Abstract
Objectives:
Evaluate whether a multimarker approach might identify patients with higher mortality and hospitalization rates after aortic valve replacement (AVR) for aortic stenosis (AS).
Background:
The society valve guidelines include accepted triggers for AVR in patients with severe asymptomatic AS, but circulating biomarkers do not have a clear role.
Methods:
From a prospective registry of patients undergoing cardiac surgery between 2000 and 2012, 665 treated with surgical AVR (441 isolated) were evaluated. Seven biomarkers were measured on blood samples obtained prior to AVR. Biomarker levels were adjusted to account for the influence of age, sex, body mass index, and renal function; the median was used to determine an elevated value. Endpoints included all-cause mortality and all-cause and cardiovascular hospitalizations. Mean follow-up was 10.7 years and 299 (45%) died.
Results:
Patients with 0–1, 2–3, 4–6, and 7 biomarkers elevated had 5-year mortality of 10%, 12%, 24%, and 33%, respectively, and 10-year mortality of 24%, 35%, 58%, and 71%, respectively (log-rank p <0.001). The association between an increasing number of elevated biomarkers and increased all-cause mortality was observed among those with minimal symptoms (NYHA I/II) and those with a low NTproBNP (p<0.01 for both). Compared to those with 0–1 biomarkers elevated, patients with 4–6 or 7 biomarkers elevated had an increased hazard of mortality after adjustment for clinical risk scores (p<0.01) and a 2–3-fold higher rate of all-cause and cardiovascular rehospitalization after AVR. Similar findings were obtained when evaluating cardiovascular mortality. Among patients with no or minimal symptoms, 42% had ≥4 biomarkers elevated.
Conclusion:
Among patients with severe AS treated with surgical AVR, an increasing number of elevated biomarkers of cardiovascular stress was associated with higher all-cause and cardiovascular mortality and a higher rate of repeat hospitalization. A multimarker approach may be useful in the surveillance of asymptomatic patients with severe AS to optimize surgical timing.
Keywords: aortic valve stenosis, biomarkers, surgical aortic valve replacement, risk stratification, mortality, hospitalization
CONDENSED ABSTRACT
In patients with severe aortic stenosis undergoing surgical aortic valve replacement, an increasing number of elevated circulating biomarkers of cardiovascular stress is independently associated with increased all-cause and cardiovascular mortality and hospitalization rates after surgery. This association was observed among those with minimal symptoms (NYHA I/II) and those with a low NTproBNP. Among patients with no or minimal symptoms, 42% had ≥4 (out of 7) biomarkers elevated. A multimarker approach may be useful in the surveillance of asymptomatic patients with severe aortic stenosis to optimize surgical timing.
INTRODUCTION
In the management of patients with severe aortic stenosis (AS), the clinician is looking for “triggers” to signal that aortic valve replacement (AVR) is appropriate. While the recommendation to undergo AVR takes into account patient risk, other comorbidities, and patient preferences, there are widely accepted guidelines for what should trigger a recommendation for or strong consideration of valve replacement.
In the absence of symptoms, class IIa triggers include very severe AS, failure to increase BP with exercise, severe valve calcification and rapid disease progression (1,2). Circulating biomarkers have no established role as a trigger for AVR in the U.S. guidelines but a IIa indication is given for a markedly elevated natriuretic peptide level in the European guidelines, albeit with level of evidence C (1,2). Most biomarker studies to date have focused on natriuretic peptides, but some have looked at troponin and other biomarkers (3–7). Limitations of these prior studies include analyzing a mixture of patients with moderate or severe AS, analyzing those treated in different ways including medical therapy or surgical or transcatheter aortic valve replacement, limited follow-up, and not accounting for factors that confound the association between biomarkers and outcomes, including age, sex, renal function, and body size.
