An immunodiagnostic assay that detects Aspergillus galactofuranose–containing antigens in urine was developed in dipstick format. Performance evaluated in a cohort of 78 subjects demonstrated good sensitivity and specificity relative to current diagnostic tests.
Keywords: galactofuranose, galactomannan, urine, diagnostics, aspergillosis
Abstract
Background
Establishing rapid diagnoses of invasive aspergillosis (IA) is a priority tests that detect galactomannan and β-d-glucan are available, but are technically cumbersome and rely on invasive sampling (blood or bronchoalveolar lavage).
Methods
We optimized a lateral flow dipstick assay using the galactofuranose-specific monoclonal antibody (mAb476), which recognizes urine antigens after Aspergillus fumigatus pulmonary infection in animals. Urine samples were obtained from a cohort of 78 subjects undergoing evaluation for suspected invasive fungal infections, and stored frozen until testing. Urine was processed by centrifugation through desalting columns and exposed to dipsticks. Reviewers blinded to clinical diagnoses graded results. Western blots were performed on urine samples from 2 subjects to characterize mAb476-reactive antigens.
Results
Per-patient sensitivity and specificity for diagnosis of proven or probable IA in the overall cohort was 80% (95% confidence interval [CI], 61.4%–92.3%) and 92% (95% CI, 74%–99%), respectively. In the subgroup with cancer, sensitivity was 89.5% (95% CI, 66.7%–98.7%) and specificity was 90.9% (95% CI, 58.7%–99.8%); among all others, sensitivity and specificity were 63.6% (95% CI, 30.8%–89.1%) and 92.9% (95% CI, 66.1%–99.8%), respectively. Eliminating lung transplant recipients with airway disease increased sensitivity in the noncancer cohort (85.7% [95% CI, 42.1%–99.6%]). Semiquantitative urine assay results correlated with serum galactomannan indices. Western blots demonstrated mAb476-reactive antigens in urine from cases, ranging between 26 kDa and 35 kDa in size.
Conclusions
Urine testing using mAb476 may be used as an aid to diagnose IA in high-risk patients.
Mammals synthesize the monosaccharide galactose in a 6-member ring form, galactopyranose [1, 2]. Nonmammalian Eukaryotes and some Prokaryotes have enzymes to convert galactopyranose into a 5-member ring form, galactofuranose [1–3]. This uniquely nonmammalian monosaccharide is a component of multiple glycoconjugates within Ascomycete fungi, where it contributes to the structure of galactomannan (GM), membrane sphingolipids, and cellular and secreted glycoproteins (O-glycans and N-glycans) [1, 3–8].
The current commercial enzyme immunoassay (EIA) used to aid diagnosis of invasive aspergillosis (IA) (BioRad Platelia Aspergillus EIA) utilizes an antibody (EB-A2) that recognizes branched β-1,5-linked galactofuranose side chains of the α-linked mannosyl backbone of the large GM polysaccharide [9–11]. EB-A2 has a binding preference for long-branched GMs that contain 4–7 residue β-(1,5)-galactofuranose oligomers that are typically found on hyphae [12, 13]. The serum Platelia assay is 61%–82% sensitive and 70%–93% specific, depending on the optical density cutoff used to interpret positivity and patient characteristics that impact circulating antigen burden, such as degree of neutropenia and prior antifungal therapy [11, 14–16].
While the Platelia assay has contributed greatly to the care of neutropenic patients, uptake of the test has been limited by complexity of laboratory processing and dependence on blood or bronchoalveolar lavage (BAL) fluid. Urine would be an easy-to-test, typically abundant body fluid, but studies evaluating use of the EB-A2 antibody on urine have reported limited utility [17]. We previously described a novel galactofuranose-specific anti–Aspergillus fumigatus monoclonal antibody (mAb476) that rapidly detects fungal antigen in urine in mice and guinea pig models of pulmonary aspergillosis, and demonstrated proof of concept for mAb476 testing in a limited numbers of human samples [18]. We have since optimized a lateral flow immunodiagnostic device in dipstick format and report performance in human subjects evaluated for invasive fungal infections (IFIs) at Johns Hopkins Medical Center.
METHODS
Development of a Lateral Flow Dipstick Immunoassay for Urine Aspergillus Antigen
The urine lateral flow dipstick immunoassay was developed in collaboration with Bioassay Works (Ijamsville, Maryland). In brief, mAb476 (capture antibody) and Bioassay Works proprietary control line solution were striped onto a nitrocellulose membrane. Antibody (mAb476) was conjugated to Bioassay Works’ Naked Gold 40 nm nanoparticles and dried on a polyester ribbon for use as detection antibody. Crude ethanol–precipitated antigen from A. fumigatus (isolate Af293) culture [18] was spiked into human urine pooled from several healthy volunteers, and used to optimize assay parameters, including membrane type and dimensions, gold conjugation conditions, blocking buffers, and test timing, in variable-element checkerboard experiments.
