Abstract
Anti-NGF therapy has shown significant promise in attenuating several types of skeletal pain. However, whether anti-NGF therapy changes the level of physical activity in individuals with or without skeletal pain is largely unknown. Here, automated day/night activity boxes monitored the effects of anti-NGF treatment on physical activity in normal young (3 months old) and aging (18–23 months old) mice and mice with bone fracture pain. Although aging mice were clearly less active and showed loss of bone mass compared to young mice, anti-NGF treatment had no effect on any measure of day/night activity in either the young or aging mice. In contrast, in mice with femoral fracture pain, anti-NGF treatment produced a clear increase (10–27%) in horizontal activity, vertical rearing, and velocity of travel compared to the fracture + vehicle group. These results suggest just as in humans, mice titrate their level of physical activity to their level of skeletal pain. The level of skeletal pain may in part be determined by the level of free NGF which appears to rise following injury but not normal aging of the skeleton. In terms of bone healing, animals that received anti-NGF showed an increase in the size of calcified callus but no increase in the number of displaced fractures or time to cortical union. As physical activity is the best non-drug treatment for many patients with skeletal pain, anti-NGF may be useful in reducing pain and promoting activity in these patients.
Keywords: nociceptors, neurotrophins, peripheral sensitization, bone, joint, anti-NGF
Summary:
Anti-NGF does not increase physical activity in young or aging naïve mice but attenuates the decline in physical activity in mice with skeletal pain.
INTRODUCTION
The problem of musculoskeletal pain
Skeletal pain is a leading cause of morbidity and mortality in both developing and developed countries [62; 64; 100]. Despite the increase in burden of musculoskeletal pain, treatment options remain limited. The two major classes of analgesics used to treat musculoskeletal pain are NSAIDs and opiates although both are accompanied by a variety of unwanted side effects. Long term NSAID use may inhibit bone formation in patients at high risk for impaired bone healing [81] as well as produce significant gastrointestinal and renal side effects [2; 23; 76; 77]. In developed market economies, the majority of prescribed opiates are for the management of chronic musculoskeletal pain. However, opiates have a variety of adverse side effects including sedation, respiratory compromise, genitourinary retention, and dependence all of which negatively impact the individual’s physical activity, functional status and ability to return to work [45; 84; 97]. The lack of efficacious and better tolerated analgesics for treating musculoskeletal pain has undoubtedly played a major role in the current opioid epidemic plaguing several developed countries, including the US.
The promise and unknowns of anti-NGF and skeletal pain
Preclinical studies have shown that administration of anti-nerve growth factor (anti-NGF) can attenuate a variety of skeletal pains including that due to osteoarthritis [10; 35; 36; 38; 51; 88; 101], hip dysplasia [53], bone fracture [43; 48; 78; 83; 99; 102], orthopedic surgery [59], bone cancer [31; 65; 67; 87], and head and neck cancer [103]. In humans, anti-NGF has been shown to significantly reduce the magnitude of osteoarthritis (OA) and low back pain [9; 17; 18; 44; 52; 69]. Anti-NGF treatment has also been shown to produce significant relief of human bone cancer pain, although this relief was only observed in patients that had high bone pain and low opiate use upon initial enrollment in the study [90].
One side effect that was observed when using anti-NGF to treat patients with OA was that a subset of patients developed an accelerated form of OA that has been previously described as rapidly progressive osteoarthritis (RPOA) [33]. Risk factors for the development of RPOA in these studies included a high dose of anti-NGF, concomitant use of NSAIDs and underlying joint pathologies [33]. A major question is whether this RPOA is due to a direct biological effect of anti-NGF on the bone and/or joint [8; 33; 34; 86] or whether in some patients the significant pain relief offered by anti-NGF may lead to increased physical activity resulting in further injury to an already compromised arthritic joint [64; 71].
Currently, relatively little is known as to whether anti-NGF therapy increases the level of physical activity in humans or animals with or without skeletal pain [20; 29; 53; 59; 71]. In the present study, this question is explored in naïve mice and mice with skeletal pain due to fracture of the femur. Activity was quantified using automated activity boxes that continuously record horizontal activity, vertical activity and velocity of movement over a 20-hour day/night period.
METHODS
Animals
Experiments were performed on 70 adult male C57Bl/6J mice (numbers represent three independent experiments) (Jackson Laboratories, Bar Harbor, ME, USA), approximately 8–10 weeks old (young adult) or 18–23 months old (aged adult), weighing 22–30g at the time of stabilizing pin implantation surgery (aged, naïve mice were 28–40g). Forty-two mice were used to establish the timeline and effect of fracture on activity, rearing and velocity of movement after anti-NGF or vehicle (16 anti-NGF, 16 vehicle, 10 naive) administration while an additional 28 naïve mice (15 young and 13 aged) were tested before and after the administration of anti-NGF. Animals were individually housed (AAALAC approved SPF facility, Lab Products IVC 750 cages, 6.75”x12.25”x5”, with ¼” corn cob bedding and nestlet) for at least 2 weeks prior to baseline recordings and continued throughout the duration of the experiment. After one week of acclimation to the housing facility, all mice were numbered via eartag. For the fracture experiment, the first thirty-two odd numbered mice were assigned to a group and even numbered mice to another group prior to any testing or surgical procedures. Fracture mice underwent stabilizing pin implantation surgery after naïve baselines were obtained. Mice were housed in accordance with National Institutes of Health Guidelines and kept in a vivarium maintained at 22°C with a 12h alternating light–dark cycle (7am-7pm) and provided food and water ad libitum. All procedures adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain and were approved by the Institutional Animal Care and Use Committee at the University of Arizona (Tucson, AZ, USA).
Stabilizing Pin Implantation Surgery
An arthrotomy was performed as described previously [60; 61]. In short, following induction of general anesthesia with ketamine/xylazine (100 mg/kg ketamine and 10 mg/kg xylazine; s.c.), a 1-centimeter incision was made in the skin above the right femur. The skin over the knee was de-gloved and the joint was exposed by transposing the patella medially via blunt dissection while the knee is flexed. A 0.5mm in diameter hole was drilled in the center of the trochlear groove of the femur using a pneumatic dental high-speed hand piece. A precut 0.015-in-diameter stainless steel wire (Small Parts, Inc., Logansport, IN) was inserted into the intramedullary canal for fracture stabilization. The drill site was sealed with a dental amalgam plug (Dentsply, Milford, DE). The knee was extended and the patella returned to its proper position. To minimize medial patella luxation, the rectus femoris and vastus medialis muscles near the knee were secured back in position using a horizontal mattress suture. Wound closure was achieved with two 7-mm auto wound clips (Becton Dickinson, Sparks, MD). Animals recovered from anesthesia on heating pads, and received injections of antibiotic (Baytril, 85mg/kg, s.c.) and sterile saline (1mL, s.c.). After recovering from surgery, animals remained individually housed to avoid fighting which increases the likelihood of displacement of the patella.
Fracture Production
A closed mid-diaphyseal fracture of the femur was produced 28 days after pin implantation. Following induction of general anesthesia with ketamine/xylazine (100 mg/kg ketamine and 10 mg/kg xylazine; s.c.), a fracture of the right femur was produced using a three-point bending device (BBC Specialty Automotive Center, Linden, NJ) based on the fracture apparatus described by Bonnarens and Einhorn (1984). The anesthetized mice were placed in a supine position with the femur (medial side up) directly over the support anvil of the bending device. The blunt guillotine blade was gently lowered onto the hind limb equidistant between the knee and the hip joints. A 168g weight was dropped onto the guillotine from a height of 18cm creating the closed fracture. Immediately after fracture, mice were radiographed to ensure localization of fracture to the mid-diaphysis of the femur (± 1.5mm).
Radiography
While under general anesthesia on the day of fracture, high-resolution x-ray images of the mediolateral plane of the ipsilateral femur were obtained using a Faxitron MX-20 digital cabinet x-ray system (Faxitron/Bioptics, Wheeling, IL). Animals also had x-ray images taken under light anesthesia (50 mg/kg ketamine and 5 mg/kg xylazine; s.c.) on day 7 post-surgery, as x-ray images easily show if patellar displacement has occurred, and days 0, 7, 14, 21, 42 and 63 post-fracture. Also using Photoshop, x-ray images, as TIFF files, were used to calculate the area of the calcified callus (mm2) around the healing femur as well as determining when cortical union occurs. The days when cortical union occurred were scored by blinded individuals other than the investigator performing the measurement of physical activity.
