Abstract
BACKGROUND
Reports on the relative importance of the diastolic and systolic blood pressures (DBP and SBP) in age-related cognitive decline are mixed. Investigating the relation between DBP/SBP and functional and structural brain changes could elucidate which of the 2 measures is more critically important for brain function and, consequently, cognitive impairment.
METHODS
We investigated the association of SBP and DBP with cortical volume, cerebral blood flow (CBF), and white matter lesions (WML), in nondemented older adults with and without mild cognitive impairment (MCI; N = 265, 185 MCI, mean age = 64 years). Brachial blood pressure was measured twice while seated, and the average of the 2 measures was used. Cortical volume, gray matter (GM) CBF, and WML were estimated using T1-weighted imaging, arterial spin labeling, and fluid attenuation inversion recovery, respectively.
RESULTS
Reduced cortical volume was associated with elevated DBP (β= −0.18, P = 0.034) but not with SBP (β = −0.10, P = 0.206). GM CBF was associated with DBP (β = −0.13, P = 0.048) but not with SBP (β = −0.07, P = 0.275). Likewise, CBF within brain regions where MCI patients showed hypoperfusion were only associated with DBP (DBP: β = −0.17, P = 0.005; SBP: β = −0.09, P = 0.120). WML volume was associated with both DBP (β = 0.20, P = 0.005) and SBP (β = 0.30, P < 0.001). For all measures, there was no interaction between DBP/SBP and cognitive status, indicating that these associations were independent of the cognitive status.
CONCLUSIONS
Independently of the cognitive status, DBP is more critically important for GM volume and perfusion, whereas WML is associated with both blood pressures, likely reflecting long-term effect of hypertension and autoregulation dysfunction.
Keywords: arterial spin labeling, blood pressure, cerebral perfusion, hypertension, mild cognitive impairment, white matter lesions
Blood pressure elevation is an important risk factor for age-related cognitive decline, mild cognitive impairment (MCI), and Alzheimer’s disease (AD).1,2 The cerebral vasculature is a system directly impacted by elevated blood pressure, which leads to perfusion changes, microvascular injury, and blood–brain barrier permeability.3 Whether elevated levels of systolic or diastolic blood pressures (SBP or DBP) have stronger impact on brain remains a matter of debate. Some studies have demonstrated an association between cognitive decline and high DBP, but not SBP, in older adults with and without cognitive impairment.4–8 Interestingly, it has been argued that both high and very low levels of DBP can predict cognitive impairment 20 years later.9 Other studies have shown a correlation between cognitive decline and high SBP in older adults,10 as well as between midlife high SBP and late-life cognitive impairment.11 The discrepancy between these findings likely reflects the complexity of the mechanisms by which blood pressure affects brain and, consequently, cognitive function. Direct estimation of the correlation between DBP/SBP and MCI-related changes in brain structure and function could shed light on these mechanisms.
Changes in the cortical volume,12 cerebral blood flow (CBF),13,14 and white matter lesions (WML)15,16 are key factors that are associated with both hypertension and cognitive decline. Reduced CBF in parts of the gray matter (GM CBF) such as the posterior cingulate cortex and the parietal lobe has been associated with cognitive decline in patients with MCI and AD, with some studies reporting more extensive hypoperfusion in the AD patients that also covers parts of the temporal and frontal lobes.13,17–19 WML is proposed to be a measure of microvascular injury,20,21 and its clinical significance in MCI/AD is supported by the observation that MCI patients with higher WML volume are at greater risk of conversion to AD.22,23 The aim of this study was therefore to estimate and compare the associations of DBP and SBP with cortical volume, CBF, and WML. We also examined whether these associations hold true in the absence of cognitive impairment. Accordingly, we tested the following hypotheses in a group of nondemented, older adults with or without MCI: (i) DBP and SBP show differential correlations with the above-mentioned measures of brain function and structure, with some cerebral measures more strongly correlated with DBP and others with SBP. Such differential correlations could be the underlying reason for the discrepancy between studies of the associations between DBP/SBP and cognitive decline. (ii) Overall, DBP will be more relevant to the cerebral/cerebrovascular measures than SBP due to its role in coronary flow.24,25 (iii) Subjects with MCI will show stronger correlation between cerebral measures and DBP/SBP compared with those with normal cognitive function.
METHODS
Participants
The ethics approval for the study was obtained from Emory Institutional Review Board. All participants signed an informed consent form. Two hundred sixty-five nondemented older adults, 50 years or older, were recruited from the greater Metro Atlanta community. MCI was diagnosed using cognitive testing (see the next section) and clinical diagnosis by study physicians or neuropsychologists, and 185 of all participants were categorized as having MCI. Blood pressure was measured in both arms while sitting and rested for 5 minutes, according to the American Heart Association guidelines,26 and the arm with higher blood pressure was selected during a screening visit. Two subsequent measures in the selected arm were then obtained on the scan day, and the average of the 2 measurements was used in this analysis. Exclusion criteria included prior diagnosis of psychiatric disorders or neurological conditions including Parkinson’s disease, multiple sclerosis, history of head injury, stroke or transient ischemic attack in the past 3 years, MRI contraindications, and women of childbearing potential. In addition, those with creatinine levels of greater than 1.99 mg/dl, serum potassium greater than 5.5 meq/l, and abnormal levels of thyroid stimulating hormone (TSH) (>10) or B12 (<250) were excluded.