Measurement of biomarkers in patients with AS could potentially be useful to minimize morbidity and mortality before and after valve replacement by optimizing decision making regarding the timing of valve replacement and identifying higher risk sub-groups who may need more careful follow-up after valve replacement to minimize heart failure symptoms and rehospitalization. Accordingly, the aim of this work is to evaluate the prognostic significance of multiple biomarkers of cardiovascular stress in a more homogeneous population of asymptomatic and symptomatic patients with severe AS undergoing surgical AVR and consider the potentially confounding effects of age, sex, renal function, and body size on these relationships. The biomarkers selected reflect diverse pathways including myocyte injury, myocyte stress, inflammation, fibrosis, and neurohormonal activation and have previously been shown to have prognostic utility in AS or non-AS heart failure populations (8–12). Our objective was not to create a more accurate risk prediction score for outcome after AVR, but rather to identify what might be a “biomarker trigger” for AVR even in the absence of symptoms. This is consistent with the approach taken in the analyses that underlie our current guideline triggers for AVR (1,2).
METHODS
Study population.
Patients were identified from a prospective clinical and biobank registry of patients undergoing cardiac surgery at Québec Heart and Lung Institute. Those with severe AS undergoing surgical AVR between March, 2000, and October, 2012, were included in this analysis. We excluded patients with a prior valve replacement of any valve or patients that underwent a concomitant mitral, tricuspid, or pulmonic valve procedure at the time of their AVR. Written informed consent was obtained from all patients.
Clinical and echocardiographic data.
Clinical data were prospectively collected and entered into a database prior to surgery. Echocardiographic measurements were made according to established guidelines.
Biomarker analyses.
Blood specimen collection before AVR was performed by venipuncture using vacutainers tubes (BD diagnostics, On, Canada). After centrifugation, plasma or serum was immediately stored at −80°C until time of assay. High sensitivity cardiac troponin T (hs-cTnT; Roche Diagnostics, REF 05092744190), human epididymis protein (HE4; Roche Diagnostics, REF 05950929190), cancer antigen 125 (CA125; Roche Diagnostics, REF 11776223322), growth differentiation factor 15 (GDF15, Roche Diagnostics, test in development), aminoterminal prohormone of B-type natriuretic peptide (NTproBNP; Roche Diagnostics, REF 04842464190) analyses were all performed on Modular Analytics E170 immunoassay module (Roche Diagnostics, Laval, Qc, Canada). These analyses have shown optimal performance as determined using QC from Roche Diagnostics as well as independent QC samples (Bio-Rad Laboratories, Montréal, Canada). All analyses achieved CV’s (intra-assay and inter-assay) below 4% at clinical level values. For GDF-15, CA125 and HE4 analyses, only one lot of reagent and calibrators were used to minimize lot-to-lot variation. In these cases, CV’s (intra-assay) were below 3%. The high sensitivity C-reactive protein (hsCRP; Roche Diagnostics, REF 04628918190) was measured on Integra800 device (Roche Diagnostics, Laval, Qc, Canada) with a typical performance below 4% for CV’s intra and inter-assay. Soluble ST2 (sST2) analysis was performed using an ELISA kit (PresageR ST2 assay, Critical Diagnostics, San Diego, CA) and shows an intra-assay CV of 2.6% and an inter-assay CV about 6.0%. All analytes (biomarkers) were measured from banked samples after only one freeze-thaw cycle.
Vital status and hospitalizations.
Data on death, reason for death, hospitalizations and reason for hospitalization were retrospectively obtained from Institut de la Statistique du Québec. To maximize the interrogation of the central database of the Institut de la Statistique du Québec, a list with multiple demographics (including first and last names, dates of birth, and social security numbers) and a delay of 1 year between interrogation and closing follow-up dates were used. Cardiovascular deaths included those determined to be from a cardiovascular cause and deaths from an unknown cause.
Statistical analysis.
All continuous and ordinal variables were presented as median (1st quartile, 3rd quartile) and nominal variables as percentages. Baseline characteristics were compared between groups defined by number of elevated biomarkers (0–1, 2–3, 4–6, and 7 elevated) using the Kruskal-Wallis test and chi-square test, respectively. Operative mortality was defined as (a) all deaths occurring during the hospitalization in which the operation was performed, even if after 30 days; and (b) all death occurring after discharge from the hospital, but before the end of the thirtieth postoperative day.