Diagnostic Performance Testing
Human subjects approval was obtained by the Johns Hopkins Medicine Institutional Review Board, under protocols that enabled 1-time or sequential collection of samples from both healthy human volunteers and patients suspected to have an IFI. Subjects were consented for collection of urine and clinical follow-up for confirmation of diagnosis. Urine samples were collected from volunteers and stored frozen at –80°C until testing. Urines were thawed and prepared as previously described [18]. In brief, urine underwent a 2-minute spin through a desalting column, and dipstick sample pads were placed into 50 μL of the desalted urine. After 10-minute incubation, 2 readers who were blinded to clinical diagnoses read test results. These results were semiquantitatively interpreted as high-positive (++), low-positive (+), or negative (–). A clinician who was blinded to test results reviewed clinical records to adjudicate certainty of diagnosis, using the published European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) definitions for IFI [19]. In brief, proven IA or other IFIs required documentation of the causative organism by culture and histopathology from invasive tissue samples (eg, lung biopsy). Invasive aspergillosis was considered “probable” with documentation of focal pulmonary consolidations or nodular infiltrates with demonstration of any of the following: (1) culture of Aspergillus species from sputum and/or BAL fluid; (2) documented positivity of BAL GM EIA with indices ≥0.7; and/or (3) documented positivity of serum GM EIA with indices ≥0.5 on at least 2 samples. Subjects in whom IA was suspected but not confirmed by at least 1 of the above criteria were considered to have “possible” IA. Fungitell β-d-glucan assay results were considered positive using the recommended cutoff of 80 pg/mL. As the EORTC/MSG definitions were developed specifically for cancer patients, some modifications were required. Underlying host criteria were expanded to include solid organ transplant recipients, people with human immunodeficiency virus (HIV)/AIDS, and receipt of other immunosuppression for autoimmune diseases. Infections caused by pathogenic yeasts (eg, Cryptococcus neoformans, Histoplasma capsulatum) were considered probable with culture and/or antigen positivity, and proven with documentation via histopathology.
Western Blots
To understand the antigen expressed in human urine samples, Western blots were performed, comparing control (pooled healthy volunteer) urine and that from 2 subjects with confirmed probable IA and high–positive dipstick results. In brief, urine samples were concentrated by approximately 95% using a 10k-Da molecular weight cutoff (MWCO) Amicon Spin concentrator (Millipore) and desalted using a 7-kDa MWCO Zeba Spin column (Pierce/ThermoFisher). Samples (20 µg protein) were run on a reducing sodium dodecyl sulphate–polyacrylamide gel electrophoresis gel, followed by transfer to a nitrocellulose membrane. The membrane was blocked with StartingBlock/PBS buffer (Pierce/ThermoFisher) supplemented with 0.05% Tween-20, probed overnight at 4°C with 1 µg/mL mAb476 in blocking buffer, and detected with horseradish peroxidase–conjugated goat antimouse immunoglobulin M (Southern Biotech). Signals were generated using SuperSignal West Femto Chemiluminescent reagent (Pierce/ThermoFisher).
Statistical Analyses
Sensitivity and specificity with 95% confidence intervals (CIs) were calculated in per-patient analyses with variable case definitions [20, 21]. Galactomannan EIA indices were plotted against corresponding semiquantitative urine assay grades, and variances were analyzed with the nonparametric Kruskal-Wallis test with Dunn posttest for multiple comparisons.
RESULTS
Urine Dipstick Prototype Development