Cognitive assessment
The cognitive tests included (i) the Montreal Cognitive Assessment, a 30-point test that assesses multiple cognitive domains: attention, executive functions, memory, language, visuospatial skills, short-term memory recall, calculations, and orientation27; (ii) logical memory test that is part of the Wechsler Memory Scale-Revised28 with a maximum score of 25; (iii) clinical dementia rating that assesses cognitive and functional performance in memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care and provides the dementia stage from 0 indicating no dementia (normal) to 3 indicating severe dementia29; (iv) functional assessment questionnaire that includes questions regarding performance in 10 categories of complex higher order activities.30 In addition, executive function was assessed using 4 tests: Trail Making Test-Part B, Digit Span and Digit Sequencing, modified Stroop interference, and Letter fluency.31
MCI diagnostic criteria included Montreal Cognitive Assessment score <26; clinical dementia rating = 0.5; and functional assessment questionnaire rating <9; abnormal logical memory scores of <11 for 16 or more years of education, <9 for 8–15 years of education, and <6 for less than 8 years of education; or a performance at the 10th percentile or below on at least 1 of 4 tests for executive function.31 The diagnosis of MCI or normal cognition was then confirmed by the study physicians and neuropsychologists via a consensus discussion.
MRI acquisition
Imaging was performed on a 3T MRI scanner equipped with a 20-element, receive-only head coil (Magnetom Prisma, Siemens, Erlangen, Germany). High-resolution, T1-weighted images were acquired using magnetization-prepared rapid gradient-echo imaging sequence: field-of-view = 256 × 256 mm2; 176 sagittal slices; isotropic voxel resolution = 1.0 mm3; repetition time/echo time/inversion time = 2,300/2.89/800 ms, respectively; flip angle = 8 degrees; and scan duration = 8 minutes and 37 seconds.
Cerebral perfusion was measured using arterial spin labeling (ASL). ASL images were acquired using a pseudocontinuous labeling method32 combined with a 2D, gradient-echo echo-planar imaging sequence with field-of-view = 220 × 220 mm2; 20 axial slices; voxel size = 3.4 × 3.4 × 5 mm3, with a 1 mm gap between slices; and repetition time/echo time = 3,500/16 ms. The pseudocontinuous labeling method consisted of a 1.5 seconds long train of radiofrequency pulses applied 9 cm below the center of the imaging volume with a mean gradient of 0.6 mT/m.33 The post-labeling delay was 1 second. A total of 60 labeled and control pairs were acquired for scan duration of 7 minutes and 4 seconds.
To detect WML, high-resolution T2-weighted fluid attenuation inversion recovery images were acquired with field-of-view = 250 × 250 mm2, 160 axial slices, voxel size = 0.5 × 0.5 × 1 mm3, acceleration factor = 2, repetition time/echo time/inversion time = 4,500/367/1,800 ms, and scan duration = 6 minutes and 20 seconds.
Image analysis
A combination of image processing methods in FSL (https://www.fmrib.ox.ac.uk/fsl) and SPM (www.fil.ion.ucl.ac.uk/spm/) packages were used. For each participant, the ASL images were motion corrected and pair-wise subtracted to generate perfusion-weighted images, which were then averaged and smoothed using an 8 mm Gaussian filter. The T1 image was coregistered with the mean ASL image and segmented, during which deformation fields were calculated. The mean perfusion image was then normalized to the Montreal Neurological Institute (MNI) template using the estimated deformations. Finally, single-compartment flow model was used to generate images of CBF.34
MNI-based GM mask was used to obtain mean GM CBF. For voxel-wise comparison between the MCI and control groups, CBF maps were scaled to the mean GM value to improve the statistical power by removing within-subject variability as well as between-subject variability due to factors such as age and sex.
Since CBF calculation is prone to partial volume effect, in order to verify whether GM volume differences between the 2 groups introduced bias in estimating CBF differences, voxel-based morphometry was implemented using the voxel-based morphometry toolbox35 within SPM12. Briefly, T1 images were corrected for bias field, segmented into GM, white matter (WM), and cerebrospinal fluid, and normalized to the MNI template using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra and smoothed using an 8 mm Gaussian filter.
Fluid attenuation inversion recovery images were processed using lesion prediction algorithm as implemented in the Lesion Segmentation Toolbox version 2.0.15 (www.statistical-modelling.de/lst.html) for SPM. This algorithm uses a combination of voxel intensity and WM probability map as well as a previously trained algorithm to estimate the WML probability for each voxel. A detailed explanation of processing steps for fluid attenuation inversion recovery images is presented in Supplementary Appendix A.
Statistical analysis
The statistical analysis was performed using R-version 3.3.3 (https://www.r-project.org/), and the significance level was set at 0.05. We used multiple regression to investigate partial correlations of CBF, cortical and WML volumes (dependent variables) with SBP/DBP (independent variables) after adjusting for confounding variables. WML volume was adjusted for brain volume and log-transformed to reduce skewness, and for cortical volume, the cortex-to-brain volume ratio was used. In order to avoid an overfitting model due to a large number of potential confounders (see Table 1), the relevant importance of each variable was first estimated (http://prof.beuth-hochschule.de/groemping/relaimpo/) by calculating its contribution to the total variance of the dependent variable (CBF, cortical volume, or WML volume). The covariates were then selected for the linear model if they contributed to 95% of the explained variance of the dependent variable. A step-by-step description of the statistical analysis for estimating the partial correlations with DBP and SBP is given in Supplementary Appendix B.
