score in Epidemic 1 was 2.64 (standard deviation [SD] 1.19), compared to 2.95 (SD 1.11) in Epidemic 2 (P = .016). In an ordinal logistic regression, in Epidemic 2 the odds of a symptom score of 4 versus a lower symptom score was 1.96 (95% CI: 1.30, 2.94) times greater than in Epidemic 1, with age, sex, and day of presentation held constant. There was no significant difference in day of presentation of cases between Epidemic 1 (1.71 days, SD 0.74) and Epidemic 2 (1.68, SD 0.83; P = .66).
Table 3.
Distribution and Odds Ratios for Symptoms of Chikungunya by Epidemic Wave
| Epidemic 1 n (%) | Epidemic 2 n (%) | P Value | Crude Odds Ratio (95% CI) | Adjusted Odds Ratioa (95% CI) | |
|---|---|---|---|---|---|
| Arthralgia | 77 (80.2) | 384 (86.7) | 0.102 | 1.61 (0.91, 2.85) | 2.05 (1.12, 3.74) |
| Fever (≥38°C) | 72 (75.0) | 356 (80.4) | 0.239 | 1.36 (0.81, 2.29) | 1.34 (0.77, 2.32) |
| Headache | 70 (72.9) | 340 (76.8) | 0.425 | 1.23 (0.74, 2.02) | 1.59 (0.94, 2.71) |
| Myalgia | 35 (36.5) | 227 (51.2) | 0.009 | 1.83 (1.16, 2.89) | 2.16 (1.34, 3.47) |
| Symptom score (mean) | 2.64 | 2.95 | 0.016 | 1.65 (1.11, 2.46) | 1.96 (1.30, 2.94) |
Statistically significant variables are presented in boldface font.
Abbreviation: CI, confidence interval.
aModels adjusted for age, sex, and days between illness onset and first medical visit. Logistic regression used for individual symptoms and ordinal logistic regression for symptom score.
Early Epidemic Growth Profiles and Reproduction Numbers
Using the early growth phases (determined by the number of new weekly cases), we estimated the scaling of growth parameter at 0.85 (95% CI: 0.56, 1.0) for Epidemic 1 and 0.95 (95% CI: 0.79, 1.0) for Epidemic 2. For both epidemic waves, the uncertainty surrounding the early growth trajectory includes the possibility of exponential growth dynamics (ie, p = 1). Based on the generalized-growth method, we estimated the reproduction number for Epidemic 1 in the range of 1.2–2.1 and for Epidemic 2 in the range of 1.6–3.5, considering a mean generation interval in the range of 1.5–2.0 weeks (Table 4).
Table 4.
Effective Reproduction Number During the Early Growth Phases of Chikungunya Epidemics 1 and 2
| Epidemic 1 | Epidemic 2 | |||
|---|---|---|---|---|
| 1.5-wk Generation Interval | 2-wk Generation Interval | 1.5-wk Generation Interval | 2-wk Generation Interval | |
| Reproduction number | 1.5 (1.2, 1.7) | 1.7 (1.2, 2.1) | 2.2 (1.6, 2.5) | 3.0 (2.0, 3.5) |
| Growth rate, r | 0.08 (0.05, 0.13) | 0.11 (0.09, 0.15) | ||
| Scaling of growth parameter, p | 0.85 (0.56, 1) | 0.95 (0.79, 1) | ||
Values shown are mean estimates (95% confidence intervals). We assumed a generation interval that follows a gamma distribution with a mean of 1.5 weeks or 2 weeks and variance of (6/7)2.