The association between individual biomarkers and mortality was examined through a series of Cox proportional hazards models. In addition to unadjusted analyses, models were created to adjust for Parsonnet score and, separately, for EuroSCORE 2. To facilitate comparisons between biomarkers, each biomarker was transformed using log base 2 and then standardized so that a one unit increase in the biomarker represented one standard deviation increase in the log-transformed biomarker.
Biomarker levels were modified to take into account the influence of age, sex, body mass index, and creatinine clearance. In separate linear regression models, each biomarker was transformed using log base 2 and then regressed against age, sex, body mass index, and creatinine clearance. Using these regression models, each patient had an expected log2 transformed biomarker value for each biomarker. A “modified” biomarker value was defined as the actual log2 transformed biomarker value divided by the expected log2 transformed biomarker value. A biomarker was considered to be elevated if the modified biomarker value was greater than the median for the whole cohort. The association between quartiles of this modified biomarker level and mortality was evaluated for each biomarker.
Kaplan-Meier curves and associated 5- and 10-year survival estimates were obtained based on the number of elevated biomarkers. Additionally, Cox proportional hazards models were created that included the number of elevated biomarkers and further adjusted for NYHA class and Parsonnet score or EuroSCORE 2. Start time was date of surgery and patients were followed until death or were censored at last available follow-up. The cause of death was only available on deaths prior to 2015, so analyses of cardiovascular death were censored after December 31, 2014. Median follow-up time (1st quartile, 3rd quartile) based on the reverse Kaplan-Meier method (13). To determine if the association between number of elevated biomarkers and mortality varied by clinical, echocardiographic, or procedural factors, the interaction between number of elevated biomarkers and each subgroup was evaluated. A separate Cox model was created for each subgroup evaluation. The hazard ratio per one elevated biomarker was obtained from these models and compared across subgroup levels based on the interaction. Cardiovascular mortality was evaluated in a competing risk model via methods by Fine and Grey (14).
Rehospitalizaton event rates per patient-year were computed to account for differences in observable time among patients. Event rates were calculated by the number of elevated biomarkers: 0–1, 2–3, 4–6, and 7. A Poisson model with offset equal to the log follow-up time and Pearson scaling to account for overdispersion was used to obtain estimates and confidence intervals. Unadjusted estimates and estimates adjusted for NYHA class and Parsonnet score or EuroSCORE 2 were obtained.
All analyses conducted in SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patient population.
Among 665 patients treated with surgical AVR for AS, 441 had an isolated AVR, 143 had AVR and coronary artery bypass grafting, and 91 underwent AVR and another procedure (10 had both coronary artery bypass grafting and another procedure). Other concomitant procedures included preventative ascending aortic replacement or repair, Maze, atrial appendage ligation, closure of inter-atrial communication, and myectomy. During this same period, there were 2949 patients who also met the inclusion and exclusion criteria of our analysis except for the fact that they did not have banked blood samples available. When we compared those 2949 patients to the 665 included in our analysis, the main difference was a higher prevalence of coronary artery disease in the patients not included (Online Table 1). The average age in our study population was 70 years, 44% were female, mean LVEF was 60%, 46% had more significant symptoms (NYHA III/IV), and the mean scores were 12.5 and 2.5 for the Parsonnet score and EuroSCORE 2, respectively. Over a median follow-up of 10.7 (6.7, 13.1) years, 299 (45%) died. Baseline characteristics of the study population before and after classification according to the number of biomarkers elevated are shown in Table 1. While there were differences in the prevalence of diabetes, coronary artery disease, LVEF, NYHA class, mitral regurgitation severity, and clinical risk score, there were no differences observed for age, sex, or renal function across the groups. Correlations between the log2 transformed biomarker levels and select clinical variables are shown in Online Table 2. For each biomarker, there was a significant association between an increasing biomarker level and lower LVEF (p<0.001 for all).