Table 1.
Participants’ demographics and characteristics
| Characteristics | Controls | MCI | P |
|---|---|---|---|
| Demographics | |||
| No. of participants | 80 | 185 | |
| Age years (SD) | 63.1 (7.2) | 64.4 (7.5) | 0.209 |
| Sex (%female) | 76% | 60% | 0.011 |
| Education years (SD) | 17.1 (2.8) | 15.2 (2.8) | <0.001 |
| Race (%black) | 27% | 62% | <0.001 |
| Clinical characteristics | |||
| History of hypertensiona (%) | |||
| Full sample (without adjusting for race) | 57% | 76% | 0.002 |
| Black | 74% | 85% | 0.200 |
| White | 51% | 61% | 0.233 |
| Diabetes (%) | 15% | 23% | 0.113 |
| High cholesterol | 45% | 48% | 0.682 |
| Alcohol use | 0% | 59% | <0.001 |
| Current smokers | 0% | 15% | 0.400 |
| Heart disease/heart attack/MI | 7% | 6% | 0.752 |
| Atrial fibrillation/irregular heart rhythm | 12% | 14% | 0.587 |
| Congestive/chronic heart failure | 2% | 2% | 0.624 |
| SBP mm Hg (SD) | 123.1 (18.3) | 137.6 (23.0) | <0.001 |
| DBP mm Hg (SD) | 73.4 (12.0) | 81.2 (14.3) | <0.001 |
| MoCA | 26.9 (2.2) | 21.6 (3.4) | <0.001 |
| TMT-B (seconds) | 80.0 (35.3) | 136.3 (78.3) | 0.004 |
| Delayed recall | 10.0 (1.8) | 7.0 (5.2) | 0.007 |
| MRI characteristics | |||
| Brain tissue volumes ml (SD): | |||
| GM | 626 (83) | 598 (88) | 0.146 |
| WM | 403 (50) | 407 (55) | 0.391 |
| CSF | 449 (211) | 536 (168) | 0.465 |
| Mean GM CBF ml/100g/min (SD) | 45.7 (8.6) | 46.4 (8.4) | 0.537 |
| Log (WML volume ml; SD) | −0.12 (1.2) | 0.34 (1.4) | 0.241 |
The mean and SD of cardiovascular parameters, cognitive scores, and MRI parameters within each of the MCI and control groups are presented. P values of between-group differences after adjusting for sex, age, and race are also shown. Cognitive scores comparisons were also adjusted for education. The brain tissues and WML volumes are also adjusted for brain volume. Abbreviations: CBF, cerebral blood flow; CSF, cerebrospinal fluid; DBP, diastolic blood pressure; GM, gray matter; MCI, mild cognitive impairment; MI, myocardial infarction; MoCA, Montreal Cognitive Assessment score; MRI, magnetic resonance imaging; SBP, systolic blood pressure; TMT-B, Trail Making Test-Part B; WM, white matter; WML, white matter lesion.
aHypertension was defined by high blood pressure (SBP > 140 mm Hg or DBP > 90 mm Hg) and/or consumption of antihypertensive drugs and/or prior diagnosis. Alcohol use was defined by drinking at least 2 units per day.
To further investigate the regional, MCI-related effects of blood pressure on CBF (as opposed to global measures of CBF), a voxel-wise comparison between the MCI and control groups was conducted to identify local perfusion differences. We then estimated the partial correlation between blood pressure measures and local CBF within regions where the MCI group showed hypoperfusion.
RESULTS
Table 1 shows the demographics of subjects with and without (controls) MCI. Compared with the Whites, Blacks had more history of hypertension (84% vs. 57%; P < 0.001), higher DBP (mean = 82.4 vs. 75.1 mm Hg; P < 0.001), and SBP (mean = 137.2 vs. 129.0 mm Hg; P < 0.001) after adjusting for age and sex.
Subjects with MCI had higher DBP and SBP compared with controls, after adjusting for age, sex, and race (Table 1). Although MCI subjects had more history of hypertension in total, this difference was not significant after adjusting for race (Table 1), suggesting that race was driving this difference and was therefore included as a covariate in all our analyses. There were no significant differences in local (voxel-wise) GM volumes between the MCI and control groups after adjusting for age, sex, and race. Likewise, total brain tissues volumes (GM, WM, and cerebrospinal fluid), mean GM CBF, and total WML volume were similar between these 2 groups (Table 1).
Partial correlation of cerebral measures with DBP/SBP
Figures 1–3 show the changes in cerebral measures with DBP and SBP as well as the contribution of each variable to the explained variance of the cerebral measures, respectively.