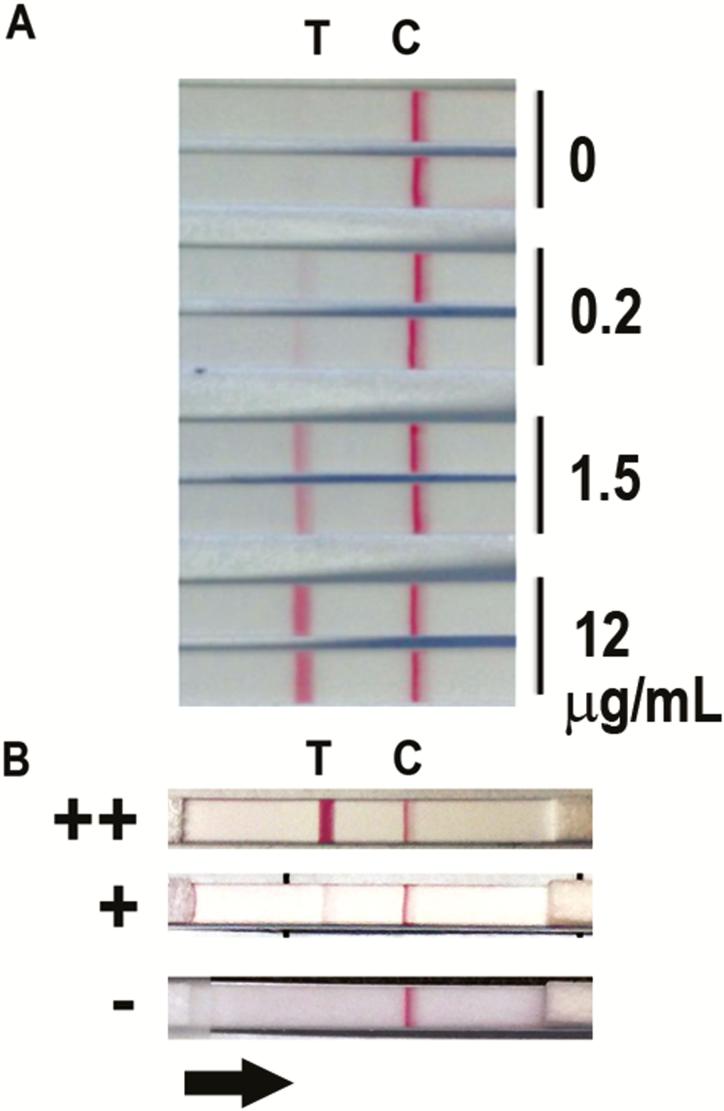
In the final version of the dipstick, reproducible visual positivity was apparent at concentrations >0.2 μg/mL ethanol precipitate spiked into urine (Figure 1A). For clinical sample testing, visual reads were considered negative (–), low positive (+), or high positive (++), as shown in representative samples in Figure 1B. Low-positive sample results visually corresponded to the lines present with ≤0.2 μg/mL ethanol precipitate spiked into urine, and high-positive samples had more robust test line positivity. The clinical sample that had the highest signal intensity (shown as ++ in Figure 1B) stably exceeded visual intensity apparent from control urine spiked with 12 µg/mL ethanol precipitate.
Figure 1.
A, Representative prototype dipstick positivity at variable concentrations (0, 0.2, 1.5, and 12 µg/mL) of ethanol precipitate antigen spiked into healthy human urine. Two replicates are shown for each antigen condition. Line “C” represents the position of the control line containing control antibody and line “T” represents the position of β-galactofuranose-recognizing mAb476 on the membrane. Binding of Aspergillus antigen within ethanol precipitate or sample marks the T-line. B, Representative test results of clinical samples showing semiquantitative visual appearance of negative (–), low-positive (+), and high-positive (++) interpretations. The black arrow represents the direction of flow of the sample in the assay.
Cohort Characteristics and Dipstick Performance
Urine samples were obtained from 78 consenting subjects who were hospitalized with suspected or confirmed IFIs. The majority of the cohort had hematological malignancies and/or had received hematopoietic stem cell transplantation (HSCT); fewer had received solid organ transplantation or biological immunosuppression for autoimmune conditions (Table 1). Thirty subjects had IA confirmed as proven or probable, involving the lungs. Two subjects also had proven dissemination to other sites, vertebrae and eye. The majority of probable IA cases were documented by GM antigen in blood and/or BAL fluid only (n = 30); 7 of 30 also had respiratory culture confirmation of A. fumigatus (n = 5), 1 Aspergillus terreus, and 1 Aspergillus flavus. Twenty-three subjects were considered to have possible IA using EORTC/MSG definitions. Controls had multiple documented IFI, bacterial infections, and viral infections. One subject with A. fumigatus cultivated from BAL was considered to have tumor colonization, and included as a control. Urine samples were collected a median of 8 days after presentation.
Table 1.
Characteristics of Subjects in the Cohort (N = 78)
| Factor | Result |
|---|---|
| Age, y, mean (range) | 52.8 (6–88) |
| Nontransplant underlying disease, No. (%)a | |
| Hematologic malignancy | 21 (55.3) |
| Solid tumor | 4 (10.5) |
| Autoimmune disease | 3 (7.9) |
| Other | 10 (26.3) |
| Transplant type, No. (%)b | |
| Allogeneic HSCT | 22 (55) |
| Autologous HSCT | 1 (2.5) |
| Kidney transplant | 6 (15) |
| Lung transplant | 4 (10) |
| Liver transplant | 2 (5.0) |
| Heart transplant | 3 (7.5) |
| Kidney + liver transplant | 2 (5.0) |
| Case diagnoses (n = 53), No. | |
| Proven IA | 3 |
| Probable IA | 27 |
| Possible IA | 23 |
| Control subjects (n = 25), No.c | |
| No fungal infection; other diagnoses | 9 |
| Proven or probable histoplasmosis | 10 |
| Proven or probable cryptococcosis | 2 |
| Proven or probable mucormycosis | 3 |
| Probable Pneumocystis jirovecii pneumonia | 1 |
| Aspergillus colonization | 1 |
| Time between presentation and urine sample, d, median (range)d | 8 (4–51) |
| Time between presentation and confirmation of diagnosis, d, median (range)d | 3 (–7 to 50) |
Abbreviations: HSCT, hematopoietic stem cell transplant; IA, invasive aspergillosis.