Table 1. Baseline Characteristics According to the Number of Biomarkers Elevated.
| All (n= 665) |
0–1 biomarkers elevated (n=108) |
2–3 biomarkers elevated (n=233) |
4–6 biomarkers elevated (n=271) |
7 biomarkers elevated (n=46) |
p-value | |
|---|---|---|---|---|---|---|
| Biomarkers levels | ||||||
| hs-cTnT (ng/L) | 12.6 (7.8,20.2) | 9.0 (6.0,11.9) | 10.6 (6.7,16.5) | 15.4 (10.3,25.0) | 27.6 (18.9,51.4) | <0.001 |
| HE4 (pmol/L) | 67.8 (53.8,91.4) | 59.7 (46.6,68.4) | 58.6 (48.1,76.9) | 79.8 (61.8,108.0) | 120.4 (90.0,162.9) | <0.001 |
| CA125 (kU/L) | 11.8 (8.3,17.4) | 9.2 (7.1,10.9) | 10.1 (7.4,14.5) | 14.4 (10.5,20.1) | 35.6 (17.9,66.1) | <0.001 |
| GDF15 (pg/mL) | 1221 (850,1861) | 893 (711,1198) | 1034 (724,1459) | 1508 (1047,2109) | 2387 (1712,4056) | <0.001 |
| NTproBNP (pg/mL) | 390 (145,924) | 150 (76,279) | 287 (117,632)) | 614 (255,1448) | 2913 (1122,5126) | <0.001 |
| hsCRP (mg/L) | 1.9 (0.8,4.8) | 0.9 (0.5,1.5) | 1.5 (0.8,2.9) | 2.9 (1.3,6.0) | 9.9 (4.8,18.2) | <0.001 |
| sST2 (ng/mL) | 18.1 (14.8,22.0) | 14.9 (12.9,17.2) | 17.4 (14.5,20.4 | 19.1 (16.0,23.8) | 26.7 (21.7,32.9) | <0.001 |
| Clinical characteristics | ||||||
| Age (years) | 71 (63,77) | 71 (63,77) | 70 (63,76) | 71 (64,78) | 72 (60,79) | 0.70 |
| Female | 44% | 38% | 41% | 49% | 41% | 0.16 |
| Diabetes | 24% | 14% | 21% | 30% | 30% | 0.003 |
| Coronary artery disease | 33% | 22% | 32% | 37% | 43% | 0.02 |
| PCI (%) | 7% | 6% | 8% | 6% | 4% | 0.84 |
| Creatinine clearance | 69 (59,82) | 68 (60,78) | 69 (60,80) | 70 (56,85) | 64 (51,81) | 0.32 |
| NYHA III/IV | 46% | 37% | 40% | 50% | 70% | <0.001 |
| LV ejection fraction | 60 (52,70) | 65 (60,73) | 60 (55,70) | 60 (50,68) | 50 (35,63) | <0.001 |
| AVA (cm2) | 0.70 (0.6,0.8) | 0.70 (0.6,0.9) | 0.70 (0.6,0.8) | 0.70 (0.6,0.8) | 0.64 (0.5,0.8) | 0.05 |
| Mean gradient (mmHg) | 43 (34,56) | 43 (38,50) | 43 (34,55) | 44 (34,56) | 45 (32,59) | 0.42 |
| Moderate/severe MR (%) | 24% | 16% | 19% | 29% | 30% | 0.019 |
| Parsonnet score | 12.5 (6.6,20) | 10.0 (5.5,16.0) | 11.5 (6.0,17.5) | 14.5 (9.0, 21.0) | 20.3 (12.0, 31.5) | <0.001 |
| EuroSCORE 2 | 2.5 (1.3,4.2) | 1.8 (1.1,3.3) | 2.2 (1.3,3.9) | 2.7 (1.5,4.5) | 3.9 (2.6,8.0) | <0.001 |
| Isolated AVR | 66% | 77% | 61% | 67% | 59% | 0.027 |
| Operative Death | 3% | 1% | 3% | 2% | 2% | 0.56 |
Data shown as % or median (25th, 75th percentiles).
Abbreviations: hs-cTnT, high sensitivity cardiac troponin T; HE4, human epididymis protein; CA125, cancer antigen 125; GDF15, growth differentiation factor 15; NTproBNP, aminoterminal prohormone of B-type natriuretic peptide; hsCRP, high sensitivity C-reactive protein; sST2, soluble ST2; PCI, percutaneous coronary intervention; NYHA, New York Heart Association; LV, left ventricular; AVA, aortic valve area; MR, mitral regurgitation; AVR, aortic valve replacement.