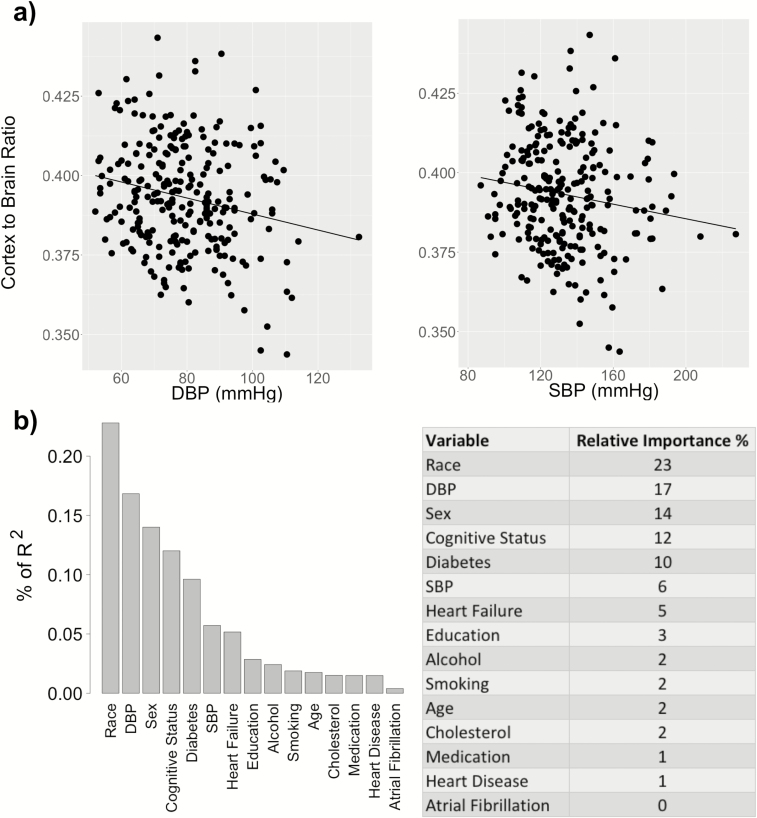
Figure 1.
Association of cortical volume with DBP and SBP. (a) Cortical volume (scaled by brain volume) is correlated with DBP (β = −0.18, P = 0.006, R2 = 0.12) but not with SBP (β = −0.10, P = 0.126, R2 = 0.10), after controlling for the selected covariates (sex, age, race, cognitive status, diabetes, smoking, alcohol use, heart failure, and education). (b) The relative importance of each variable is normalized to sum 100% of the explained portion of the total variance of the cortical volume. Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
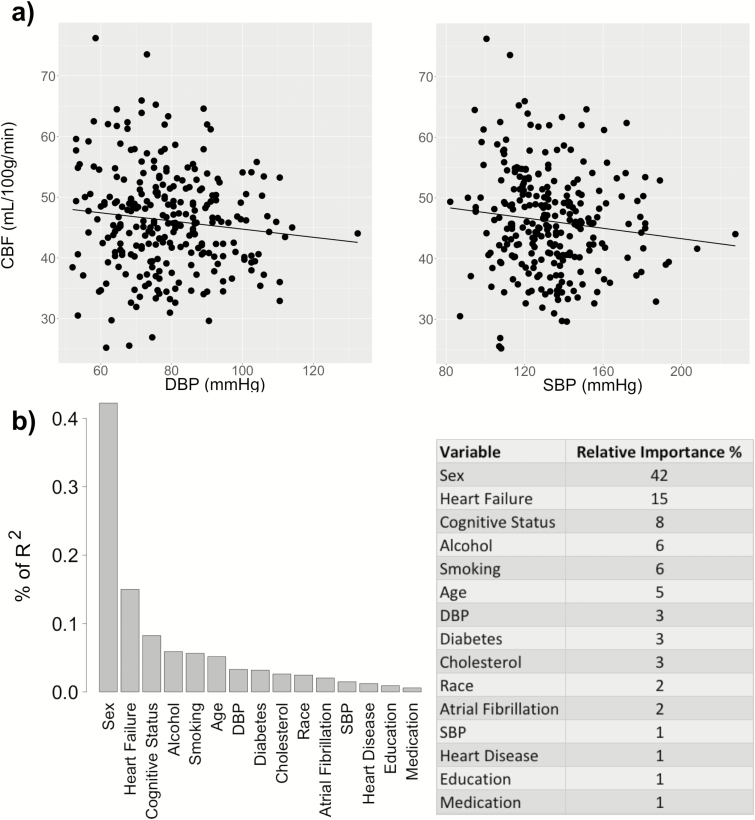
Figure 2.
Association of mean CBF with DBP and SBP. (a) CBF is correlated with DBP (β = −0.13, P = 0.048, R2 = 0.13) but not with SBP (β = −0.07, P = 0.275, R2 = 0.12), after controlling for the selected covariates (sex, age, race, cognitive status, diabetes, smoking, alcohol use, heart failure, and cholesterol). (b) The relative importance of each variable is normalized to sum 100% of the explained portion of the total variance of the CBF. Abbreviations: CBF, cerebral blood flow; DBP, diastolic blood pressure; SBP, systolic blood pressure.