Environmental and Vector Factors
The mean daily temperature in the 3 weeks preceding and the weeks during the epidemic growth periods was 26.9°C (95% CI: 26.7, 27.0) in Epidemic 1 and 28.2°C (95% CI: 28.2, 28.6) in Epidemic 2. Likewise, the maximum temperature in Epidemic 2 was greater than that in Epidemic 1 (33.5°C [95% CI: 33.3, 33.8] vs. 31.4°C [95% CI: 31.1, 31.7]) and the minimum temperature was higher in Epidemic 2 than in Epidemic 1 (24.7°C [95% CI: 24.6, 24.8] vs. 23.3°C [95% CI: 23.0, 23.6]). Intensive vector control activities began in early 2014 and continued throughout the epidemic and inter-epidemic periods. In Ministry of Health larval surveys in the study area, the Breteau index (the number of positive containers per 100 houses sampled) was 4.6 in Epidemic 1 and 1.0 in Epidemic 2. Neighborhood population density around cases and infections did not differ between Epidemics 1 and 2 (P = .62 and P = .38, respectively).
DISCUSSION
In this study, we examined the characteristics of 2 successive epidemic waves of chikungunya during the introduction of the disease into Nicaragua. We found that over the 2 epidemic waves, 24.9% of children were infected with CHIKV. Of those infected, more than half were detected as a symptomatic case. Epidemic 2 was both stronger, as evidenced by a higher infection rate and higher reproductive number, and more severe, as evidenced by a higher proportion of symptomatic infections and higher odds of systemic symptoms in cases.
A relationship between the force of infection and the proportion of symptomatic infections has been observed in other vector-borne infectious diseases, including dengue and malaria [37, 38]. In a cohort of Thai schoolchildren, a higher incidence of DENV infection was strongly associated with a higher proportion of symptomatic infections [38, 39]. In the PDCS and in another cohort study in Vietnam, this relationship was also observed, although the association was weaker than in the Thailand studies [40, 41]. In relation to dengue, it has been postulated that this may be due to the pre-existing antibody titers in the population [38]. In areas with an extremely high force of infection, incidence of clinical malaria has been limited to the transmission periods, despite the fact that children exhibit parasitemia year-round. Thus, clinical disease is likely caused by new infections or superinfections, so a higher-force infection is thought to lead to multiple exposures, thereby increasing the likelihood of clinical disease. Also, the probability of a mosquito transmitting Plasmodium shows a clear dose-response relationship with parasite density in the mosquito salivary gland, and the time to infection is shorter as the dose increases [42].
A possible explanation for the difference in the S:I ratios between the 2 epidemics observed in our study is that the force of infection may be related to the exposure dose. In experimental infections of humans with DENV, while a high dose resulted in typical symptomatic infection in all volunteers, a lower exposure dose resulted in inapparent or less symptomatic infection in half of the volunteers [43]. Thus, in the second Nicaraguan chikungunya epidemic, where both the overall incidence and the effective reproductive number were higher, it is possible that mosquitoes delivered a higher exposure dose. Temperature has been shown to affect vector competence of Ae. aegypti mosquitoes for transmitting both CHIKV and DENV [44, 45]. While it is possible that the pathogenicity of the circulating CHIKV strains differed between the 2 epidemics, preliminary whole genome sequence analyses do not indicate that there were significant phylogenetic differences between viruses in the 2 epidemics.
In Epidemic 2, there was a linear increase in infection incidence with age. While this pattern is typically due to accumulated risk throughout a lifetime, in this case, since all participants were over the age of 2 and the epidemic occurred over a 2-year period, the risk must instead be due to other factors associated with age. The relationship between age and risk might be, at least in part, due to mosquito biting preferences. It has previously been shown that a larger body size attracts more mosquitoes, possibly due to exhaling more CO2 [46–48]. It is also possible that mobility, which increases with age, could affect the risk of exposure to CHIKV-positive mosquitoes in urban Managua. Further, there was a large proportion of children that were asymptomatic, and the role of asymptomatic infections in transmission remains to be elucidated.
Here, we estimate the mean reproductive number to range from 1.2 to 2.1 in Epidemic 1 and 1.6 to 3.5 in Epidemic 2. Our estimates of the reproduction number are sensitive to the length of the generation interval and are in broad agreement with previously reported estimates for the Caribbean (~ 1.8–4) [49, 50].