Mortality by NYHA class and NTproBNP level.
NYHA class was not associated with all-cause mortality in this population (Figure 1A). While a higher raw NTproBNP level was associated with increased all-cause mortality (Figure 1B), after the NTproBNP level was adjusted for the influence of age, sex, renal function, and body size, this association was no longer significant (Figure 1C).
Figure 1. NYHA class, NTproBNP, and mortality after surgical aortic valve replacement.
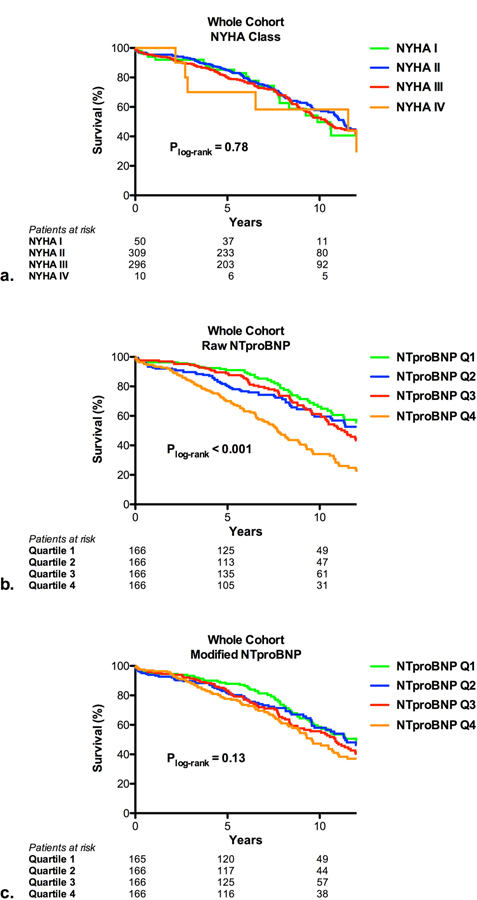
Kaplan-Meier curves are shown based on NYHA class (a), raw level of NTproBNP (b), and modified level of NTproBNP (c) for patients treated with surgical aortic valve replacement. The modified level of NTproBNP accounted for the influence of age, sex, body mass index, and renal function on NTproBNP levels.
Individual biomarkers and mortality.
The association between each individual biomarker and all-cause mortality is shown in Online Table 3, reported as the standardized log2 transformed raw level of the biomarker both unadjusted and adjusted for the Parsonnet score and EuroSCORE 2. When analyzed in this way, an elevation in the raw level of each biomarker was associated with increased all-cause mortality before and after adjustment for clinical risk scores. Then each biomarker level was modified to account for the influence of age, sex, renal function, and body size as described in the methods. The association between quartiles of the modified log2 transformed value and mortality is shown before and after adjustment for clinical risk scores in Online Table 4. The highest hazards for mortality were seen with higher levels of hs-cTnT, HE4, and GDF15. For each biomarker, survival curves based on quartiles of the raw level and modified level are shown in Online Figures 1–6.
Multiple biomarkers in combination and mortality.
We considered the number of biomarkers elevated without regard to which specific biomarker was elevated. As shown in Table 2 and Online Figure 7, as the number of biomarkers elevated increased, survival at 5 and 10 years declined. To simplify subsequent analyses and based on the similarities in 5 and 10 year survival rates, we defined 4 groups according to the number of biomarkers elevated: 0–1, 2–3, 4–6, and 7. Survival curves for these groups are shown in Figure 2. The association between an increasing number of biomarkers elevated and increased all-cause mortality is seen in the whole cohort and when the population is broken down into those with minimal symptoms (NYHA I/II) versus more severe heart failure symptoms (NYHA III/IV). Notably, 42% of subjects with minimal symptoms had 4 or more biomarkers elevated (Table 2). In an ancillary analysis, we found that an increasing number of elevated biomarkers (not including NTproBNP) was associated with increased all-cause mortality among those with or without an elevated NTproBNP level (Online Figure 8).