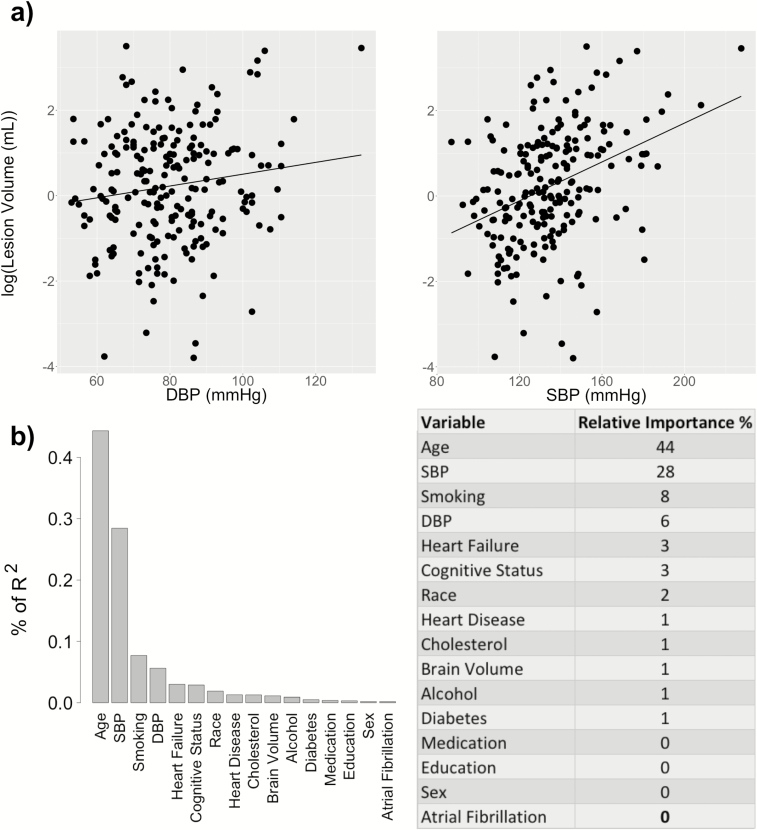
Figure 3.
Association of WML volume with DBP and SBP. (a) WML volume (log-transformed) is correlated with both DBP (β = 0.20, P = 0.005, R2 = 0.23) and with SBP (β = 0.30, P < 0.001, R2 = 0.27), after controlling for the selected covariates (age, race, cognitive status, smoking, heart failure, and brain volume). (b) The relative importance of each variable is normalized to sum 100% of the explained portion of the total variance of the WML volume. Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure; WML, white matter lesion.
Table 2 shows the selected covariates and regression results after adjusting for these covariates. For each regression analysis corresponding to a cerebral measure as the dependent variable and either DBP or SBP as the independent variable, b refers to the regression coefficient for DBP/SBP and β represents the standardized coefficient, i.e., the change in SD of the dependent variable per SD increase in DBP/SBP. As shown by Table 2, reduced cortical volume ratio (cortex-to-brain volume ratio) was associated with elevated DBP (β = −0.18, P = 0.034) but not with SBP (β = −0.10, P = 0.206). Similarly, decreased mean GM CBF was associated with elevated DBP (β = −0.13, P = 0.048) but not with SBP (β = −0.07, P = 0.275). In contrast, increased WML volume (log-transformed) was associated with elevated DBP (β = 0.20, P = 0.005) and SBP (β = 0.30, P < 0.001). For all cerebral measures (cortical volume, CBF, and WML), there was no significant interaction between the cognitive status (MCI or control) and either DBP or SBP, indicating that the associations between the cerebral and blood pressure measures were not affected by the cognitive status.
Table 2.
Partial correlation of the cortical volume, CBF, and WML volume with DBP and SBP
| Cerebral measures | DBP | SBP | ||||||
|---|---|---|---|---|---|---|---|---|
| b (95% CI) | β | P | R 2 | b (95% CI) | β | P | R 2 | |
| Cortical volume ratio | −1.8 (−3.3, −0.3) × 10–4 | −0.18 | 0.034 | 0.12 | −6.7 (−17.7, 4.3) × 10–5 | −0.10 | 0.206 | 0.10 |
| CBF ml/100 g/min | −0.08 (−0.14, −0.02) | −0.13 | 0.048 | 0.13 | −0.03 (−0.07, 0.01) | −0.07 | 0.275 | 0.12 |
| Local CBF (Control > MCI) (scaled to mean value) | −0.16 (−0.26, −0.06) | 0.17 | 0.005 | 0.16 | −0.05 (−0.11, 0.01) | −0.09 | 0.120 | 0.13 |
| Log (WML volume ml) | 0.02 (0.01, 0.03) | 0.20 | 0.005 | 0.23 | 0.02 (0.01, 0.03) | 0.30 | <0.001 | 0.27 |
The regression parameters for each cerebral measure as the dependent variable and either DBP or SBP as the independent variable are presented. For each regression analysis, the regression coefficient (b) and its 95% CI, standardized coefficient (β), P value, and R2 of the multiple regression are presented. For each regression, the covariates were chosen so as to account for 95% of the explained variance of the dependent variable: cortical volume ratio = sex + age + race + cognitive status + diabetes +smoking + alcohol + heart failure + education + (DBP or SBP); CBF = sex + age + race + cognitive status + diabetes + smoking + alcohol + heart failure + cholesterol + (DBP or SBP); log (WML volume) = age + race + cognitive status + smoking + heart failure + brain volume. Abbreviations: CBF, cerebral blood flow; CI, confidence interval; DBP, diastolic blood pressure; MCI, mild cognitive impairment; SBP, systolic blood pressure; WML, white matter lesion.