Exclusion Criteria
Animals were excluded from the experiment if any of these conditions occurred: surgical complications, a loss of more than 20% of their pre-surgery weight, if patella displacement had occurred as identified through radiography, fractures located too far from the mid-diaphyseal region of the femur, dislodged pin, nonvisible fracture after impact or fragmentation of the bone. The final number of mice used for the fracture experiment was 30 (one mouse was lost due to bone fragmentation and one mouse was removed due to fracture located too distally; both mice were from the Fracture + anti-NGF group).
Assessment of Locomotor Activity
For the fracture experiments, mice were assessed at day −7 (baseline pre-surgery), day 21 post-surgery (to insure the return to baseline levels) and on days 1, 3, 7 and 14 post-fracture for locomotor activity (horizontal activity and velocity) and vertical rearing episodes. For the anti-NGF in naïve mice experiment, mice were assessed at naïve baseline and 4 days post-anti-NGF administration for locomotor activity (horizontal activity and velocity) and vertical rearing episodes. Animals were placed individually in plexiglass boxes (16×16×11.75 inches) containing a thin layer of bedding and a 1-inch square of Napa Nectar (Systems Engineering, Napa CA, USA). Locomotor activity and rearing episodes were assessed via open field monitoring by arrays of photo-beam sensors that use beam breaks to determine the location of each animal at all times (Omnitech Electronics, Columbus OH, USA). Locomotor activity and rearing episodes were monitored for 20 hours beginning at 1200 hours (noon) in a light (12-hour light/dark, 7am-7pm) and temperature controlled (22◦−26◦ C) testing room that remained closed to any other activity. Fusion software (Omnitech Electronics) was used to analyze and store the above parameters.
Drug Treatment
NGF sequestering antibody (mAb911 at a dose of 10mg/kg (i.p.), a kind gift from Dr. David Shelton and Dr. Kris Poulsen, Rinat/Pfizer, San Francisco CA, USA) or vehicle (PBS, 10ml/kg) was given 1 hour following bone fracture and once every five days thereafter until day 15. This dose was chosen as it significantly reduced evoked pain behaviors in preclinical models of skeletal pain (McCaffrey, 2014; Majuta, 2015; Guedon, 2016). Mice were assigned to a group via even or odd numbered eartags. Anti-NGF and vehicle solutions were prepared in equal volumes and labeled A and B by an individual not associated with testing the animals. The individual giving the injections was blinded as to the identity of each solution.
Statistical Analysis
All values are expressed as means ± SEM. ANOVA was performed on data from each day (0,1,3,7,14) separately for the “exploratory hour” and for the “first three hours of dark” variables for horizontal distance, vertical episodes, and average velocity. All variables were log transformed before AUC computation. Analyses of variance with post-hoc t-tests (unadjusted for multiple comparisons) were used to compare the three mouse groups (Naive, Fracture + Vehicle, Fracture + anti-NGF). Two-group t-tests were used when comparing the Fracture + Vehicle and Fracture + anti-NGF groups on callus area as well as for the naïve before and after anti-NGF administration. Union results were calculated using Kruskal-Wallis one-way nonparametric ANOVA. Significance level was set at p ≤ 0.05.
RESULTS
The fracture model and bone healing
Fracture pain was generated in animals by implantation of a stabilizing pin followed 28 days later by the production of a closed mid-diaphyseal fracture of the femur using a three-point bending device. Figure 1 shows a naïve femur, the stages of fracture production (pin placement and fracture) and fracture healing (mineralized callus and fracture union) using high-resolution x-ray images (Figure 1A-E respectively). The fracture (Figure 1C-arrows) must occur within 1.5mm of the mid-diaphysis. The mineralized callus is radiographically apparent by day 14 post-fracture (Figure 1D).
Figure 1. Radiographic images of the mouse model of bone fracture pain.
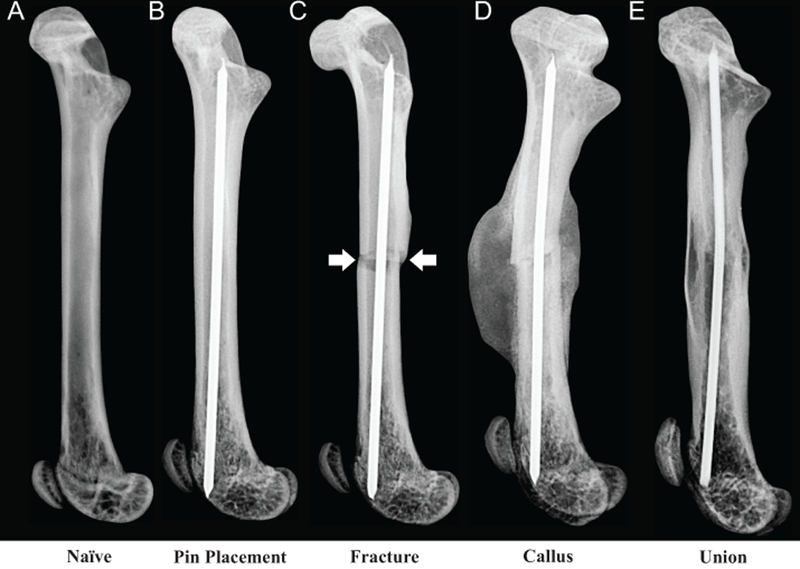
A 0.5-mm hole was drilled in the center of the trochlear groove of naïve C57Bl/6J male mice (A), 8 to 9 weeks old. A pin was then placed (B) into the intramedullary canal, the drill site was sealed with a dental amalgam plug and the mice were allowed to recover for 3 weeks before pre-fracture activity was measured. A closed mid-diaphyseal fracture (arrows) of the femur (C) was performed using a 3-point impact device. Formation of a large mineralized callus (D) occurs at 14-days post-fracture and union of the cortical bone (E) is apparent at day 63 post-fracture.
The importance of monitoring spontaneous activity and rearing when animals are normally the most active
In mice, there are marked diurnal variations in horizontal and vertical activity (Figure 2A-B respectively) as well as velocity (Figure 2C) for all groups (naïve, Frac + anti-NGF and Frac + Vehicle) on day 3 post-fracture. When mice are first placed in the activity boxes during the light phase at noon (1200 hours), mice actively explore the novel environment for approximately one hour. After this initial exploration, most mice are at rest or have a marked reduction in activity until the start of the dark phase (1900 hours) when mice again show significant increases in locomotor activity during the hours of night (1900–0700 hours). This period of increased activity during the daytime (1200–1300 hours) is consistent with the behavior of mice that rapidly explore a novel environment for potential threats, whereas the increased activity of the mice immediately after the room becomes dark at 1900 hours is consistent with mice grooming, eating, drinking, making nests, exploring their environment and foraging for food.
Figure 2. Day/night physical activity in naive mice and mice with skeletal pain with and without anti-NGF.
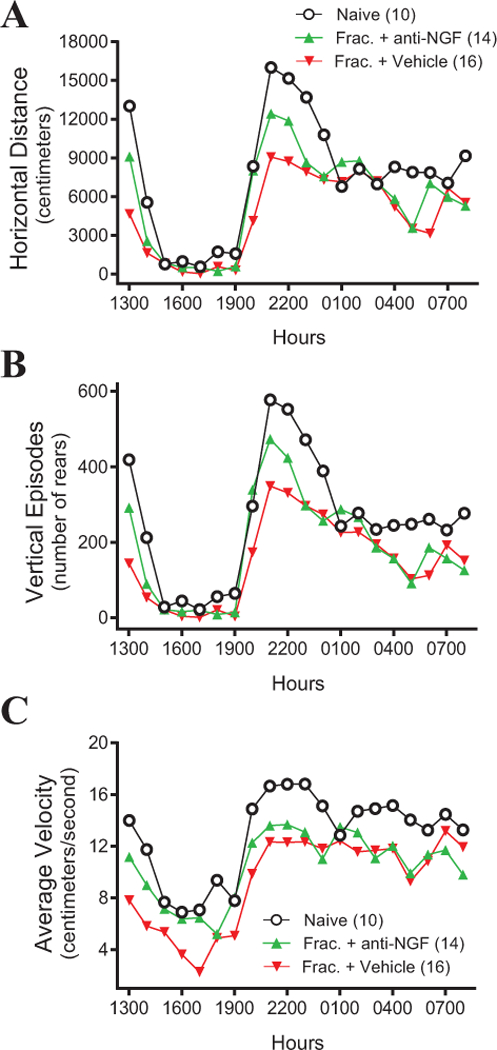
In naive mice (black circles) and mice with fracture + vehicle (upside down red triangles) or fracture + anti-NGF (green triangles) the horizontal and vertical activity and average velocity (A-C respectively) of the mice was continuously recorded for 20 hours, from 1200 (noon) to 0800 hours the following morning under a 12-hour light/dark cycle (0700–1900 hours light and 1900–0700 hours dark). Note that the greatest level of activity and the greatest differences between the three groups was during the first hour the mice were placed in the activity box (1200 to 1300 hours) and the first 3 hours of dark (1900–2100).