Table 2. Survival at 5 and 10 years according to the number of biomarkers elevated.
| Biomarkers elevated | Patients | 5 year survival | 10 year survival | ||
|---|---|---|---|---|---|
| Total no. (%) |
NYHA I/II no. (%) |
NYHA III/IV no. (%) |
|||
| Overall | 665 (100%) | 359 (54%) | 306 (46%) | 82% (79%, 85%) | 55% (50%, 59%) |
| 0 | 27 (4%) | 16 (4%) | 11 (4%) | 96% (76%, 99%) | 73% (46%, 88%) |
| 1 | 81 (12%) | 52 (15%) | 29 (10%) | 88% (79%, 94%) | 77% (63%, 86%) |
| 2 | 111 (17%) | 63 (18%) | 48 (16%) | 92% (85%, 96%) | 66% (55%, 76%) |
| 3 | 122 (18.5%) | 77 (22%) | 45 (15%) | 85% (77%, 90%) | 64% (54%, 73%) |
| 4 | 108 (16.5%) | 56 (16%) | 52 (17%) | 79% (69%, 86%) | 42% (30%, 55%) |
| 5 | 89 (13.5%) | 46 (13%) | 43 (14%) | 76% (65%, 84%) | 43% (31%, 54%) |
| 6 | 74 (11%) | 33 (9%) | 41 (14%) | 74% 62%, 82%) | 41% (29%, 54%) |
| 7 | 46 (7%) | 14 (4%) | 32 (11%) | 67% (51%, 78%) | 29% (15%, 45%) |
|
Elevated biomarker categories |
|||||
| 0–1 | 108 (16%) | 68 (19%) | 40 (13%) | 90% (83%, 95%) | 76% (64%, 84%) |
| 2–3 | 233 (35%) | 140 (39%) | 93 (31%) | 88% (83%, 92%) | 65% (58%, 72%) |
| 4–6 | 271 (41%) | 135 (38%) | 136 (45%) | 76% (71%, 81%) | 42% (35%, 49%) |
| 7 | 46 (7%) | 14 (4%) | 32 (11%) | 67% (51%, 78%) | 29% (15%, 45%) |
Abbreviations: NYHA, New York Heart Association
Figure 2. Multiple biomarkers and mortality after surgical aortic valve replacement.
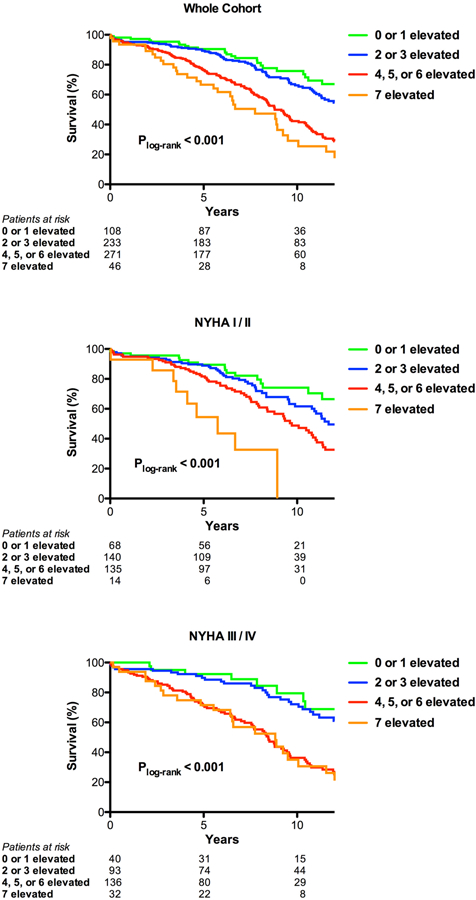
Kaplan-Meier curves are shown for number of biomarkers elevated (0–1, 2–3, 4–6, or 7) for patients treated with surgical aortic valve replacement. Curves are shown for all patients (a), those with NYHA I/II (b), and those with NYHA III/IV (c).
Compared to those with 0–1 biomarkers elevated, patients with 4–6 or 7 biomarkers elevated had an increased hazard of all-cause mortality in unadjusted analyses and after adjustment for NYHA class and the Parsonnet score or EuroSCORE 2 (Table 3). For each biomarker elevated, there was an approximately 20% increase in all-cause mortality before and after adjustment (Table 3). After additional adjustment for comorbidities that differed across the biomarker groups in Table 1 (diabetes, coronary disease, and mitral regurgitation severity), the results were similar. The association between an increased number of biomarkers elevated and increased all-cause mortality was consistent across multiple sub-groups, including age, sex, renal function, diabetes, coronary disease, LVEF, mitral regurgitation severity, NYHA class, isolated versus combined AVR, and clinical risk (Online Table 5).
