Porphyrins are a class of organic molecules with a macrocyclic tetrapyrrole core and different substituents. Porphyrins have remarkable photo-, catalytic-, electro-, and biochemical properties.1 They are extensively used in self-assembling processes to prepare monolayers and thin films.2,3 Multiporphyrin arrays are prepared both by organic synthesis and self-assembling techniques,1–4 and self-organized nanomaterials (radius ≈ 3 nm) composed of porphyrins have also been prepared.4
The properties of many nanoscaled particles are substantially different than those of bulk materials composed of the same atoms or molecules.5 Nanometer-scale particles composed of metals, metal oxides, and other inorganic materials have been reported5 as have a few composed of organic molecules.6–12 Organic molecules also are used in self-assembling processes to prepare “soft” nanostructures such as spheres and tubes.2,3 Thus, nanoscaled particles composed of porphyrins are expected to have chemical activities significantly different from those of the free porphyrins or of those immobilized onto/into supports. Porphyrin nanoparticles are promising components of advanced materials because of the rich photochemistry, stability, and proven catalytic activity.1 In analogy to inorganic and other organic nanoparticles, it is expected that nanoparticles of porphyrins will have unique photonic properties not obtainable by larger-scaled materials containing the macrocycle, or by the molecules themselves.4 We report the formation of nanoparticles of catalytic porphyrins with enhanced stability and catalytic rate because of the structure of the aggregate and the greater surface area.
Here we report the first synthesis and characterization of porphyrin nanoparticles that are neither self-assembled by designed intermolecular interactions nor encapsulated in an external matrix. Porphyrin nanoparticles were prepared using mixing solvent techniques.7–12 (Polyetheylene)glycol (PEG) derivatives have been widely used in nanoparticle preparations to prevent agglomeration and precipitation of amorphous solids, such as for CdS.13 PEG was used in experiments to prepare nanoparticles composed of meso-substituted tetraphenylporphyrins, Table 1. The procedure to prepare nanoparticles composed of hydrophilic porphyrin Fe(III)1 was to dissolve 0.6–3 mg of porphyrin in 0.035–0.2 mL of water, followed by rapid addition of 5 mL of CH3CN. For nanoparticles composed of hydrophobic/amphipathic porphyrins, 50 µL of (triethyleneglycol)monomethyl ether was mixed into a 0.4 mL (0.28–1.2 mM) solution of the porphyrin in DMSO, and then 5 mL of water was added rapidly. In cases where DMSO was not a suitable solvent, the porphyrin was dissolved in pyridine. The four ethyleneglycol monomethyl ether derivatives appended to porphyrin Fe(III)1 aids in the formation and stabilization of nanoparticles. Control experiments without PEG yielded no stable porphyrin nanoparticles. Consistent with other nanoparticle preparations, the stabilizer prevents agglomeration and is a factor in determining nanoparticle size.13
Table 1.
Hydrodynamic Radii of Nanoparticles Formed from Hydrophobic, Hydrophilic, Amphipathic, and Bis-functionalized Porphyrins, by DLS; = Represents the Distributions

| R | M | Average radius (nm) |
|
|---|---|---|---|
| 1 |  |
Fe(III) | 27(±6) |
| 2 | 4-carboxylpheny1 | 2H+ | 46(±40) |
| 3 | 4-carboxylpheny1, methy1 ester | 2H+ | 58(±47) |
| 4 | Pheny1 | 2H+ | 11(±4) |
| 5 | 4-methoxypheny1 | 2H+ | 34(±30) |
| 6 | 4-pyridy1 | 2H+ | 70(±50) |
| 7 | 5,15-(mesity1)-10,20-(4-bromo-pheny1) | 2H+ | 24(±6) |
Dynamic light scattering (DLS) was used for initial characterization of the porphyrin nanoparticles, Table 1. Figure 1A shows the DLS measurement of a nanoparticle size of Fe(III)1. The average particle radius is 27 nm, and the size distribution of the nanoparticles is remarkably narrow. Since many applications of these materials require them to be on surfaces, atomic force microscopy (AFM) was used to evaluate the integrity of the nanoparticles on glass in the absence of solvent, Figure 2. The heights of the nanoparticles measured by AFM are 30–65 nm, Figure 1B, which is in general agreement with the DLS result, but this is not necessarily the case for each entry in Table 1. Consistent with other nanoparticle preparations,7–12 different water-to-acetonitrile ratios affect the size of porphyrin nanoparticles, Figure 3.
Figure 1.