aThirty-eight subjects had not received transplants. Underlying diseases classified as hematological included lymphoma (n = 9) acute myelogenous leukemia/myelodysplastic syndromes (n = 9), acute lymphocytic leukemia (n = 2), and aplastic anemia (n = 1). Solid tumors included histiocytic sarcoma (n = 1), melanoma (n = 1), glioblastoma (n = 1), and small-cell lung cancer (n = 1). Autoimmune conditions include systemic lupus erythematosus (n = 1), rheumatoid arthritis (n = 1), and myasthenia gravis (n = 1). Other underlying diseases included aging-related frailty (n = 2), chronic lung disease (n = 1), diabetes (n = 1), and human immunodeficiency virus/AIDS (n = 1). Five subjects in the other category had no underlying diseases noted.
bPercentages shown relative to 40 subjects who had undergone transplantation. Two organ transplant recipients were also neutropenic from cancer therapy at diagnosis.
cIncludes 1 subject with combined diagnosis of probable cryptococcosis and proven histoplasmosis, assessed by positive Cryptococcus antigen and Histoplasma capsulatum recovered from biopsy. Other diagnoses included bacterial pneumonia and viral pneumonitis. One subject with Aspergillus colonization of a necrotic lung cancer was included as control.
dDay of presentation interpreted as the day on which patient had first sign or symptom attributable to current diagnosis. Day of confirmation of diagnosis was the day on which the sample was obtained, not resulted. This analysis excluded 1 patient in whom diagnosis was not confirmed for several months after presentation, during which period of time he received antifungals empirically but progressed clinically.
Sensitivity and specificity of the dipstick test using different case definitions are shown in Table 2. In these analyses, subjects who were considered to have possible IA were not included, given inability to determine diagnosis. Amongst the overall cohort, 24 of 30 subjects with proven or probable IA had positive dipstick results, yielding sensitivity of 80% (95% confidence interval [CI], 61.4%–92.3%). High-positive (++) results were seen in 15 cases with probable IA, 2 of 3 cases with proven IA, and 3 people who had possible IA by clinical testing. Two of 25 controls also had positive dipstick results, generating specificity of 92% (95% CI, 74%–99%). Both controls with false-positive assays had clinical diagnoses of histoplasmosis, with positive serum and urine histoplasma EIA results (MiraVista); there were 8 additional subjects in the control group who did not have positive urine IA assays. False positivity did not correlate with histoplasma antigen levels (data not shown).
Table 2.
Performance of the Urinary Galactofuranose-Antigen Detection Dipstick Assay
| IA Diagnosis | Subjects With Positive Tests, No. (%) | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| Overall cohort (n = 78)a | |||
| Proven/probable IA (n = 30) | 24 (80) | 80 (61.4–92.3) | 92 (74–99) |
| Controls (n = 25) | 2 (8) | ||
| Overall cohort, with β-d-glucan (n = 78)b | |||
| Proven/probable (n = 40) | 32 (80) | 80 (64.4–90.9) | 92 (74–99) |
| Controls (n = 25) | 2 (8) | ||
| Subgroup: hematological malignancy/BMT/neutropenia (n = 50)c | |||
| Proven/probable IA (n = 19) | 17 (89.5) | 89.5 (66.7–98.7) | 90.9 (58.7–99.8) |
| Controls (n = 11) | 1 (9.1) | ||
| Subgroup: noncancer (n = 28)c | |||
| Proven/probable IA (n = 11) | 7 (63.6) | 63.6 (30.8–89.1) | 92.9 (66.1–99.8) |
| Controls (n = 14) | 1 (7.1) | ||
Abbreviations: BMT, bone marrow transplant; CI, confidence interval; IA, invasive aspergillosis.
aExcludes 23 subjects with possible IA. Controls include no invasive fungal infection (IFI) or non-Aspergillus IFI.
bCase definitions considering β-d-glucan results, upgrading 10 subjects with possible IA to probable. Controls include no IFI or non-Aspergillus IFI.
cCancer subgroup excludes 20 subjects with possible IA and noncancer subgroup excludes 3 subjects with possible IA. Cancer subgroup includes 2 solid organ transplant recipients who had concurrent cancer with neutropenia.
When Fungitell β-d-glucan results were included in the case definition for proven or probable IFI, 10 subjects with possible IA were upgraded to probable IFI, based on β-d-glucan values >80 pg/mL, and appropriate radiographic abnormalities. Sensitivity and specificity of the assay remained the same (Table 2). Analyses restricted to people with hematological malignancies or other cancers as their underlying disease yielded an estimated sensitivity of 89.5% (95% CI, 66.7%–98.7%) and specificity of 90.9% (95% CI, 58.7%–99.8%). Sensitivity from the small subgroup with other underlying diseases was lower, at 63.6% (95% CI, 30.8%–89.1%). Three of 4 of the false-negative results were in lung transplant recipients, in whom noninvasive airway infection occurs. Eliminating these subjects changed sensitivity of the overall cohort to 88.5% (95% CI, 69.9%–97.6%) and of the limited noncancer cohort to 85.7% (95% CI, 42.1%–99.6%). Specificities did not change, at 92% and 92.9%, respectively.