Limitations of this study include that it is restricted to one location and to a pediatric population aged 2 years and older. However, our data are in concordance with what was seen at a national level [51]. Given that seroprevalence rates were greater in the adult population than in the pediatric cohort population following Epidemic 1 and that adults tend to be more symptomatic, our estimates of the reproductive number are likely conservative [17, 33]. In addition, the CHIKV ELISA could potentially be cross-reactive with other alphaviruses, leading to misclassification of other alphaviral infections as CHIKV infections. However, ELISA results showing that all children were CHIKV-naive in the 2014 blood sample suggest that no cross-reactive alphaviruses were endemic in the population, and there were no reports of introduction of other alphaviruses during the study period.
In summary, we found large differences in both the incidence of infection and the severity of disease manifestation in 2 sequential epidemics of chikungunya in children. We hypothesize that the force of infection and the clinical presentation may be related to the exposure dose. However, additional investigation is required to further investigate the potential relationship between force of infection and exposure dose in mosquito-borne diseases.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank past and present members of the study team, based at the Health Center Sócrates Flores Vivas, the National Virology Laboratory in the Centro Nacional de Diagnóstico y Referencia, and the Sustainable Sciences Institute in Nicaragua, for their dedication and high-quality work. We are grateful to the study participants and their families.
Disclaimer. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants R01AI099631 to A. B., K02TW009483 to A. G, and P01AI106695 and U19AI118610 to E. H).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pan American Health Organization. Geographic spread of chikungunya in the Americas, December 2013-December 2017, 2018. Available at: http://ais.paho.org/phip/viz/ed_chikungunya_amro.asp. Accessed 4 December 2018. [Google Scholar]
- 2. Rosario en Multinoticias (9 de Julio de 2014). El 19 digital, 2014. Accessed 7 September 2014 https://www.el19digital.com/articulos/ver/titulo:20342-rosario-en-multinoticias-9-de-julio-de-2014. [Google Scholar]
- 3. Renault P, Balleydier E, D’Ortenzio E, Bâville M, Filleul L. Epidemiology of chikungunya infection on Reunion Island, Mayotte, and neighboring countries. Med Mal Infect 2012; 42:93–101. [DOI] [PubMed] [Google Scholar]
- 4. Sergon K, Njuguna C, Kalani R, et al. . Seroprevalence of chikungunya virus (CHIKV) infection on Lamu Island, Kenya, October 2004. Am J Trop Med Hyg 2008; 78:333–7. [PubMed] [Google Scholar]
- 5. Sergon K, Yahaya AA, Brown J, et al. . Seroprevalence of chikungunya virus infection on Grande Comore Island, union of the Comoros, 2005. Am J Trop Med Hyg 2007; 76:1189–93. [PubMed] [Google Scholar]
- 6. Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 2007; 88:2363–77. [DOI] [PubMed] [Google Scholar]
- 7. Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. Chikungunya in the Americas. Lancet 2014; 383:514. [DOI] [PubMed] [Google Scholar]
- 8. Costa-da-Silva AL, Ioshino RS, Petersen V, et al. . First report of naturally infected Aedes aegypti with chikungunya virus genotype ECSA in the Americas. PLoS Negl Trop Dis 2017; 11:e0005630. doi: 10.1371/journal.pntd.0005630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cunha MS, Cruz NVG, Schnellrath LC, et al. . Autochthonous transmission of East/Central/South African genotype chikungunya virus, Brazil. Emerg Infect Dis 2017; 23:1737–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Souza TM, Azeredo EL, Badolato-Correa J, et al. . First report of the East-Central South African genotype of chikungunya virus in Rio de Janeiro, Brazil. PLoS Curr 2017; 9. doi: 10.1371/currents.outbreaks.d62ccaa454599cd2735727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lanciotti RS, Valadere AM. Transcontinental movement of Asian genotype chikungunya virus. Emerg Infect Dis 2014; 20:1400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet 2012; 379:662–71. [DOI] [PubMed] [Google Scholar]