Representative tracings of distance and the pattern of horizontal locomotor activity and episodes of rearing from a naïve, fracture + vehicle and fracture + anti-NGF mouse obtained over a 15-minute period during the most active portion of the dark phase (2100–2115 hours) are shown in Figure 3. Visually significant reductions in horizontal activity and rearing episodes are apparent for the fracture + vehicle mouse (Figure 3B and E) three days post-fracture when compared with naive (Figure 3A and D). When compared to the fracture + vehicle mouse, horizontal activity and the number of rearing episodes are increased with the fracture + anti-NGF mouse (Figure 3C and F).
Figure 3. Tracings from a single representative mouse from the naïve, fracture + vehicle and fracture + anti-NGF groups showing the effect of anti-NGF on horizontal movement and vertical rears.
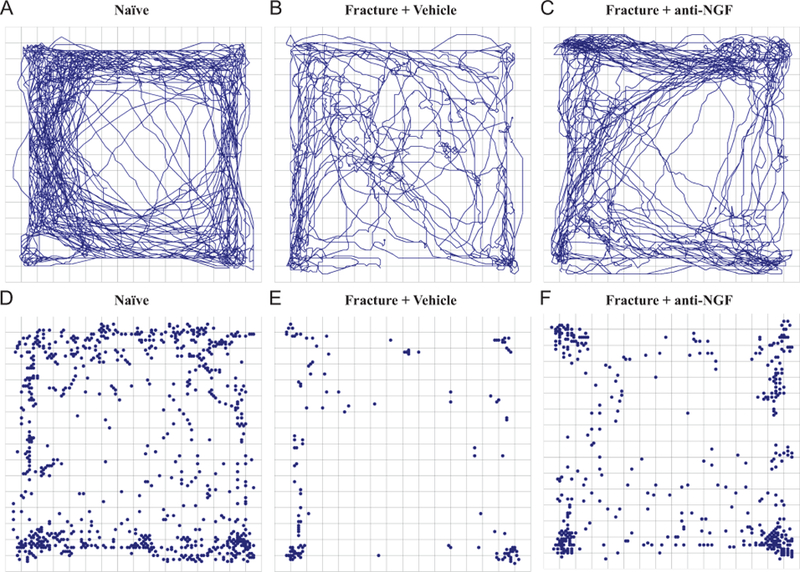
Representative tracings of horizontal activity (A-C) and vertical rearing events (C-F) of a naïve mouse (A and D), a fracture + vehicle mouse (B and E) and a fracture + anti-NGF mouse (C and F) in the activity box 3 days after fracture during the 15-minute nighttime period when mice normally display the greatest spontaneous activity (from 2100 to 2115 hours). Note that compared with a naïve mouse (A & D), there is a marked reduction in horizontal and vertical activity in the fracture + vehicle mouse (B & E) and the fracture induced decline in both horizontal (C) and vertical activity (F) is attenuated by administration of anti-NGF.
The fracture–induced reduction in horizontal distance traveled, number of vertical episodes, and average velocity are attenuated by administration of anti–nerve growth factor
Anti-NGF (10 mg/kg, i.p.) or vehicle was administered on the day of fracture and on days 5, 10 and 15 post-fracture. Comparisons of the initial exploratory hour (1200–1300 hours) and the combined time over the first three hours of the dark phase (1900–2200 hours) are presented in figure 4. Mice treated with anti-NGF (Frac + Anti-NGF) traveled a significantly greater horizontal distance on days 1, 3 and 7 post-fracture during the initial exploratory hour when compared with vehicle-treated (Frac + Vehicle) animals (Figure 4A; vehicle day 1 = 4227 ± 544 cm, anti-NGF day 1 = 5763 ± 374 cm, P = 0.012; vehicle day 3 = 4671 ± 615 cm, and anti-NGF day 3 = 9027 ± 798 cm, P = 0.00004; vehicle day 7 = 7750 ± 898 cm, and anti-NGF day 7 = 9766 ± 557 cm, P = 0.038; vehicle day 14 = 10458 ± 929 cm, and anti-NGF day 14 = 12097 ± 639 cm, P = 0.1). The number of vertical rearing episodes for the Fracture + Anti-NGF animals on days 1 and 3 post-fracture during the initial exploratory hour were also significantly increased when compared with Fracture + Vehicle (Figure 4C; vehicle day 1 = 101 ± 20.3, anti-NGF day 1 = 155 ± 12.6, P = 0.03; vehicle day 3 = 144 ± 27.4, anti-NGF day 3 = 292 ± 22.1, P = 0.0001; ; vehicle day 7 = 300 ± 45.6, and anti-NGF day 7 = 349 ± 21.0, P = 0.08; vehicle day 14 = 388 ± 44.8, and anti-NGF day 14 = 445 ± 22.0, P = 0.1). As with horizontal distance traveled, the average velocity of the Fracture + Anti-NGF animals on days 1, 3 and 7 post-fracture during the initial exploratory hour were also significantly increased when compared with Fracture + Vehicle (Figure 4E; vehicle day 1 = 7.9 ± 0.4 cm/s, anti-NGF day 1 = 9.2 ± 0.3 cm/s, P = 0.02; vehicle day 3 = 7.8 ± 0.5 cm/s, and anti-NGF day 3 = 11.2 ± 0.4 cm/s, P = 0.000008; vehicle day 7 = 10.4 ± 0.5 cm/s, and anti-NGF day 7 = 11.9 ± 0.3 cm/s, P = 0.02; vehicle day 14 = 12.1 ± 0.6 cm/s, and anti-NGF day 14 = 13.0 ± 0.4 cm/s, P = 0.2).
Figure 4. Changes in horizontal distance, vertical rears and velocity of travel at increasing times in naïve mice, mice with fracture + vehicle and mice with fracture + anti-NGF.
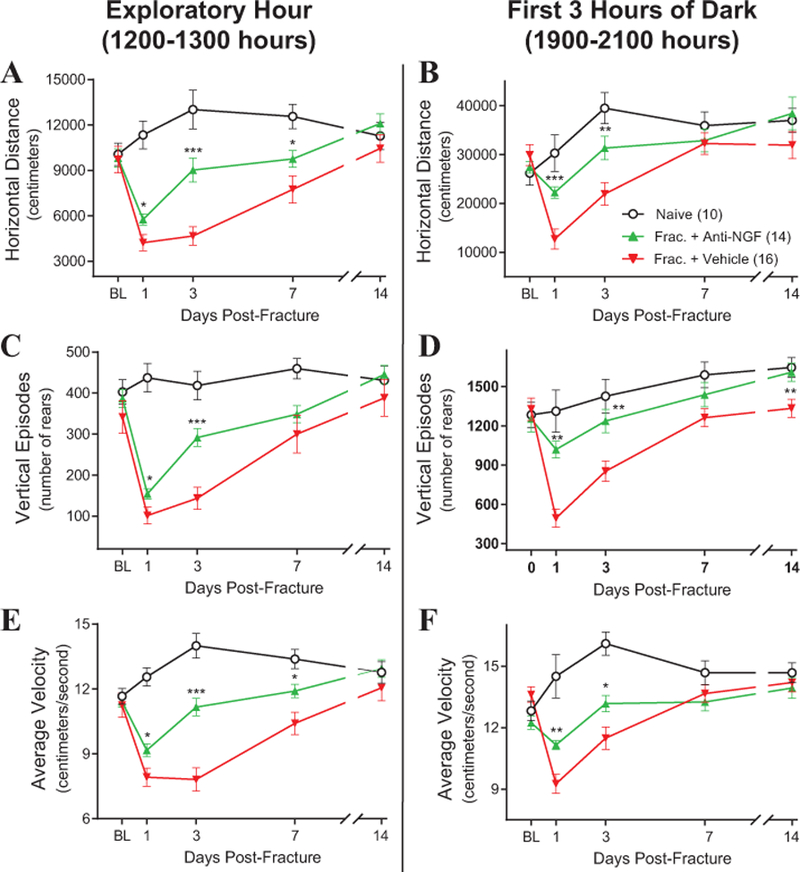
Fracture of the femur induces a marked decline in horizontal distance traveled (A,B), vertical rearing episodes (C,D) and average velocity of horizontal travel (E,F) at the times during the day (A,C,E) & night (B,D,F) when the mice are normally most active. These declines peak at day 1 post-fracture and return to naïve levels by day 14 when the callus has significantly stabilized the fracture site. Note that anti-NGF partially reverses this decline in activity at days 1, 3, and in some cases at day 7 post-fracture. However, by day 14, differences in the activity measures between the three groups have largely disappeared. Statistical significant differences between Fracture + Vehicle animals vs Fracture + Anti-NGF animals during the initial exploratory hour (A, C, E) and the first 3 hours of dark activity (B, D, F) for each day are indicated by *, **, *** = P< 0.05, 0.01 and 0.001, respectively.