While these analyses are characteristically performed only among subjects who meet proven or probable case definitions, we also assessed dipstick positivity within the subgroup of subjects who had possible IA without either GM EIA or β-d-glucan antigen positivity. Urine samples of 5 of 13 (38.5%) possible IA cases were positive via the dipstick, with most considered to be low intensity. Several of the subjects with possible IA also had positive β-d-glucan assays, supporting the diagnosis of an IFI.
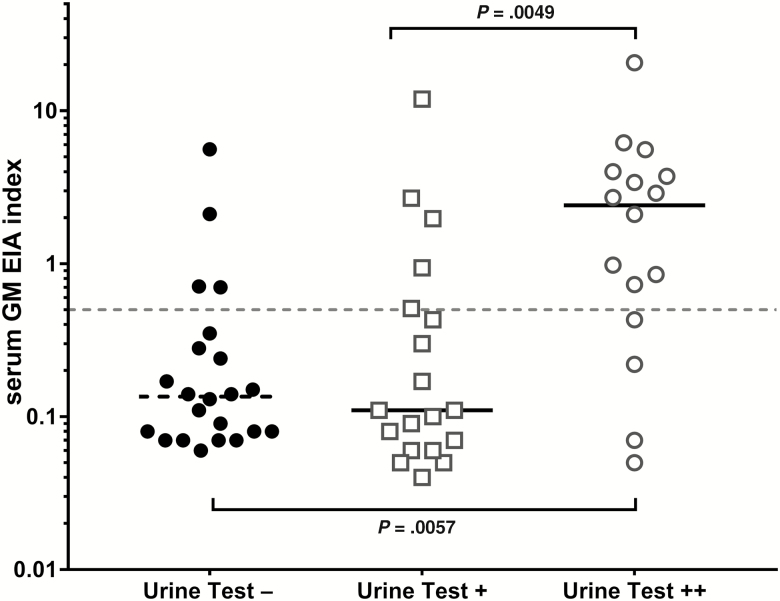
Semiquantitative urine assay grades were plotted relative to GM EIA indices to assess potential correlation among markers that reflect relative fungal burden (Figure 2). Serum GM EIA indices were significantly higher in people who had high-intensity urine assay results compared to either low-positive or negative urine results.
Figure 2.
Urine test results relative to maximum serum galactomannan enzyme immunoassay (GM EIA) optical density index measured by Platelia. P values calculated by Kruskal-Wallis test with adjustment for multiple comparisons are shown.
Representative Cases
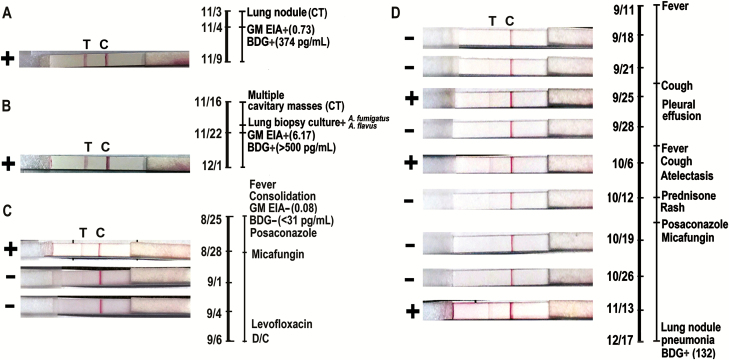
Representative cases are shown in Figure 3. Figure 3A illustrates dipstick results from a subject with HIV/AIDS, who developed a cavitary pulmonary lesion with tree-in-bud inflammatory nodules after presenting with pancytopenia secondary to ayptical hemolytic uremic syndrome with thrombotic thrombocytopenic purpura. The high-positive result in Figure 3B was observed in a subject with proven disseminated IA that developed during corticosteroid therapy for glioblastoma multiforme. Results of serial tests from subjects with hematological malignancies are shown in Figure 3C and 3D. Both of these subjects lacked definitive diagnoses using conventional clinical testing. In the subject schematized in Figure 3C, serial urine samples were tested, with the first demonstrating low-level positivity. This 82-year-old woman was admitted with a new diagnosis of acute leukemia, and chest computed tomography (CT) demonstrated focal consolidations and bilateral nodular opacities. The subject was treated empirically with broad antimicrobials and had negative diagnostic tests, without undergoing BAL. She was treated for presumed bacterial pneumonia and died without a clear diagnosis. The urine assay was positive 3 days after admission, and resolved to negative while the patients was on antifungal therapy. Figure 3D illustrates the course of a man who developed fever of 38.8°C early after HSCT, with lung CT showing subcentimeter mediastinal lymphadenopathy. With cough, and initiation of prednisone for graft-vs-host disease, repeat CT scan showed bilateral centrilobular nodules with ground glass opacities and, later, a positive β-d-glucan test. During this interval, his urine tests were intermittently low-positive.