- 13. Sissoko D, Ezzedine K, Moendandze A, Giry C, Renault P, Malvy D. Field evaluation of clinical features during chikungunya outbreak in Mayotte, 2005–2006. Trop Med Int Health 2010; 15:600–7. doi: 10.1111/j.365-3156.2010.02485.x. [DOI] [PubMed] [Google Scholar]
- 14. Gérardin P, Guernier V, Perrau J, et al. . Estimating chikungunya prevalence in La Réunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis 2008; 8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moro ML, Gagliotti C, Silvi G, et al. ; Chikungunya Study Group Chikungunya virus in North-Eastern Italy: a seroprevalence survey. Am J Trop Med Hyg 2010; 82:508–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar NP, Suresh A, Vanamail P, et al. . Chikungunya virus outbreak in Kerala, India, 2007: a seroprevalence study. Mem Inst Oswaldo Cruz 2011; 106:912–6. [DOI] [PubMed] [Google Scholar]
- 17. Ritz N, Hufnagel M, Gérardin P. Chikungunya in children. Pediatr Infect Dis J 2015; 34:789–91. [DOI] [PubMed] [Google Scholar]
- 18. Sissoko D, Moendandze A, Malvy D, Giry C, Ezzedine K, Solet JL, Pierre V Seroprevalence and risk factors of chikungunya virus infection in Mayotte, Indian Ocean, 2005–2006: a population-based survey. PLoS One 2008; 3:e3066. doi: 10.1371/journal.pone.0003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewthwaite P, Vasanthapuram R, Osborne JC, et al. . Chikungunya virus and central nervous system infections in children, India. Emerg Infect Dis 2009; 15:329–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robin S, Ramful D, Le Seach’ F, Jaffar-Bandjee MC, Rigou G, Alessandri JL. Neurologic manifestations of pediatric chikungunya infection. J Child Neurol 2008; 23:1028–35. [DOI] [PubMed] [Google Scholar]
- 21. Robin S, Ramful D, Zettor J, et al. . Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr 2010; 169:67–72. [DOI] [PubMed] [Google Scholar]
- 22. Gérardin P, Barau G, Michault A, et al. . Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Réunion. PLoS Med 2008; 5:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Torres JR, Falleiros-Arlant LH, Duenas L, Pleitez-Navarrete J, Salgado DM, Castillo JB. Congenital and perinatal complications of chikungunya fever: a Latin American experience. Int J Infect Dis 2016; 51:85–88. doi: 10.1016/j.ijid.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 24. Gérardin P, Sampériz S, Ramful D, et al. . Neurocognitive outcome of children exposed to perinatal mother-to-child chikungunya virus infection: the CHIMERE cohort study on Reunion Island. PLoS Negl Trop Dis 2014; 8:e2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balmaseda A, Gordon A, Gresh L, et al. . Clinical attack rate of chikungunya in a cohort of Nicaraguan children. Am J Trop Med Hyg 2016; 94:397–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuan G, Gordon A, Avilés W, et al. . The Nicaraguan pediatric dengue cohort study: study design, methods, use of information technology, and extension to other infectious diseases. Am J Epidemiol 2009; 170:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gordon A, Kuan G, Aviles W, et al. . The Nicaraguan pediatric influenza cohort study: design, methods, use of technology, and compliance. BMC Infect Dis 2015; 15:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization. Technical guides for diagnosis, treatment, surveillance, prevention and control of dengue haemorrhagic fever. Geneva, Switzerland: World Health Organization; 1975. [Google Scholar]