Measuring the activity of mice during the dark phase (1900–0700 hours) enables the capture of more spontaneous behaviors. Animals treated with anti-NGF after fracture also exhibited significantly higher horizontal distance traveled on days 1 and 3 post-fracture during peak nocturnal activity (first 3 hours of dark, 1900–2200 hours) compared with fracture + vehicle animals (Figure 4B; vehicle day 1 = 12723 ± 2061 cm, anti-NGF day 1 = 22237 ± 1180 cm, P = 0.0002; vehicle day 3 = 21935 ± 2314 cm, and anti-NGF day 3 = 31359 ± 2422 cm, P = 0.006; vehicle day 7 = 32274 ± 2211 cm, and anti-NGF day 7 = 32878 ± 2346 cm, P = 0.9; vehicle day 14 = 31957 ± 2778 cm, and anti-NGF day 14 = 38451 ± 3319 cm, P = 0.1). The number of vertical rearing episodes for the anti-NGF animals on days 1, 3 and 14 post-fracture during peak nocturnal activity were also significantly increased when compared with Fracture + Vehicle (Figure 4D; vehicle day 1 = 494 ± 68.8, anti-NGF day 1 = 1020 ± 64.1, P = 0.002; vehicle day 3 = 853 ± 77.4, anti-NGF day 3 = 1236 ± 88.9, P = 0.004; vehicle day 7 = 1262 ± 68.2, and anti-NGF day 7 = 1438 ± 92.5, P = 0.2; vehicle day 14 = 1333 ± 69.3, and anti-NGF day 14 = 1609 ± 69.6, P = 0.009). Again as with horizontal distance traveled, the average velocity of the Fracture + Anti-NGF animals on days 1 and 3 post-fracture during peak nocturnal activity were also significantly increased when compared with Fracture + Vehicle (Figure 4F; vehicle day 1 = 9.3 ± 0.5 cm/s, anti-NGF day 1 = 11.2 ± 0.2 cm/s, P = 0.002; vehicle day 3 = 11.5 ± 0.6 cm/s, and anti-NGF day 3 = 13.2 ± 0.4 cm/s, P = 0.014; vehicle day 7 = 13.7 ± 0.4 cm/s, and anti-NGF day 7 = 13.3 ± 0.4 cm/s, P = 0.52; vehicle day 14 = 14.2 ± 0.5 cm/s, and anti-NGF day 14 = 13.9 ± 0.5 cm/s, P = 0.7).
The effect of anti-NGF on horizontal distance traveled, number of vertical episodes, and average velocity in young naïve mice versus aged naïve mice
Previous reports have shown that bone loss occurs with aging in C57Bl/6 mice (Figure 5A) [25]. This loss includes a decrease in cortical thickness of the femur as well as loss of trabecular bone in the proximal and distal femur. In this experiment, anti-NGF (10 mg/kg, i.p.) was administered to young and aged, naïve mice after baseline activity recordings (Figure 5B) were made and re-tested again four days later. Their naïve horizontal distance traveled, number of rearing episodes and average velocity during the initial exploratory hour (first hour of testing, 1200–1300 hours) and during peak nocturnal activity (first 3 hours of dark, 1900–2200 hours) were compared with those values after anti-NGF (Figure 5C-H respectively). For both the young and aged mice, no significant difference was found between naïve and naïve + anti-NGF for any activity (Horizontal distance Explor. naïve young = 12444 ± 676cm, anti-NGF = 13026 ± 780cm, P = 0.939; Horizontal distance Explor. naïve aged = 8916 ± 750cm, anti-NGF = 8225 ± 621cm, P = 0.920; Horizontal distance Dark naïve young = 36193 ± 2126cm, anti-NGF = 36463 ± 2158cm, P = 0.9998; Horizontal distance Dark naïve aged = 29086 ± 3145cm, anti-NGF = 28566 ± 2544cm, P = 0.9991; Vertical episodes Explor. naïve young = 411 ± 27, anti-NGF = 434 ± 26, P = 0.918; Vertical episodes Explor. naïve aged = 287 ± 25, anti-NGF = 260 ± 19, P = 0.895; Vertical episodes Dark naïve young = 1559 ± 79, anti-NGF = 1614 ± 82, P = 0.975; Vertical episodes Dark naïve aged = 1027 ± 94, anti-NGF = 1126 ± 118, P = 0.895; Average velocity Explor. naïve young = 13.1 ± 0.4cm/s, anti-NGF = 13.4 ± 0.4cm/s, P = 0.980; Average velocity Explor. naïve aged = 10.0 ± 0.6cm/s, anti-NGF = 9.3 ± 0.4cm/s, P = 0.786; Average velocity Dark naïve young = 14.7 ± 0.4cm/s, anti-NGF = 14.5 ± 0.4cm/s, P = 0.992; Average velocity Dark naïve aged = 12.4 ± 0.4cm/s, anti-NGF = 12.6 ± 0.4cm/s, P = 0.991). In contrast, the naïve and anti-NGF data, when comparing young vs. aged, clearly shows significant differences between the young and the aged mice for horizontal distance traveled (exploratory hour only), number of rearing episodes and average velocity (Figure 5C-H respectively, Horizontal distance Explor: naïve young vs. naïve aged P = 0.0.0078, young anti-NGF vs. aged anti-NGF P = 0.0002; Horizontal distance Dark: naïve young vs. naïve aged P = 0.222, young anti-NGF vs. aged anti-NGF P = 0.147; Vertical episodes Explor: naïve young vs. naïve aged P = 0.0.0072, young anti-NGF vs. aged anti-NGF P = <0.0001; Vertical episodes Dark: naïve young vs. naïve aged P = 0.0016, young anti-NGF vs. aged anti-NGF P = 0.0044; Average velocity Explor: naïve young vs. naïve aged P = 0.0.0002, young anti-NGF vs. aged anti-NGF P = <0.0001; Average velocity Dark: naïve young vs. naïve aged P = 0.0030, young anti-NGF vs. aged anti-NGF P = 0.0158).
Figure 5. As with most mammals, normal aging is accompanied by significant loss of bone mass and an overall decline in physical activity yet anti-NGF treatment does not increase physical activity in either the young or aged mouse.
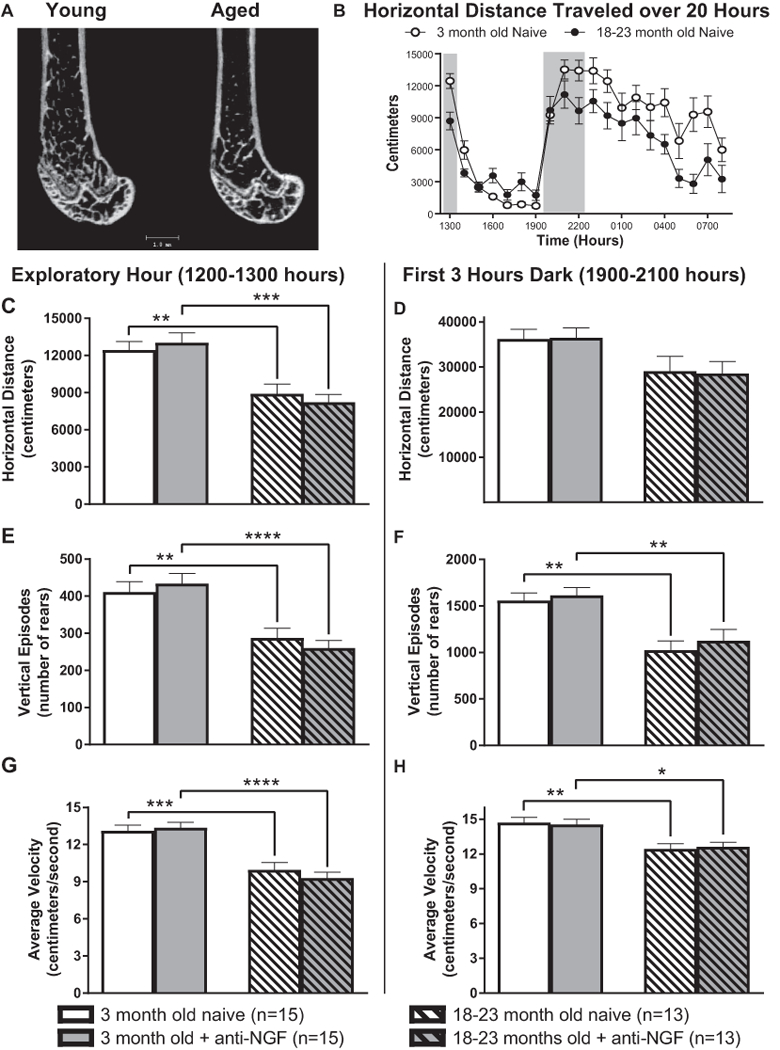
Micro-CT images (A: Kindly provided by Dr. Vaida Glatt, University of Texas Health Science Center San Antonio) of the young (3-month old) and aged (20-month old) distal femur of the male C57Bl/6 mouse show that with aging there is loss of trabecular bone and thinning of the cortical wall. For the time points examined (B), there is also a significant decrease in physical activity in the aged vs. young mice (C, E-H). However, anti-NGF itself does not increase physical activity as measured by horizontal (C & D), vertical rears (E & F) or velocity of travel (G & H) in either the young or aged animals.