Figure 3.
Dipstick visual results and correlating clinical findings in 4 subjects in which single or serial assays were performed (A–D). Timeline on the right of each subject reflects clinical evaluation. Abbreviations: BDG, β-d-glucan; C, control line; CT, computed tomography; D/C, discharged; EIA, enzyme immunoassay; GM, galactomannan; T, test line.
Western Blots Showing mAb476-Antigen Recognition in Urine
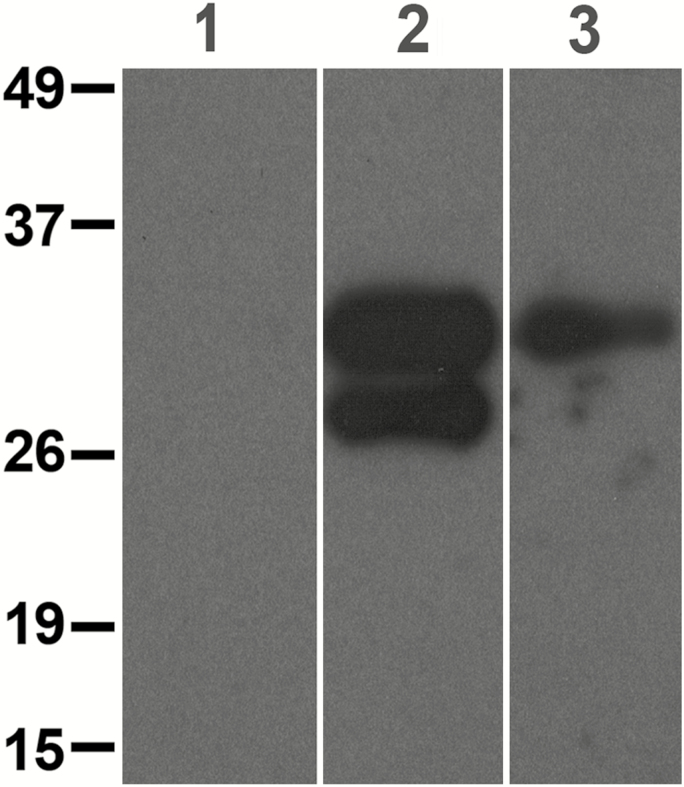
Antibody reactivity was assessed on urine from 2 subjects with high-positive (++) dipstick results and from healthy controls (Figure 4). Results demonstrate that the mAb476-recognized antigens that migrated as 1–2 bands between 26 and 35 kDa. Healthy control urine lacked any mAb476-reactive bands.
Figure 4.
Western blot showing galactofuranose-specific monoclonal antibody (mAb476)–reactive bands. Lane 1 = pooled urine from 4 healthy controls; lanes 2 and 3 = urine samples from 2 subjects with probable invasive aspergillosis and high-positive dipstick results. Molecular weight standards (kDa) are shown on left panel.
DISCUSSION
Establishing a diagnosis of IA has presented a long-time clinical challenge, as these organisms are difficult to cultivate, radiographic abnormalities are nonspecific, and fungal burden can be low despite significant pulmonary compromise. While the Platelia GM immunoassay has offered much improvement compared to culture and histopathology, results of meta-analyses illustrate complexities in analysis and inconsistencies in performance. The most recent Cochrane Library review of 54 studies published prior to 2014 concluded 61%–82% sensitivity and 81%–93% specificity, with variations according to optical density index cutoffs [14]. Studies have also reported that frequent monitoring may assist in preventing establishment of severe disease in highly immunosuppressed patients, but this practice has not become widespread [22–25]. Encouraged by the results of animal studies that suggested that the novel antibody mAb476 detects antigens that are rapidly excreted in urine after inhaled infection with A. fumigatus [18], we created an immobilized assay and tested performance in patients suspected to have IA. Results suggest that the urine assay demonstrates good performance, with early positivity and ease of testing suggesting a potential for application as a screening assay in patients at risk.
The assay shown here uses a novel antibody (mAb476) that is specific to galactofuranose-containing antigens. Using 99mTc-labeled mAb476, we previously demonstrated that the antibody was localized into the bladder rapidly after immunosuppressed mice were infected with A. fumigatus [18]. This behavior is distinct from reported characteristics of the EB-A series of antibodies (EB-A1 and EB-A2), which have been highly studied as part of current commercial assays [12]. The EB-A series have a binding preference for complex large-polymer GM that contains long, 4–7 residue β-(1,5)-galactofuranose oligomer side-chains. Kudoh and colleagues showed that this type of GM is made by Aspergillus species that are grown in certain culture conditions, but not others [13]. The antigen typically migrates as a high-molecular-weight smear (>80–100 kDa) on Western blots of A. fumigatus extracts [7, 9] and can be found in blood and BAL fluid in infected patients.