Table 3. Multivariable Models for All-Cause and Cardiovascular Mortality.
| All-cause mortality | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1* | Model 2** | ||||
|
HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
HR (95% CI) |
p-value | |
|
Biomarkers elevated (referent is 0–1 elevated) |
||||||
| 2–3 biomarkers elevated |
1.31 (0.87, 1.99) |
0.20 |
1.19 (0.78, 1.80) |
0.42 |
1.35 (0.89, 2.05) |
0.16 |
| 4–6 biomarkers elevated |
2.51 (1.69, 3.72) |
<0.001 |
2.08 (1.40, 3.10) |
<0.001 |
2.47 (1.66, 3.66) |
<0.001 |
| 7 biomarkers elevated |
3.36 (2.02, 5.57) |
<0.001 |
2.12 (1.24, 3.64) |
0.006 |
2.56 (1.50, 4.38) |
<0.001 |
|
Biomarkers elevated (per additional biomarker elevated) |
1.24 (1.17, 1.32) |
<0.001 |
1.17 (1.10, 1.25) |
<0.001 |
1.21 (1.14, 1.29) |
<0.001 |
| Cardiovascular mortality | ||||||
| Unadjusted | Model 1* | Model 2** | ||||
|
HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
HR (95% CI) |
p-value | |
|
Biomarkers elevated (referent is 0–1 elevated) |
||||||
| 2–3 biomarkers elevated |
1.87 (0.90, 3.89) |
0.09 |
1.70 (0.81, 3.56) |
0.16 |
1.89 (0.91, 3.94) |
0.09 |
| 4–6 biomarkers elevated |
2.78 (1.38, 5.63) |
0.004 |
2.24 (1.09, 4.58) |
0.03 |
2.68 (1.32, 5.44) |
0.007 |
| 7 biomarkers elevated |
6.11 (2.74, 13.62) |
<0.001 |
3.82 (1.63, 8.94) |
0.002 |
5.01 (2.13, 11.78) |
<0.001 |
|
Biomarkers elevated (per additional biomarker elevated) |
1.29 (1.17, 1.42) |
<0.001 |
1.20 (1.08, 1.33) |
<0.001 |
1.25 (1.13, 1.39) |
<0.001 |
Model 1 adjusts for NYHA class (III/IV vs. I/II) and the Parsonnet score.
Model 2 adjusts for NYHA class (III/IV vs. I/II) and the EuroSCORE 2.
Abbreviations: HR, hazard ratio; CI, confidence interval.
With respect to cardiovascular mortality (n=127, 42% of all deaths), the results were similar to those for all-cause mortality, but the hazard associated with biomarker elevations for cardiovascular mortality was more pronounced than for all-cause mortality (Table 3). Kaplan-Meier curves for cardiovascular mortality are shown for groups based on the number of biomarkers elevated in Online Figure 9.
As a sensitivity analysis, we evaluated the association between an increasing number of elevated biomarkers (per biomarker elevated and based on groups: 2 or 3 elevated; 4, 5, or 6 elevated; and 7 elevated) adjusting for any variables from Table 1 with a univariable association with mortality (p<0.10) (age, creatinine clearance, transvalvular mean gradient, diabetes, atherosclerosis, moderate or severe mitral regurgitation, and isolated AVR; Parsonnet score and EuroSCORE 2 were excluded from these sensitivity analyses). In each of these additional models, an increasing number of elevated biomarkers was significantly associated with increased all-cause and cardiovascular mortality with adjusted hazard ratios comparable to those shown in Table 3.
Multiple biomarkers in combination and rehospitalization.