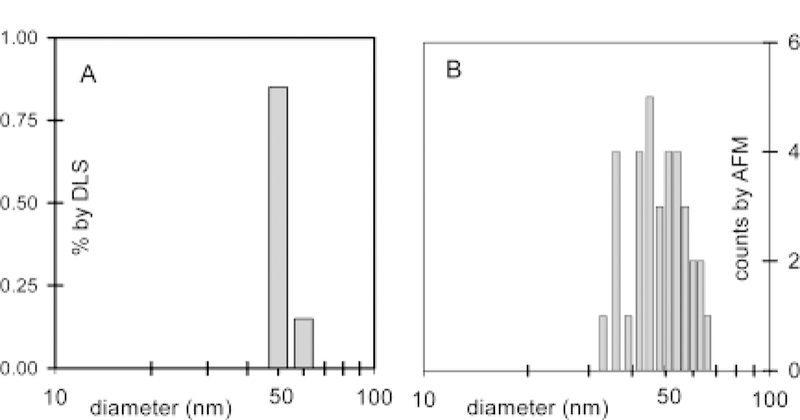
(A) DLS characterization of porphyrin nanoparticles, average diameter = 54 nm, made by adding 5 mL of acetonitrile to a solution containing 0.6 mg of Fe(III)1 in 0.035 mL of water. (B) Height distributions determined by AFM of the same solution see Figure 2.
Figure 2.

Topographic AFM image of porphyrin nanoparticles of Fe(III)1 on glass. The particles are from the same solution as that used in the DLS measurement.
Figure 3.

Relationship between particle radius of porphyrin Fe(III)1 and water content of solvent determined by DLS; the error bars represent the distributions.
As expected, the UV-vis spectra of porphyrin nanoparticles are significantly different compared to the spectra of the corresponding porphyrin solutions. Soret bands were found to be broadened and split. Figure 4 presents the optical spectra of nanoparticles of a free base hydrophilic porphryin 2, and of its component molecule in water. The arrangement of macrocycles in aggregates generally fall into two types, “J” (edge-to-edge) and “H” (face-to-face). Each type, J and H, have distinctive spectral features that can be exploited or utilized.14 The split Soret band together with the broadened and red-shifted Q-bands in the optical spectra suggest both types of interactions in the nanoparticles.6,14 These spectra are consistent with other porphyin nanoscaled aggregates encapsulated in MCM-416 and are well-understood to be indicative of electronic coupling of the chromophores.1,4,14
Figure 4.
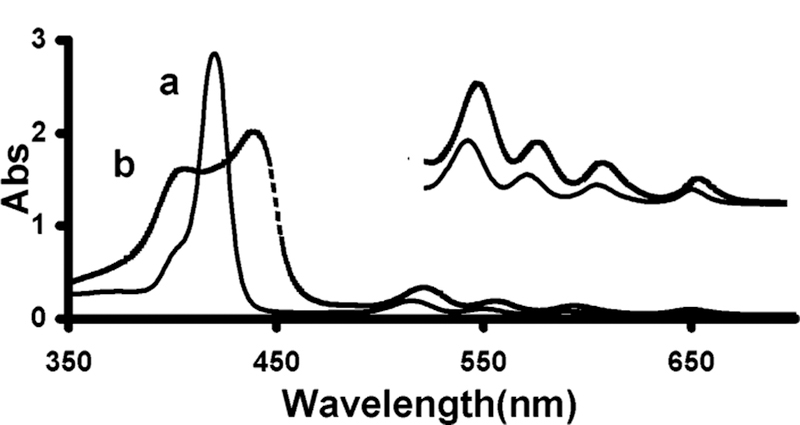
UV-vis spectra, 2H2 in DMSO (a) and its nanoparticle in water (b). The inset absorbance scale is ×5.
The porphyrin nanoparticles are likely held together by hydro- phobic and π-stacking effects.14 In polar solvents such as water, these intermolecular forces become stronger, and no mater the substitution, the porphyrin core remains hydrophobic. The initial dispersion in the solvent system may contain hundreds of porphyrins π-stacked together. If the particle is more than one porphyrin wide in any dimension, as observed by DLS and AFM data, the aggregation may be cooperative-resulting in more thermodynamically stable particles than expected from the ∼5 kcal/mol per porphyrin face π-stacking energy.14 The porphyrin nanoparticles are exceptionally stable as judged by the unchanging optical spectrum and DLS after months of storage.
Preliminary results on the catalytic activity of Fe porphyrin nanoparticles, Fe(III)1, or Fe(III)7 reveal that they have a ∼70- fold greater turnover number and a 10-fold greater rate than the individual porphyrin in solution using a standard catalytic epoxidation of cyclohexene.15
In conclusion, we have developed a general method for preparation of stable 20–200nm diameter porphyrin nanoparticles from a wide variety of meso-arylporphyrins. The elegance of the method lies in its simplicity. This work shows that the agent used to prevent agglomeration can be covalently attached to the dye forming the particle or as part of the solvent system. It also demonstrates that these and other types of dyes with a range of photonic properties do not need to be prepared by encapsulation in matrices or by designed self-assembly a priori. The matrix may severely limit the functionality of the particles in the former case, and at present, this size of particle is difficult to achieve in the latter. A “green” synthesis of porphyrins16 will also make these materials more economically feasible.