Local CBF differences between MCI and control groups
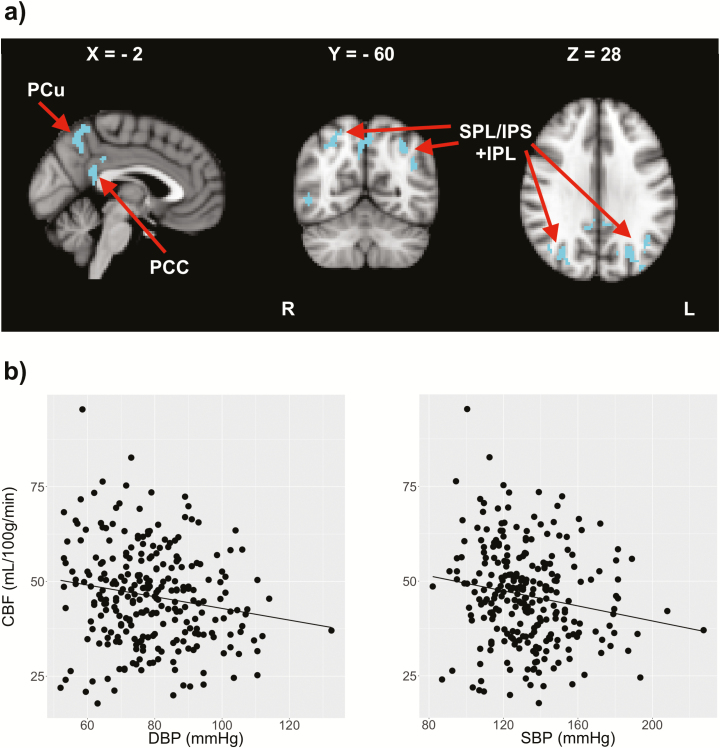
Voxel-wise CBF comparison showed reduced CBF in the posterior cingulate cortex, precunus, inferior, and superior parietal lobule of the MCI group, as shown by Figure 4 and Table 3. The mean CBF within these voxels showed significant correlation with DBP (β = −0.17, P = 0.005), but not with SBP (β = −0.09, P = 0.120). MCI subjects did not exhibit hyperperfusion in any brain regions.
Figure 4.
Voxel-wise CBF comparison between MCI patients and controls. (a) The clusters show areas with reduced CBF in the MCI group compared with controls. The coordinates are also shown in MNI template. (b) Changes in mean CBF within the same voxels with DBP (β = −0.17, P = 0.005) and SBP (β = −0.09, P = 0.120).Abbreviations: CBF, cerebral blood flow; DBP, diastolic blood pressure; IPL, inferior parietal lobule; IPS, intraparietal sulcus; MCI, mild cognitive impairment; MNI, Montreal Neurological Institute; PCC, posterior cingulate cortex; Pcu, precuneus; SBP, systolic blood pressure; SPL, superior parietal lobule.
Table 3.
Clusters corresponding to hypoperfusion in MCI patients
| No. of voxels | X (mm) | Y (mm) | Z (mm) | Region | P (cluster) |
|---|---|---|---|---|---|
| 1,752 | −27 | −70 | 32 | L IPL | <0.001 |
| 172 | 56 | −48 | −12 | R ITG/MTG | <0.001 |
| 69 | −2 | −46 | 14 | L PCC | <0.001 |
| 57 | 6 | −48 | 18 | R PCC | <0.001 |
| 49 | −2 | −38 | 32 | L PCC | <0.001 |
| 17 | 6 | −54 | 60 | R PCu | <0.001 |
The number of voxels and MNI coordinates of peak voxels within each cluster and the anatomical regions are presented. P values correspond to clusters and are corrected using family-wise error. Abbreviations: MCI, mild cognitive impairment; MNI, Montreal Neurological Institute; MTG, middle temporal gyrus; IPL, inferior parietal lobule; ITG, inferior temporal gyrus; PCC, posterior cingulate cortex; PCu, precuneus.
DISCUSSION
The main finding of this study was that DBP is associated with cerebral/cerebrovascular measures among nondemented older adults. Our results suggest that across the cognitive spectrum from normal aging to MCI, elevated DBP is associated with reduced cortical volume and perfusion. Reduced cortical volume is a key factor in age-related cognitive decline and is also shown to be associated with hypertension.12 Decreased perfusion may be the earliest sign of conversion into MCI16–18,36, and its relation to elevated DBP offers additional information regarding brain aging from the cerebrovascular perspective. We also found the same pattern of correlation when we compared the associations of DBP and SBP with CBF in the hypoperfused regions in the MCI group: namely, parts of the posterior cingulate cortex, precunus, inferior, and superior parietal lobule: as before, CBF in these regions was only associated with DBP and not with SBP. Hypoperfusion in these areas is in line with previous studies of MCI patients and has been attributed to impaired memory.13,17,19,36 Consequently, these results suggest that significant contribution of DBP (but not SBP) to cerebral perfusion is also evident in brain regions affected by memory and cognitive impairment, confirming that DBP has a rather broad role in cerebral perfusion regardless of the coexisting cognitive impairment. Indeed, previous studies have demonstrated the role of vascular risk burden in future cognitive decline among older adults with or without normal cognitive function.1,2
WML volume was associated with both DBP and SBP across the disease spectrum, although SBP showed slightly stronger correlation. WML is considered to be a surrogate for cerebral microvascular injuries typically seen in MCI patients.1,16,22 It is also shown that WML is associated with dysfunction in cerebral autoregulation, i.e., maintaining a constant cerebral perfusion despite changes in blood pressure, and amyloid deposition.37 These findings suggest that high DBP and SBP are associated with autoregulatory dysfunction and development of cerebral microvascular injuries. The observation that increased WML is associated with both DBP and SBP is likely due to long-term damage to regions of the WM that are most vulnerable to chronic hypoperfusion. Taken together, reduced cortical volume and perfusion and increased WML volume (hence cerebral microvascular injuries) are likely initiated by different mechanisms.