Urine has previously been investigated as a diagnostic source in both humans and animals, but with limited success. In 1987, Dupont and colleagues described A. fumigatus antigens in human subjects and rabbits after experimental infection [26]. Haynes subsequently confirmed this finding and added that these small molecular weight antigens, ranging between 10 and 31 kDa in molecular weight, were not recognized by EB-A1, but instead were recognized by a polyclonal antibody prepared against A. fumigatus cell wall extract [27]. Consistent with these animal models, 6 small clinical studies subsequently reported low sensitivities for urine antigen detection using EB-A antibodies [17]. The mAb476 behaves more similarly to the anti-cell wall polyclonals as compared to the EB-A series, recognizing small-molecular-weight antigens in patient urines; with this in mind, it appears uniquely positioned for use in the urine diagnostic test.
Results generated here suggest that the urine assay is both sensitive and specific. No false positivity has been observed in healthy donor urines. However, the assay does appear prone to cross-reactivity caused by highly related Ascomycetes such as Histoplasma capsulatum, much like other immunoassays that detect shared β-galactofuranose epitopes [2, 28]. More studies are necessary to understand the variability in false positivity witnessed between histoplasmosis cases.
In this study, the highest sensitivity was noted in people with cancer, including hematological malignancies and HSCT. Lower sensitivities were observed in the cohort of subjects with other underlying diseases, and this was especially notable in lung transplant recipients. This is consistent with the presence of infection restricted to the airway in these patients, as with other assays [29]. Larger studies will be necessary to understand performance of urine testing in sequential samples obtained from large cohorts of organ transplant recipients.
Similar to prior studies, subjects with “possible IA” were not included in calculations of performance, since the clinical syndrome causing symptoms remain unknown. Close examination of these cases suggests that a large proportion may have had actual IA that was not detected using the GM EIA test, with concurrent positivity with β-d-glucan testing. Initial positivity with the urine assay and gradual progression to negativity upon serial testing with concurrent antifungal therapy raises the likelihood that this test may be able to detect early infection. More longitudinal testing will be needed to define utility as a screening assay to prevent development of severe disease.
The dipstick technology requires minimal sample preparation with visual interpretation within 30 minutes. Unlike the more complex EIA, little laboratory skill is required. Semiquantitative visual reads correlated with serum GM positivity, suggesting that urine antigen levels vary with circulating fungal burden; development of the assay using a quantitative platform may further assist interpretation. This simple dipstick technology may enable development of multiplexed urine assays that can distinguish between infections caused by other galactofuranose-producing microbes, using antibodies with enhanced epitope specificities.
This study has limitations. The cohort size restricted subgroup analysis in some of the less common underlying diseases. Some subjects had only 1 sample for assessment; performance could be different with multiple samples tested, enabling analysis with per-sample calculations. Urine was tested a median of 8 days after presentation, so most people were already receiving mold-active antifungals; we do not know how this impacted performance. The per-patient analysis does not provide an understanding of the impact of time-dependent treatment variables, such as antifungal therapies [30]. Additional studies will be necessary to determine the impact of other clinical variables, such as renal diseases.
In summary, we present favorable proof-of-concept data to support use of a lateral flow dipstick assay used on urine as an aid to diagnose IA. If results are confirmed, urine testing may provide great clinical utility in establishing diagnosis of IA in people with consistent signs and symptoms, and for developing screening strategies for prevention of infection in high-risk patients.
Notes
Acknowledgments. The authors thank past employees of MycoMed Technologies, BioAssay Works, clinical research personnel at Johns Hopkins University, and the patients who have donated samples and data.
Financial support. This work was supported by the Maryland Technology Development Corporation (grant number 1112-006) and the National Institutes of Health (NIH) (award number R41AI115866), both to K. A. M. and MycoMed Technologies; antibodies were generated with support from the NIH (award number R21AI065745 to M. L. F.).