Supplementary Material
Acknowledgment.
C.M.D. thanks NSF, CHE-0135509, and the Israel-U.S. Binational Science Foundation.
Footnotes
Supporting Information Available: Preparation methods, DLS histograms, UV-vis, and AFM data for porphyrin nanoparticles in Table 1. (PDF) This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).(a) See: Chou J-H; Kosal ME; Nalwa HS; Rakow NA; Suslick KS In The Porphyrin Handbook; Kadish KM, Smith KM, Guilard R, Eds.; Academic Press: New York, 2000; Vol. 6, pp 43–131 [Google Scholar]; (b) Chambron J-C; Heitz V; Sauvage J-P In The Porphyrin Handbook; Kadish KM, Smith KM, Guilard R, Eds.; Academic Press: New York, 2000; Vol. 6, pp 1–42. [Google Scholar]
- (2).Self-assembly reviews: (a) Lehn J-M Pure Appl. Chem 1994, 66, 1961– 1966. [Google Scholar]; (b) Lindsey JS New J. Chem 1991, 15, 153–180. [Google Scholar]; (c) Stang PJ; Olenyuk B Acc. Chem. Res 1997, 30, 502–518. [Google Scholar]
- (3).Belanger S; Hupp JT Angew. Chem., Int. Ed 1999, 38, 2222–2224. [PubMed] [Google Scholar]; (b) Crossley MJ; Prashar JK Tetrahedron Lett 1997, 38, 6751– 6754. [Google Scholar]
- (4).Drain CM; Nifiatis F; Vasenko A; Batteas JD Angew. Chem., Int. Ed 1998, 37, 2344–2347. [DOI] [PubMed] [Google Scholar]; (b) Drain CM; Lehn J-M J. Chem. Soc., Chem. Commun 1994, 2313–2315.; (c) Milic TN; Chi N; Yablon DG; Flynn GW; Batteas JD; Drain CM Angew. Chem., Int. Ed 2002, 42, 2117–2119. [PubMed] [Google Scholar]
- (5).Dagani R Chem. Eng. News 1998, 76, 70–78. [Google Scholar]; (b) Dagani R Chem. Eng. News 1998, 76, 1–32. [Google Scholar]; (c) Dagani R Chem. Eng. News 1999, 77, 25–37. [Google Scholar]
- (6).Xu W; Guo H; Akins DL J. Phys Chem B 2001, 105, 1543–1546. [Google Scholar]
- (7).Keuren EV; Georgieva E; Adrian J Nano Lett 2001, 1, 141–144. [Google Scholar]
- (8).Wiese H; Horn D Ber. Bunsen-Ges. Phys. Chem 1993, 97, 1589– 1597. [Google Scholar]
- (9).Debuigne F; Jeunieau L; Wiame LJ; Nagy JB Langmuir 2000,16, 7605–7611 [Google Scholar]
- (10).Fu HB; Yao JN J. Am. Chem. Soc 2001, 123, 1434–1439. [Google Scholar]
- (11).Matsuda H; Van Keuren E; Masaki A; Yase K; Mito A; Takahashi C; Kasai H; Kamatani H; Okada S; Nakanishi H Nonlinear Opt 1995, 10, 123–128. [Google Scholar]
- (12).Li M; Jiang M; Zhu L; Wu C Macromolecules 1997, 30, 2201– 2203 [Google Scholar]
- (13).Qi L; Colfen H; Antonietti M Nano Lett 2001, 1, 61–65. [Google Scholar]
- (14).Maiti NC; Mazumdar S; Periasamy N, J. Phys. Chem. B 1998, 102, 1528–1538 [Google Scholar]; (b) Hunter CA; Sanders JK M. J. Am. Chem. Soc 1990, 112, 5525–5534. [Google Scholar]; (c) Kano K; Minamizono H; Kitae T; Negi SJ Phys. Chem. A 1997, 101, 6118–6124. [Google Scholar]; (d) Drain CM; Batteas JD; Flynn GW; Milic T; Chi N; Yablon DG; Sommers H Proc. Natl. Acad. Sci. U.S.A 2002, 99, 6498–6502. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Purrello R; Monsu’ Scolaro L; Bellacchio E; Gurrieri S; Romeo A Inorg. Chem 1998, 37, 3647– 3648. [DOI] [PubMed] [Google Scholar]
- (15).Groves JT; Nemo TE J. Am. Chem. Soc 1983, 105, 5786–5791. [Google Scholar]
- (16).Drain CM; Gong X Chem. Commun 1997, 2117–2118.JA027405Z
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