The effects of anti-NGF on bone healing following fracture
X-ray images of the healing fractures on days 0, 14, 21, 42 and 63 were used to determine the size of the callus as well as when union has occurred (Figure 6). The callus areas for the fracture + anti-NGF mice were significantly higher on days 14, 21 and 42 post-fracture when compared to fracture + vehicle, but were not significantly different at day 63 (Figure 6A; vehicle day 14 = 5.99 ± 0.4 mm2, anti-NGF day 14 = 9.97 ± 0.6 mm2, P = 0.000006; vehicle day 21 = 6.10 ± 0.5 mm2, anti-NGF day 21 = 8.89 ± 0.5 mm2, P = 0.0006; vehicle day 42 = 3.28 ± 0.4 mm2, anti-NGF day 42 = 5.62 ± 0.4 mm2, P = 0.0004; vehicle day 63 = 3.15 ± 0.4 mm2, anti-NGF day 63 = 4.11 ± 0.3 mm2, P = 0.0596). There was no significant difference between the fracture + vehicle and fracture + anti-NGF groups for the percentage of mice with cortical union on any day post-fracture (Figure 6B; vehicle day 21 = 16.67% ± 9.3, anti-NGF day 21 = 30.77% ± 13.3, P = 0.384; vehicle day 42 = 60.0% ± 13.0, anti-NGF day 42 = 80.77% ± 10.7, P = 0.239; vehicle day 63 = 100% ± 0, anti-NGF day 63 = 100% ± 0).
Figure 6. The effects of anti-NGF on bone healing following fracture.
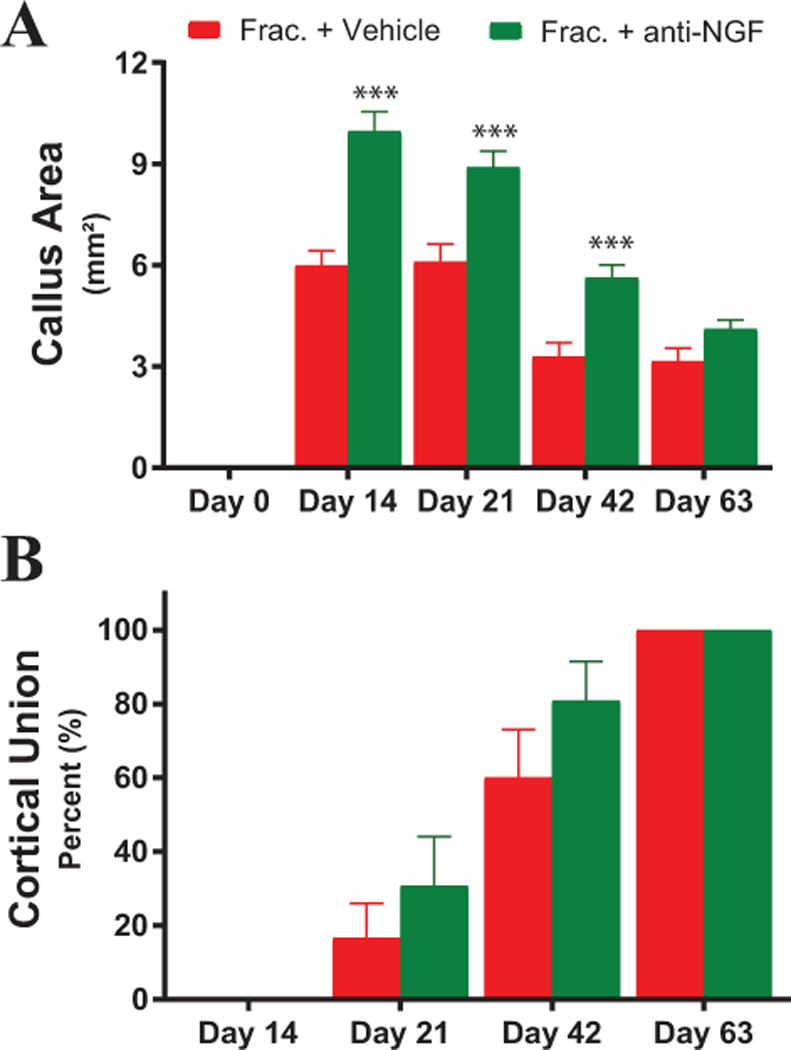
Note that while anti-NGF increased the size of the calcified callus at days 14, 21 and 42 post-fracture (A) by day 63 post-fracture the difference between the two groups had disappeared. Also, note anti-NGF did not appear to impact cortical union (B). For (A-B), the level of significance between Fracture + Vehicle and Fracture + Anti-NGF animals are indicated by *, **, *** = P< 0.05, 0.01 and 0.001, respectively.
DISCUSSION
Changes in physical activity with anti-NGF
In the present study, anti-NGF administration in mice with significant skeletal pain due to bone fracture, produced a 10–27% increase in horizontal activity, rears and velocity of movement as compared to mice that had fracture + vehicle. The most parsimonious explanation of the increased physical activity is that anti-NGF blocks NGF-induced sensitization of nociceptors that innervate the bone [37; 43; 48; 72]. Thus, similar to humans, mice appear to be titrating their level of physical activity to their level of pain so that when anti-NGF administration reduces skeletal pain, there is a concomitant increase in physical activity.
In most animals, normal aging is accompanied by significant loss of mass/strength and a decrease in physical activity. One possibility is that as bone mass and strength deteriorates with aging there would be a concomitant increase in the expression and release of NGF as is seen in acutely injured tissues [15; 54; 55]. This gradual increase of NGF in the aging bone would in turn drive the decrease in physical activity that is observed with normal aging. However, acute treatment of normal young or aging animals with anti-NGF did not increase physical activity in either group. These results suggest that the age-related decline in physical activity that is generally seen in normal, healthy humans and animals is probably driven more by a CNS driven increase in fatigue rather than peripherally driven NGF-induced bone pain.
The expression and release of NGF in the normal and injured skeleton
Recent data suggest that in the normal, young mouse femur there is significant expression of NGF by a remarkably restricted set of cells in bone and cartilage [12]. Thus, while less than 2% of all cells in the femur display any NGF expression, robust NGF immunoreactivity was observed in mostly CD-31 negative blood vessel-associated cells that have pericyte like morphology, a small subset of CD-31+ endothelial cells, an unidentified group of cells located at the subchondral bone/articular cartilage interface, and a few isolated, single cells in the bone marrow [12]. Interestingly, most of the NGF expressing blood vessel associated cells were surrounded by both TrkA+ and p75+ sensory and sympathetic nerve fibers. These data show that there is a close association between NGF+ blood vessel-associated cells and TrkA+ and p75+ nerve fibers in the naïve bone [7; 11; 12; 21; 41; 42]. The present finding that anti-NGF treatment does not alter activity in naïve mice suggests that there is little ongoing basal release of NGF and little free NGF in the normal, uninjured skeleton. However, following significant skeletal injury (here bone fracture) NGF is released resulting in a marked NGF induced sensitization and activation of the TrkA and p75 sensory nerve fibers. This NGF-induced sensitization of sensory nerve fibers is reduced when anti-NGF is present.
The above data suggest that there is significant expression, but little release, of free NGF in the normal uninjured femur. However, a largely unanswered question is whether there is marked expansion of the types of cells expressing and/or releasing NGF following injury, disease or aging of the skeleton. Previous studies have shown that there is a marked inflammatory and immune response following injury to the skeleton [43; 58; 66]. However, which specific cells in vivo express and release NGF in the injured skeleton is largely unknown. It has been reported that mast cells, macrophages, T- and B-lymphocytes, neutrophils, fibroblasts, pericytes, endothelial cells, stem cells, osteoblasts, osteoclasts and osteocytes express NGF [5; 24; 27; 28; 70; 75; 96]. Many of the above studies showing NGF expression in bone were performed in vitro and do not thoroughly assess the specificity of the immunostaining so the specific cells that express and release authentic NGF in the injured skeleton still remains largely undefined [15; 54; 55]. Understanding whether the expression and release of NGF changes with injury, disease or aging in the in vivo skeleton would significantly increase our understanding of the mechanisms that drive skeletal pain.