The fact that DBP has a more encompassing effect on all neuroimaging markers than SBP suggests that DBP may be more critically relevant in aging, and an early indicator of the cognitive decline before long-term and more severe damages is evident. Indeed, a study of a group of nondemented elderly subjects has shown that high DBP could predict atrophy in the hippocampus and amygdala in 5 years.38
We did not observe a J-curve effect of DBP on cortical volume, CBF, or WML. However, our sample included older adults mostly with normal to high levels of DBP and SBP. Therefore, we could not fully examine possible effects of very low DBP. Few but not all prior studies have suggested negative effects of low DBP on cardiovascular disease24,25 and cognitive impairment,9 particularly in patients with cardiovascular disease.
The main limitation to our study is the cross-sectional design.39 Although this limits our ability to identify future risk stratification by DBP and SBP, it allows us to investigate the concurrent importance of blood pressure on cerebral vasculature. These associations may have a clinical relevance as DBP may need to be evaluated as a risk factor for cognitive impairment. Furthermore, we used the average of 2 blood pressure measurements on the day of MRI scan. Although this is a commonly used practice in hypertension studies, more measurements might reveal additional associations. Another limitation is the use of a relatively low post-labeling delay, which could lead to inaccurate estimation of CBF as well as intraluminar signal due to presence of labeled spins in arteries. To address these potential issues, each CBF map was visually inspected and those that failed the visual QC were excluded from further analysis. To some extent, the short post-labeling delay is expected to have introduced a systemic error considering that participants were in the same age group (see Table 1). To further reduce these errors and intersubject variability, we used normalized CBF maps (scaled to mean GM value) for voxel-wise comparison between the MCI and control groups. This is a generally accepted approach provided that mean GM CBF is similar between the 2 groups, which was a valid assumption for our sample. Finally, the CBF maps were not corrected for partial volume effect. Partial volume correction is usually performed to compensate for low spatial resolution in ASL, which results in additional signal form WM and cerebrospinal fluid. However, these methods can cause blurring40 and can be prone to misregistration between ASL and T1 images. Instead, we performed voxel-based morphometry analysis to verify whether differences in GM volume between the MCI and control groups introduced a bias in interpretation of perfusion differences. However, no differences in local or total GM volume were found between the MCI and control groups in our study group.
In conclusion, these results suggest that elevated DBP is a critically important measure in assessing reduction in GM volume and perfusion in healthy and early stages of cognitive impairment and may offer a more expansive overview of cerebrovascular health. However, both DBP and SBP are equally critical for microvascular injury. These findings offer improved insight into mechanisms by which blood pressure elevation might be affecting cerebrovascular function.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by The National Institute on Aging grants #R01AG042127, AG051633, and AG057470 (to I.H.).
DISCLOSURE
The author(s) declared no conflict of interest.
REFERENCES
- 1. Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR Jr, Jagust W, Morris JC, Petersen RC, Saykin AJ, Shaw LM, Toga AW, Trojanowski JQ; Alzheimer’s Disease Neuroimaging Initiative . Recent publications from the Alzheimer’s Disease Neuroimaging Initiative: reviewing progress toward improved AD clinical trials. Alzheimers Dement 2017; 13:e1–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning: a perspective in historical context. Hypertension 2012; 60:260–268. [DOI] [PubMed] [Google Scholar]
- 3. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 2011; 12:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cacciatore F, Abete P, Ferrara N, Paolisso G, Amato L, Canonico S, Maggi S, Varricchio M, Rengo F. The role of blood pressure in cognitive impairment in an elderly population. Osservatorio Geriatrico Campano Group. J Hypertens 1997; 15:135–142. [DOI] [PubMed] [Google Scholar]
- 5. Sharifi F, Hedayat M, Fakhrzadeh H, Mahmoudi MJ, Ghaderpanahi M, Mirarefin M, Tajalizadekhoob Y, Badamchizade Z, Larijani B. Hypertension and cognitive impairment: Kahrizak Elderly Study. Int J Gerontol. 2011; 5:212–216. [Google Scholar]
- 6. Tsivgoulis G, Alexandrov AV, Wadley VG, Unverzagt FW, Go RC, Moy CS, Kissela B, Howard G. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology 2009; 73:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roussotte FF, Siddarth P, Merrill DA, Narr KL, Ercoli LM, Martinez J, Emerson ND, Barrio JR, Small GW. In vivo brain plaque and tangle burden mediates the association between diastolic blood pressure and cognitive functioning in nondemented adults. Am J Geriatr Psychiatry 2018; 26:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glynn RJ, Beckett LA, Hebert LE, Morris MC, Scherr PA, Evans DA. Current and remote blood pressure and cognitive decline. JAMA 1999; 281:438–445. [DOI] [PubMed] [Google Scholar]