Potential conflicts of interest. K.A.M. has been a consultant to Amplyx, Advaxis, Chimerix, Cidara, F2G, Merck, and Vical, and is a co-founder of MycoMed Technologies. K. A. M., K. D., and M. L. F. are coinventors on patents owned by Johns Hopkins University and licensed by MycoMed Technologies. J. F. and M. R. are employees of MycoMed Technologies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tefsen B, Ram AF, van Die I, Routier FH. Galactofuranose in eukaryotes: aspects of biosynthesis and functional impact. Glycobiology 2012; 22:456–69. [DOI] [PubMed] [Google Scholar]
- 2. Marino C, Rinflerch A, de Lederkremer RM. Galactofuranose antigens, a target for diagnosis of fungal infections in humans. Future Sci OA 2017; 3:FSO199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Komachi Y, Hatakeyama S, Motomatsu H, et al. . GfsA encodes a novel galactofuranosyltransferase involved in biosynthesis of galactofuranose antigen of O-glycan in Aspergillus nidulans and Aspergillus fumigatus. Mol Microbiol 2013; 90:1054–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fontaine T, Simenel C, Dubreucq G, et al. . Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J Biol Chem 2000; 275:27594–607. [DOI] [PubMed] [Google Scholar]
- 5. Leitao EA, Bittencourt VC, Haido RM, et al. . Beta-galactofuranose-containing O-linked oligosaccharides present in the cell wall peptidogalactomannan of Aspergillus fumigatus contain immunodominant epitopes. Glycobiology 2003; 13:681–92. [DOI] [PubMed] [Google Scholar]
- 6. Morelle W, Bernard M, Debeaupuis JP, Buitrago M, Tabouret M, Latgé JP. Galactomannoproteins of Aspergillus fumigatus. Eukaryot Cell 2005; 4:1308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmalhorst PS, Krappmann S, Vervecken W, et al. . Contribution of galactofuranose to the virulence of the opportunistic pathogen Aspergillus fumigatus. Eukaryot Cell 2008; 7:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Latgé JP. Galactofuranose containing molecules in Aspergillus fumigatus. Med Mycol 2009; 47:S104–9. [DOI] [PubMed] [Google Scholar]
- 9. Latgé JP, Kobayashi H, Debeaupuis JP, et al. . Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect Immun 1994; 62:5424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stynen D, Goris A, Sarfati J, Latgé JP. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J Clin Microbiol 1995; 33:497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J Infect Dis 2004; 190:641–9. [DOI] [PubMed] [Google Scholar]
- 12. Stynen D, Sarfati J, Goris A, et al. . Rat monoclonal antibodies against Aspergillus galactomannan. Infect Immun 1992; 60:2237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kudoh A, Okawa Y, Shibata N. Significant structural change in both O- and N-linked carbohydrate moieties of the antigenic galactomannan from Aspergillus fumigatus grown under different culture conditions. Glycobiology 2015; 25:74–87. [DOI] [PubMed] [Google Scholar]
- 14. Leeflang MM, Debets-Ossenkopp YJ, Wang J, et al. . Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev 2015:CD007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis 2005; 40:1762–9. [DOI] [PubMed] [Google Scholar]
- 16. Mennink-Kersten MA, Klont RR, Warris A, Op den Camp HJ, Verweij PE. Bifidobacterium lipoteichoic acid and false ELISA reactivity in aspergillus antigen detection. Lancet 2004; 363:325–7. [DOI] [PubMed] [Google Scholar]
- 17. Klont RR, Mennink-Kersten MA, Verweij PE. Utility of Aspergillus antigen detection in specimens other than serum specimens. Clin Infect Dis 2004; 39:1467–74. [DOI] [PubMed] [Google Scholar]
- 18. Dufresne SF, Datta K, Li X, et al. . Detection of urinary excreted fungal galactomannan-like antigens for diagnosis of invasive aspergillosis. PLoS One 2012; 7:e42736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Pauw B, Walsh TJ, Donnelly JP, et al. . European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Altman DG. Practical statistics for medical research. London, UK: Chapman & Hall, 1995. [Google Scholar]
- 21. Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Statist Sci 2001; 16:101–33. [Google Scholar]
- 22. von Eiff M, Roos N, Schulten R, Hesse M, Zühlsdorf M, van de Loo J. Pulmonary aspergillosis: early diagnosis improves survival. Respiration 1995; 62:341–7. [DOI] [PubMed] [Google Scholar]
- 23. Maertens J, Verhaegen J, Lagrou K, Van Eldere J, Boogaerts M. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood 2001; 97:1604–10. [DOI] [PubMed] [Google Scholar]
- 24. Maertens J, Van Eldere J, Verhaegen J, Verbeken E, Verschakelen J, Boogaerts M. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis 2002; 186:1297–306. [DOI] [PubMed] [Google Scholar]
- 25. Maertens J, Theunissen K, Lodewyck T, Lagrou K, Van Eldere J. Advances in the serological diagnosis of invasive Aspergillus infections in patients with haematological disorders. Mycoses 2007; 50:2–17. [DOI] [PubMed] [Google Scholar]
- 26. Dupont B, Huber M, Kim SJ, Bennett JE. Galactomannan antigenemia and antigenuria in aspergillosis: studies in patients and experimentally infected rabbits. J Infect Dis 1987; 155:1–11. [DOI] [PubMed] [Google Scholar]
- 27. Haynes KA, Latge JP, Rogers TR. Detection of Aspergillus antigens associated with invasive infection. J Clin Microbiol 1990; 28:2040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wheat LJ, Hackett E, Durkin M, et al. . Histoplasmosis-associated cross-reactivity in the BioRad Platelia Aspergillus enzyme immunoassay. Clin Vaccine Immunol 2007; 14:638–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Husain S, Kwak EJ, Obman A, et al. . Prospective assessment of Platelia Aspergillus galactomannan antigen for the diagnosis of invasive aspergillosis in lung transplant recipients. Am J Transplant 2004; 4:796–802. [DOI] [PubMed] [Google Scholar]
- 30. Marr KA, Crippa F, Leisenring W, et al. . Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood 2004; 103:1527–33. [DOI] [PubMed] [Google Scholar]