Effects of anti-NGF on bone healing and physical activity
In terms of short-term bone healing, anti-NGF treatment clearly does increase the size of the calcified callus although this is in the context of a 10–27% increase in physical activity. At day 63 post-fracture, the size of the calcified callus in mice with fracture + anti-NGF is the same as in mice with fracture + vehicle. These data together with our findings showing that fracture + anti-NGF had no increase in number of displaced fractures and no change in cortical union are in agreement with previous data showing that anti-NGF treatment does not impair fracture healing of bone or the mechanical strength of newly formed bone at the site of fracture [48; 78]. In other studies, a similar increase in size of the calcified callus following fracture was also observed in animals that had received neonatal capsaicin, resulting in a 50% reduction in sensory nerve fibers innervating the skeleton and a 50% reduction in pain behaviors (guarding and flinching) following bone fracture [40]. These and other studies [32; 73], suggest that increased callus size is in part due to increased loading and use of the affected limb [64] and further research into the potential effects of NGF on bone formation and healing are clearly needed [33; 34; 78; 80; 95; 96].
The present report shows that anti-NGF increases physical activity in mice with facture pain but not in normal young or aging mice. However, is this increase in activity desirable in terms of skeletal healing, skeletal health, and improving the functional status of patients with skeletal pain? Although it may be counter-intuitive, previous studies have repeatedly shown that physical activity is the best non-drug treatment for improving pain and the functional status of patients with a variety of chronic skeletal pains including osteoarthritis, low back pain, fibromyalgia, and bone fracture [6; 26; 29; 47; 74; 85]. Loading of bone has been shown to decrease the expression of sclerostin, a protein expressed by bone osteocytes that inhibits bone formation [56; 68; 92; 93]. Previous data have also shown that loading of the bone following fracture increases callus formation, promotes fracture healing and decreases bone and muscle loss [13; 22; 39]. While overuse of the injured skeleton can result in further injury to the skeleton, moderate exercise and use of the skeleton is a key component to maintaining both bone and muscle mass.
Translating preclinical rodent data into human clinical studies
Currently, the most common endpoint used to measure skeletal pain in rodents is mechanical hyperalgesia of the skin of the hind paw [1; 19; 63; 89]. Skin hypersensitivity clearly does occur in some animals [3; 46; 79; 94] and humans [3; 4; 14; 50] with skeletal pain conditions. However, it remains unclear what specific mechanisms generate skin hypersensitivity and whether relief of skin hypersensitivity accurately predicts the extent of the relief of the underlying skeletal pain. Previous studies have shown that a therapy can relieve skin hypersensitivity but not reduce the underlying skeletal pain [30]. In rodent models of osteoporosis, there is both cutaneous and deep musculoskeletal pain but these two types of pain are differentially sensitive to pharmacological interventions [91]. Additionally, few human studies use skin hypersensitivity as a primary endpoint to predict the relief of skeletal pain.
Previous data collected in humans with musculoskeletal pain have shown that monitoring day/night activity using accelerometers provide an unbiased objective and more complete assessment of skeletal pain than simply asking patients, physicians or caregivers for their daily assessment of the pain [16; 57; 74; 82; 98]. Thus, while the patient assessment of pain is still important, it is frequently done at a single time point each day and is impacted by recency, mood, depression, and memory [49; 74]. In contrast, automated recording of day/night physical activity data tends to be much more specific (date, time, extent) and reliable than observational data alone and may be used to compare and contrast automated day/night physical activity obtained in preclinical models with human clinical trials using accelerometers.
A therapy that reduces skeletal pain, allowing for increased activity, with minimal organ or tissue side effects would be highly desirable for a large clinical population. This study supports the use of activity monitoring as a means to assess skeletal pain and highlights the importance of evaluating both outcomes together since each is significantly affected by the other. With new technologies now available to obtain continuous monitoring of activity and more frequent assessment of pain, such an approach is feasible and will lead to a better understanding of pain mechanisms and their modulation.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health (grant number NS023970) to Patrick Mantyh. The authors thank Dr. Viada Glatt (University of Texas Health Science Center, San Antonio, TX, USA) for generously providing image 5A. The anti-NGF used in this study was a kind gift from Drs. Kris Poulsen and David Shelton (Rinat/Pfizer, San Francisco, CA, USA). Dr. Mantyh has served as a consultant and/or received research grants from Abbott (Abbott Park, IL), Adolor (Exton, PA), Array Biopharma (Boulder, CO), Johnson and Johnson (New Brunswick, NJ), Merck (White Plains, New York), Pfizer (New York, NY), Plexxikon (Berkeley, CA), Rinat (South San Francisco, CA), and Roche (Palo Alto, CA). All other authors report no conflict of interest.
REFERENCES
- [1].Abe Y, Iba K, Sasaki K, Chiba H, Kanaya K, Kawamata T, Oda K, Amizuka N, Sasaki M, Yamashita T. Inhibitory effect of bisphosphonate on osteoclast function contributes to improved skeletal pain in ovariectomized mice. J Bone Miner Metab 2015;33(2):125–134. [DOI] [PubMed] [Google Scholar]
- [2].Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma 1995;9(5):392–400. [DOI] [PubMed] [Google Scholar]
- [3].Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149(3):573–581. [DOI] [PubMed] [Google Scholar]
- [4].Arendt-Nielsen L, Skou ST, Nielsen TA, Petersen KK. Altered Central Sensitization and Pain Modulation in the CNS in Chronic Joint Pain. Curr Osteoporos Rep 2015;13(4):225–234. [DOI] [PubMed] [Google Scholar]
- [5].Asaumi K, Nakanishi T, Asahara H, Inoue H, Takigawa M. Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone 2000;26(6):625–633. [DOI] [PubMed] [Google Scholar]
- [6].Baker K, McAlindon T. Exercise for knee osteoarthritis. Curr Opin Rheumatol 2000;12(5):456–463. [DOI] [PubMed] [Google Scholar]
- [7].Bloom AP, Jimenez-Andrade JM, Taylor RN, Castaneda-Corral G, Kaczmarska MJ, Freeman KT, Coughlin KA, Ghilardi JR, Kuskowski MA, Mantyh PW. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J Pain 2011;12(6):698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain 2012;13(8):790–798. [DOI] [PubMed] [Google Scholar]
- [9].Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum 2013;65(7):1795–1803. [DOI] [PubMed] [Google Scholar]
- [10].Bryden LA, Nicholson JR, Doods H, Pekcec A. Deficits in spontaneous burrowing behavior in the rat bilateral monosodium iodoacetate model of osteoarthritis: an objective measure of pain-related behavior and analgesic efficacy. Osteoarthritis Cartilage 2015;23(9):1605–1612. [DOI] [PubMed] [Google Scholar]
- [11].Castaneda-Corral G, Jimenez-Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, Ghilardi JR, Mantyh PW. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience 2011;178:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chartier SR, Mitchell SA, Majuta LA, Mantyh PW. Immunohistochemical localization of nerve growth factor, tropomyosin receptor kinase A, and p75 in the bone and articular cartilage of the mouse femur. Mol Pain 2017;13:1744806917745465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 2012;8(3):133–143. [DOI] [PubMed] [Google Scholar]
- [14].Curatolo M, Arendt-Nielsen L. Central hypersensitivity in chronic musculoskeletal pain. Phys Med Rehabil Clin N Am 2015;26(2):175–184. [DOI] [PubMed] [Google Scholar]
- [15].Denk F, Bennett DL, McMahon SB. Nerve Growth Factor and Pain Mechanisms. Annu Rev Neurosci 2017;40:307–325. [DOI] [PubMed] [Google Scholar]
- [16].Dunlop DD, Song J, Semanik PA, Chang RW, Sharma L, Bathon JM, Eaton CB, Hochberg MC, Jackson RD, Kwoh CK, Mysiw WJ, Nevitt MC, Hootman JM. Objective physical activity measurement in the osteoarthritis initiative: Are guidelines being met? Arthritis Rheum 2011;63(11):3372–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ekman E, Gimbel J, Bello A, Smith M, Keller D, Annis K, Brown M, West C, Verburg K. Efficacy and safety of tanezumab in comparison to placebo and naproxen in the treatment of osteoarthritis knee or hip pain: results of two phase 3 studies (NCT00830063 and NCT00863304), Proceedings of the American pain society, 30th annual scientific meeting, 2011. [Google Scholar]