- 9. Taylor C, Tillin T, Chaturvedi N, Dewey M, Ferri CP, Hughes A, Prince M, Richards M, Shah A, Stewart R. Midlife hypertensive status and cognitive function 20 years later: the Southall and Brent revisited study. J Am Geriatr Soc 2013; 61:1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Budge MM, de Jager C, Hogervorst E, Smith AD; Oxford Project To Investigate Memory and Ageing (OPTIMA) . Total plasma homocysteine, age, systolic blood pressure, and cognitive performance in older people. J Am Geriatr Soc 2002; 50:2014–2018. [DOI] [PubMed] [Google Scholar]
- 11. Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol 2014; 71:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beauchet O, Celle S, Roche F, Bartha R, Montero-Odasso M, Allali G, Annweiler C. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J Hypertens 2013; 31:1502–1516. [DOI] [PubMed] [Google Scholar]
- 13. Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and Alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 2009; 250:856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI; SMART Study Group . Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol 2012; 71:825–833. [DOI] [PubMed] [Google Scholar]
- 15. Scott JA, Braskie MN, Tosun D, Thompson PM, Weiner M, DeCarli C, Carmichael OT; Alzheimer’s Disease Neuroimaging Initiative . Cerebral amyloid and hypertension are independently associated with white matter lesions in elderly. Front Aging Neurosci 2015; 7:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Altheimer’s disease: do white matter hyperintensities matter?Dialogues Clin Neurosci 2009; 11:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alexopoulos P, Sorg C, Förschler A, Grimmer T, Skokou M, Wohlschläger A, Perneczky R, Zimmer C, Kurz A, Preibisch C. Perfusion abnormalities in mild cognitive impairment and mild dementia in Alzheimer’s disease measured by pulsed arterial spin labeling MRI. Eur Arch Psychiatry Clin Neurosci 2012; 262:69–77. [DOI] [PubMed] [Google Scholar]
- 18. Wang Z, Das SR, Xie SX, Arnold SE, Detre JA, Wolk DA; Alzheimer’s Disease Neuroimaging Initiative . Arterial spin labeled MRI in prodromal Alzheimer’s disease: a multi-site study. Neuroimage Clin 2013; 2:630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schroeter ML, Stein T, Maslowski N, Neumann J. Neural correlates of Alzheimer’s disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage 2009; 47:1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011; 82:126–135. [DOI] [PubMed] [Google Scholar]
- 21. Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke 2009; 40:S48–S52. [DOI] [PubMed] [Google Scholar]
- 22. Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM; Alzheimer’s Disease Neuroimaging Initiative . White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease?JAMA Neurol 2013; 70:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tosto G, Zimmerman ME, Carmichael OT, Brickman AM; Alzheimer’s Disease Neuroimaging Initiative . Predicting aggressive decline in mild cognitive impairment: the importance of white matter hyperintensities. JAMA Neurol 2014; 71:872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG; CLARIFY Investigators . Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388:2142–2152. [DOI] [PubMed] [Google Scholar]
- 26. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716. [DOI] [PubMed] [Google Scholar]
- 27. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699. [DOI] [PubMed] [Google Scholar]
- 28. Wechsler D. Manual for the Wechsler Memory Scale-Revised. The Psychological Corporation: San Antonio, TX, 1987. [Google Scholar]
- 29. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566–572. [DOI] [PubMed] [Google Scholar]
- 30. Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982; 37:323–329. [DOI] [PubMed] [Google Scholar]
- 31. Pa J, Boxer A, Chao LL, Gazzaley A, Freeman K, Kramer J, Miller BL, Weiner MW, Neuhaus J, Johnson JK. Clinical-neuroimaging characteristics of dysexecutive mild cognitive impairment. Ann Neurol 2009; 65:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008; 60:1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu WC, Fernández-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med 2007; 58:1020–1027. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Alsop DC, Song HK, Maldjian JA, Tang K, Salvucci AE, Detre JA. Arterial transit time imaging with flow encoding arterial spin tagging (FEAST). Magn Reson Med 2003; 50:599–607. [DOI] [PubMed] [Google Scholar]
- 35. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38:95–113. [DOI] [PubMed] [Google Scholar]
- 36. Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, Weiner MW. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord 2010; 24:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brickman AM, Guzman VA, Gonzalez-Castellon M, Razlighi Q, Gu Y, Narkhede A, Janicki S, Ichise M, Stern Y, Manly JJ, Schupf N, Marshall RS. Cerebral autoregulation, beta amyloid, and white matter hyperintensities are interrelated. Neurosci Lett 2015; 592:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, Koudstaal PJ, Breteler MMB, Van Dijk EJ. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology 2005; 64:263–267. [DOI] [PubMed] [Google Scholar]
- 39. Jennings JR, Heim AF, Sheu LK, Muldoon MF, Ryan C, Gach HM, Schirda C, Gianaros PJ. Brain regional blood flow and working memory performance predict change in blood pressure over 2 years. Hypertension 2017; 70:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liang X, Connelly A, Calamante F. Improved partial volume correction for single inversion time arterial spin labeling data. Magn Reson Med 2013; 69:531–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.