- 29. World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 30. Waggoner JJ, Ballesteros G, Gresh L, et al. . Clinical evaluation of a single-reaction real-time RT-PCR for pan-dengue and chikungunya virus detection. J Clin Virol 2016; 78:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanciotti RS, Kosoy OL, Laven JJ, Panella AJ, Velez JO, Lambert AJ, Campbell GL. Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis 2007; 13:764–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galo SS, González K, Téllez Y, et al. . Development of in-house serological methods for diagnosis and surveillance of chikungunya. Rev Panam Salud Publica 2017; 41:e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuan G, Ramirez S, Gresh L, et al. . Seroprevalence of anti-chikungunya virus antibodies in children and adults in Managua, Nicaragua, after the first chikungunya epidemic, 2014–2015. PLoS Negl Trop Dis 2016; 10:e0004773. doi: 10.1371/journal.pntd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishiura H, Chowell G. The effective reproduction number as a prelude to statistical estimation of time-dependent epidemic trends. In: Chowell G, Hyman JM, Bettencourt LMA, Castillo-Chavez C, eds. Mathematical and statistical estimation approaches in epidemiology. Dordrecht, The Netherlands: Springer, 2009:103–21. [Google Scholar]
- 35. Viboud C, Simonsen L, Chowell G. A generalized-growth model to characterize the early ascending phase of infectious disease outbreaks. Epidemics 2016; 15:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chowell G, Viboud C Simonsen L, Moghadas SM. Characterizing the reproduction number of epidemics with early sub-exponential growth dynamics. J R Soc Interface 2016; 13:20160659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greenwood BM. The epidemiology of malaria. Ann Trop Med Parasitol 1997; 91:763–9. [DOI] [PubMed] [Google Scholar]
- 38. Endy TP, Anderson KB, Nisalak A, et al. . Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in Kamphaeng Phet, Thailand. PLoS Negl Trop Dis 2011; 5:e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoon IK, Srikiatkhachorn A, Hermann L, et al. . Characteristics of mild dengue virus infection in Thai children. Am J Trop Med Hyg 2013; 89:1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tien NT, Luxemburger C, Toan NT, et al. . A prospective cohort study of dengue infection in schoolchildren in Long Xuyen, Viet Nam. Trans R Soc Trop Med Hyg 2010; 104:592–600. [DOI] [PubMed] [Google Scholar]
- 41. Balmaseda A, Standish K, Mercado JC, et al. . Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis 2010; 201:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Churcher TS, Sinden RE, Edwards NJ, et al. . Probability of transmission of malaria from mosquito to human is regulated by mosquito parasite density in naive and vaccinated hosts. PLoS Pathog 2017; 13:e1006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg 1952; 1:30–50. [DOI] [PubMed] [Google Scholar]
- 44. Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg 1987; 36:143–52. [DOI] [PubMed] [Google Scholar]
- 45. Mbaika S, Lutomiah J, Chepkorir E, et al. . Vector competence of Aedes aegypti in transmitting chikungunya virus: effects and implications of extrinsic incubation temperature on dissemination and infection rates. Virol J 2016; 13:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Muirhead-Thomson RC. The distribution of anopheline mosquito bites among different age groups; a new factor in malaria epidemiology. Br Med J 1951; 1:1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Port GFLB, Bryan JH. The relationship of host size to feeding by mosquito of the Anopheles gambiae Giles complex (Diptera: Culicidae). Bull Entomol Res 1980; 70 :133–44. [Google Scholar]
- 48. Liebman KA, Stoddard ST, Reiner RC Jr, et al. . Determinants of heterogeneous blood feeding patterns by Aedes aegypti in Iquitos, Peru. PLoS Negl Trop Dis 2014; 8:e2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cauchemez S, Ledrans M, Poletto C, Quenel P, de Valk H, Colizza V, Boelle PY. Local and regional spread of chikungunya fever in the Americas. Euro Surveill 2014; 19:20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Riou J, Poletto C, Boëlle PY. A comparative analysis of chikungunya and Zika transmission. Epidemics 2017; 19:43–52. [DOI] [PubMed] [Google Scholar]
- 51. Ministerio del Poder Ciudadano para la Salud de Nicaragua. Chikungunya seroprevalence and clinical case rate in Nicaragua, 2014–2015. Rev Panam Salud Publica 2017; 41:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.