- [18].Evans RJ, Moldwin RM, Cossons N, Darekar A, Mills IW, Scholfield D. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol 2011;185(5):1716–1721. [DOI] [PubMed] [Google Scholar]
- [19].Fan HB, Zhang T, Sun K, Song SP, Cao SB, Zhang HL, Shen W. Corticotropin-releasing factor mediates bone cancer induced pain through neuronal activation in rat spinal cord. Tumour Biol 2015;36(12):9559–9565. [DOI] [PubMed] [Google Scholar]
- [20].Frost CO, Hansen RR, Heegaard AM. Bone pain: current and future treatments. Curr Opin Pharmacol 2016;28:31–37. [DOI] [PubMed] [Google Scholar]
- [21].Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Mantyh WG, Bloom AP, Kuskowski MA, Mantyh PW. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain 2010;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury 2007;38 Suppl 4:S3–6. [DOI] [PubMed] [Google Scholar]
- [23].Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ, De Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br 2000;82(5):655–658. [DOI] [PubMed] [Google Scholar]
- [24].Gigante A, Bevilacqua C, Pagnotta A, Manzotti S, Toesca A, Greco F. Expression of NGF, Trka and p75 in human cartilage. Eur J Histochem 2003;47(4):339–344. [PubMed] [Google Scholar]
- [25].Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res 2007;22(8):1197–1207. [DOI] [PubMed] [Google Scholar]
- [26].Greaney ML, Riebe D, Ewing Garber C, Rossi JS, Lees FD, Burbank PA, Nigg CR, Ferrone CL, Clark PG. Long-term effects of a stage-based intervention for changing exercise intentions and behavior in older adults. Gerontologist 2008;48(3):358–367. [DOI] [PubMed] [Google Scholar]
- [27].Grills BL, Schuijers JA. Immunohistochemical localization of nerve growth factor in fractured and unfractured rat bone. Acta Orthop Scand 1998;69(4):415–419. [DOI] [PubMed] [Google Scholar]
- [28].Grimsholm O, Guo Y, Ny T, Forsgren S. Expression patterns of neurotrophins and neurotrophin receptors in articular chondrocytes and inflammatory infiltrates in knee joint arthritis. Cells Tissues Organs 2008;188(3):299–309. [DOI] [PubMed] [Google Scholar]
- [29].Gruen ME, Alfaro-Cordoba M, Thomson AE, Worth AC, Staicu AM, Lascelles BD. The Use of Functional Data Analysis to Evaluate Activity in a Spontaneous Model of Degenerative Joint Disease Associated Pain in Cats. PLoS One 2017;12(1):e0169576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Guedon JM, Longo G, Majuta LA, Thomspon ML, Fealk MN, Mantyh PW. Dissociation between the relief of skeletal pain behaviors and skin hypersensitivity in a model of bone cancer pain. Pain 2016;157(6):1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, Rosol TJ, Boustany L, Shelton DL, Mantyh PW. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res 2005;65(20):9426–9435. [DOI] [PubMed] [Google Scholar]
- [32].Hao Y, Ma Y, Wang X, Jin F, Ge S. Short-term muscle atrophy caused by botulinum toxin-A local injection impairs fracture healing in the rat femur. J Orthop Res 2012;30(4):574–580. [DOI] [PubMed] [Google Scholar]
- [33].Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 2015;23 Suppl 1: S18–21. [DOI] [PubMed] [Google Scholar]
- [34].Hochberg MC, Tive LA, Abramson SB, Vignon E, Verburg KM, West CR, Smith MD, Hungerford DS. When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis Rheumatol 2016;68(2):382–391. [DOI] [PubMed] [Google Scholar]
- [35].Hoffman EM, Zhang Z, Anderson MB, Schechter R, Miller KE. Potential mechanisms for hypoalgesia induced by anti-nerve growth factor immunoglobulin are identified using autoimmune nerve growth factor deprivation. Neuroscience 2011;193:452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ishikawa G, Koya Y, Tanaka H, Nagakura Y. Long-term analgesic effect of a single dose of anti-NGF antibody on pain during motion without notable suppression of joint edema and lesion in a rat model of osteoarthritis. Osteoarthritis Cartilage 2015;23(6):925–932. [DOI] [PubMed] [Google Scholar]
- [37].Ivanusic JJ. Molecular Mechanisms That Contribute to Bone Marrow Pain. Front Neurol 2017;8:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Iwakura N, Ohtori S, Orita S, Yamashita M, Takahashi K, Kuniyoshi K. Role of low-affinity nerve growth factor receptor inhibitory antibody in reducing pain behavior and calcitonin gene-related Peptide expression in a rat model of wrist joint inflammatory pain. J Hand Surg Am 2010;35(2):267–273. [DOI] [PubMed] [Google Scholar]
- [39].Jagodzinski M, Krettek C. Effect of mechanical stability on fracture healing--an update. Injury 2007;38 Suppl 1:S3–10. [DOI] [PubMed] [Google Scholar]
- [40].Jimenez-Andrade JM, Bloom AP, Mantyh WG, Koewler NJ, Freeman KT, Delong D, Ghilardi JR, Kuskowski MA, Mantyh PW. Capsaicin-sensitive sensory nerve fibers contribute to the generation and maintenance of skeletal fracture pain. Neuroscience 2009;162(4):1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jimenez-Andrade JM, Ghilardi JR, Castaneda-Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011;152(11):2564–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, Vanderah TW, Mantyh PW. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone 2010;46(2):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jimenez-Andrade JM, Martin CD, Koewler NJ, Freeman KT, Sullivan LJ, Halvorson KG, Barthold CM, Peters CM, Buus RJ, Ghilardi JR, Lewis JL, Kuskowski MA, Mantyh PW. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain 2007;133(1–3):183–196. [DOI] [PubMed] [Google Scholar]
- [44].Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, Brown MT. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011;152(10):2248–2258. [DOI] [PubMed] [Google Scholar]
- [45].Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am 2009;91(4):919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Knazovicky D, Helgeson ES, Case B, Gruen ME, Maixner W, Lascelles BD. Widespread somatosensory sensitivity in naturally occurring canine model of osteoarthritis. Pain 2016;157(6):1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. BMJ 2006;332(7555):1430–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez-Andrade JM, Ghilardi JR, Peters CM, Sullivan LJ, Kuskowski MA, Lewis JL, Mantyh PW. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res 2007;22(11):1732–1742. [DOI] [PubMed] [Google Scholar]
- [49].Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice AS, Rief W, Sluka AK. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016;157(7):1382–1386. [DOI] [PubMed] [Google Scholar]
- [50].Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain 2000;4(3):229–238. [DOI] [PubMed] [Google Scholar]
- [51].Kras JV, Kartha S, Winkelstein BA. Intra-articular nerve growth factor regulates development, but not maintenance, of injury-induced facet joint pain & spinal neuronal hypersensitivity. Osteoarthritis Cartilage 2015;23(11):1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010;363(16):1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lascelles BD, Knazovicky D, Case B, Freire M, Innes JF, Drew AC, Gearing DP. A canine-specific anti-nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease-associated pain. BMC Vet Res 2015;11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lewin GR, Lechner SG, Smith ES. Nerve growth factor and nociception: from experimental embryology to new analgesic therapy. Handb Exp Pharmacol 2014;220:251–282. [DOI] [PubMed] [Google Scholar]
- [55].Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci 1994;6(12):1903–1912. [DOI] [PubMed] [Google Scholar]
- [56].Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 2009;24(10):1651–1661. [DOI] [PubMed] [Google Scholar]
- [57].Lin CW, McAuley JH, Macedo L, Barnett DC, Smeets RJ, Verbunt JA. Relationship between physical activity and disability in low back pain: a systematic review and meta-analysis. Pain 2011;152(3):607–613. [DOI] [PubMed] [Google Scholar]
- [58].Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone 2016;86:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Majuta LA, Guedon JG, Mitchell SA, Ossipov MH, Mantyh PW. Anti-nerve growth factor therapy increases spontaneous day/night activity in mice with orthopedic surgery induced pain. Pain 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Majuta LA, Guedon JG, Mitchell SA, Ossipov MH, Mantyh PW. Anti-nerve growth factor therapy increases spontaneous day/night activity in mice with orthopedic surgery-induced pain. Pain 2017;158(4):605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Majuta LA, Longo G, Fealk MN, McCaffrey G, Mantyh PW. Orthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factor. Pain 2015;156(1):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mantyh P Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 2013;154 Suppl 1:S54–62. [DOI] [PubMed] [Google Scholar]
- [63].Mantyh PW. Bone cancer pain: from mechanism to therapy. Curr Opin Support Palliat Care 2014;8(2):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mantyh PW. The neurobiology of skeletal pain. Eur J Neurosci 2014;39(3):508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 2010;171(2):588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Marsell R, Einhorn TA. The biology of fracture healing. Injury 2011;42(6):551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].McCaffrey G, Thompson ML, Majuta L, Fealk MN, Chartier S, Longo G, Mantyh PW. NGF blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer Res 2014;74(23):7014–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, Price JS. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int 2012;23(4):1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nagashima H, Suzuki M, Araki S, Yamabe T, Muto C, Tanezumab I. Preliminary assessment of the safety and efficacy of tanezumab in Japanese patients with moderate to severe osteoarthritis of the knee: a randomized, double-blind, dose-escalation, placebo-controlled study. Osteoarthritis Cartilage 2011;19(12):1405–1412. [DOI] [PubMed] [Google Scholar]
- [70].Nakanishi T, Takahashi K, Aoki C, Nishikawa K, Hattori T, Taniguchi S. Expression of nerve growth factor family neurotrophins in a mouse osteoblastic cell line. Biochem Biophys Res Commun 1994;198(3):891–897. [DOI] [PubMed] [Google Scholar]
- [71].Nencini S, Ivanusic JJ. The Physiology of Bone Pain. How Much Do We Really Know? Front Physiol 2016;7:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nencini S, Ringuet M, Kim DH, Chen YJ, Greenhill C, Ivanusic JJ. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol Pain 2017;13:1744806917697011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Park S-H, O’connor KM, McKellop H. Interaction between active motion and exogenous transforming growth factor Beta during tibial fracture repair. Journal of orthopaedic trauma 2003;17(1):2–10. [DOI] [PubMed] [Google Scholar]
- [74].Patel KV, Dansie EJ, Turk DC. Impact of chronic musculoskeletal pain on objectively measured daily physical activity: a review of current findings. Pain Manag 2013;3(6):467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pecchi E, Priam S, Gosset M, Pigenet A, Sudre L, Laiguillon MC, Berenbaum F, Houard X. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis pain. Arthritis Res Ther 2014;16(1):R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Petre BM, Roxbury CR, McCallum JR, DeFontes III KW, Belkoff SM, Mears SC. Pain reporting, opiate dosing, and the adverse effects of opiates after hip or knee replacement in patients 60 years old or older. Geriatric orthopaedic surgery & rehabilitation 2012;3(1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pountos I, Georgouli T, Bird H, Giannoudis P. Nonsteroidal anti-inflammatory drugs: prostaglandins, indications, and side effects. International Journal of Interferon, Cytokine and Mediator Research 2011;3(1):19–27. [Google Scholar]
- [78].Rapp AE, Kroner J, Baur S, Schmid F, Walmsley A, Mottl H, Ignatius A. Analgesia via blockade of NGF/TrkA signaling does not influence fracture healing in mice. J Orthop Res 2015;33(8):1235–1241. [DOI] [PubMed] [Google Scholar]
- [79].Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Preclinical Pain C, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain 2008;139(2):243–247. [DOI] [PubMed] [Google Scholar]
- [80].Roemer FW, Miller CG, West CR, Brown MT, Sherlock SP, Kompel AJ, Diaz L, Galante N, Crema MD, Guermazi A. Development of an imaging mitigation strategy for patient enrolment in the tanezumab nerve growth factor inhibitor (NGF-ab) program with a focus on eligibility assessment. Semin Arthritis Rheum 2017. [DOI] [PubMed] [Google Scholar]
- [81].Rogers DJ RM; Rushing CJ. NSAIDs and Bone Healing: What the research reveals. Podiatry Today 2018;31(5):52–57. [Google Scholar]
- [82].Ryan CG, Grant PM, Dall PM, Gray H, Newton M, Granat MH. Individuals with chronic low back pain have a lower level, and an altered pattern, of physical activity compared with matched controls: an observational study. Aust J Physiother 2009;55(1):53–58. [DOI] [PubMed] [Google Scholar]
- [83].Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain 2008;138(1):47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Savage SR. Long-term opioid therapy: assessment of consequences and risks. J Pain Symptom Manage 1996;11(5):274–286. [DOI] [PubMed] [Google Scholar]
- [85].Schepens SL, Braun ME, Murphy SL. Effect of tailored activity pacing on self-perceived joint stiffness in adults with knee or hip osteoarthritis. Am J Occup Ther 2012;66(3):363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Schnitzer TJ, Ekman EF, Spierings EL, Greenberg HS, Smith MD, Brown MT, West CR, Verburg KM. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis 2015;74(6):1202–1211. [DOI] [PubMed] [Google Scholar]
- [87].Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, Kubota K, Kuskowski MA, Boustany L, Shelton DL, Mantyh PW. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 2005;115(1–2):128–141. [DOI] [PubMed] [Google Scholar]
- [88].Shelton DL, Zeller J, Ho WH, Pons J, Rosenthal A. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain 2005;116(1–2):8–16. [DOI] [PubMed] [Google Scholar]
- [89].Shi X, Guo TZ, Wei T, Li WW, Clark DJ, Kingery WS. Facilitated spinal neuropeptide signaling and upregulated inflammatory mediator expression contribute to postfracture nociceptive sensitization. Pain 2015;156(10):1852–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sopata M, Katz N, Carey W, Smith MD, Keller D, Verburg KM, West CR, Wolfram G, Brown MT. Efficacy and safety of tanezumab in the treatment of pain from bone metastases. Pain 2015;156(9):1703–1713. [DOI] [PubMed] [Google Scholar]
- [91].Suzuki M, Millecamps M, Naso L, Ohtori S, Mori C, Stone LS. Chronic Osteoporotic Pain in Mice: Cutaneous and Deep Musculoskeletal Pain Are Partially Independent of Bone Resorption and Differentially Sensitive to Pharmacological Interventions. J Osteoporos 2017;2017:7582716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Thompson ML, Chartier SR, Mitchell SA, Mantyh PW. Preventing painful age-related bone fractures: Anti-sclerostin therapy builds cortical bone and increases the proliferation of osteogenic cells in the periosteum of the geriatric mouse femur. Mol Pain 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tian X, Jee WS, Li X, Paszty C, Ke HZ. Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone 2011;48(2):197–201. [DOI] [PubMed] [Google Scholar]
- [94].Tomas A, Marcellin-Little DJ, Roe SC, Motsinger-Reif A, Lascelles BD. Relationship between mechanical thresholds and limb use in dogs with coxofemoral joint oa-associated pain and the modulating effects of pain alleviation from total hip replacement on mechanical thresholds. Vet Surg 2014;43(5):542–548. [DOI] [PubMed] [Google Scholar]
- [95].Tomlinson RE, Li Z, Li Z, Minichiello L, Riddle RC, Venkatesan A, Clemens TL. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc Natl Acad Sci U S A 2017;114(18):E3632–E3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tomlinson RE, Li Z, Zhang Q, Goh BC, Li Z, Thorek DLJ, Rajbhandari L, Brushart TM, Minichiello L, Zhou F, Venkatesan A, Clemens TL. NGF-TrkA Signaling by Sensory Nerves Coordinates the Vascularization and Ossification of Developing Endochondral Bone. Cell Rep 2016;16(10):2723–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine (Phila Pa 1976) 2007;32(19):2127–2132. [DOI] [PubMed] [Google Scholar]
- [98].Weering M, Vollenbroek‐Hutten M, Tönis T, Hermens H. Daily physical activities in chronic lower back pain patients assessed with accelerometry. European journal of pain 2009;13(6):649–654. [DOI] [PubMed] [Google Scholar]
- [99].Wei T, Guo TZ, Li WW, Kingery WS, Clark JD. Acute versus chronic phase mechanisms in a rat model of CRPS. J Neuroinflammation 2016;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Woolf AD, Erwin J, March L. The need to address the burden of musculoskeletal conditions. Best Pract Res Clin Rheumatol 2012;26(2):183–224. [DOI] [PubMed] [Google Scholar]
- [101].Xu L, Nwosu LN, Burston JJ, Millns PJ, Sagar DR, Mapp PI, Meesawatsom P, Li L, Bennett AJ, Walsh DA, Chapman V. The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthritis Cartilage 2016;24(9):1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Yasui M, Shiraishi Y, Ozaki N, Hayashi K, Hori K, Ichiyanagi M, Sugiura Y. Nerve growth factor and associated nerve sprouting contribute to local mechanical hyperalgesia in a rat model of bone injury. Eur J Pain 2012;16(7):953–965. [DOI] [PubMed] [Google Scholar]
- [103].Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan JC, Gibbs JL, Schmidt BL. Nerve growth factor links oral cancer progression, pain, and cachexia. Mol Cancer Ther 2011;10(9):1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