All-cause and cardiovascular rehospitalization rates are shown in Table 4. There were 2412 all-cause and 718 cardiovascular hospitalizations during follow-up. Compared to those with 0–1 biomarkers elevated, patients with 4 or more biomarkers elevated had a 2–3-fold higher rate of all-cause and cardiovascular rehospitalization after AVR. After adjustment for NYHA class and clinical risk score, most of these associations remained significant.
Table 4. All-cause and cardiovascular rehospitalizations.
| All-cause Rehospitalizations | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted for NYHA class and Parsonnet score |
Adjusted for NYHA class and EuroSCORE 2 |
||||||
|
# of elevated biomarkers |
Patient years |
Number of events |
Event rate per patient year |
95% CI |
Event rate per patient year |
95% CI |
Event rate per patient year |
95% CI |
| 0–1 | 888.32 | 295 | 0.33 | (0.26, 0.42) | 0.36 | (0.29, 0.46) | 0.34 | (0.27, 0.43) |
| 2–3 | 1889.87 | 760 | 0.40 | (0.35, 0.47) | 0.41 | (0.36, 0.47) | 0.41 | (0.36, 0.48) |
| 4–6 | 1872.12 | 1139 | 0.61 | (0.54, 0.69) | 0.57 | (0.50, 0.64) | 0.60 | (0.53, 0.68) |
| 7 | 294.11 | 218 | 0.74 | (0.56, 0.98) | 0.54 | (0.41, 0.71) | 0.60 | (0.45, 0.80) |
|
Relative event rate increase (vs. 0–1) |
Relative event rate increase (vs. 0–1) |
Relative event rate increase (vs. 0–1) |
||||||
| 2–3 | 1.21, p=0.19 | 1.13, p=0.36 | 1.20, p=0.19 | |||||
| 4–6 | 1.83, p<0.001 | 1.56, p<0.001 | 1.74, p<0.001 | |||||
| 7 | 2.23, p<0.001 | 1.48, p=0.034 | 1.74, p=0.004 | |||||
| Cardiovascular Rehospitalizations | ||||||||
|
Unadjusted |
Adjusted for NYHA class and Parsonnet score |
Adjusted for NYHA class and EuroSCORE 2 |
||||||
|
# of elevated biomarkers |
Patient years |
Number of events |
Event rate per patient year |
95% CI |
Event rate per patient year |
95% CI |
Event rate per patient year |
95% CI |
| 0–1 | 888.32 | 77 | 0.09 | (0.06, 0.13) | 0.10 | (0.06, 0.14) | 0.09 | (0.06, 0.14) |
| 2–3 | 1889.87 | 197 | 0.10 | (0.08, 0.14) | 0.11 | (0.08, 0.14) | 0.11 | (0.08, 0.14) |
| 4–6 | 1872.12 | 372 | 0.20 | (0.16, 0.24) | 0.18 | (0.15, 0.22) | 0.19 | (0.16, 0.24) |
| 7 | 294.11 | 72 | 0.24 | (0.16, 0.38) | 0.16 | (0.11, 0.26) | 0.19 | (0.12, 0.30) |
|
Relative event rate increase (vs. 0–1) |
Relative event rate increase (vs. 0–1) |
Relative event rate increase (vs. 0–1) |
||||||
| 2–3 | 1.20, p=0.48 | 1.10, p=0.68 | 1.20, p=0.47 | |||||
| 4–6 | 2.29, p<0.001 | 1.87, p=0.005 | 2.17, p<0.001 | |||||
| 7 | 2.82, p<0.001 | 1.71, p=0.08 | 2.12, p=0.018 | |||||
Abbreviations: NYHA, New York Heart Association
DISCUSSION
Among patients with severe AS treated with surgical AVR, an increasing number of elevated biomarkers of cardiovascular stress was associated with higher mortality and a higher rate of all-cause and cardiovascular hospitalization after surgery. The association between an increasing number of elevated biomarkers and increased mortality was consistent across several sub-groups, including both those who were minimally symptomatic (NYHA I/II) and those with more significant symptoms (NYHA III/IV) as well as those with a low or high natriuretic peptide level. Notably, 42% of patients with no or minimal heart failure symptoms had 4 or more biomarkers elevated, so having multiple biomarkers elevated despite having no to minimal symptoms is fairly common. Collectively, these data suggest that multiple biomarkers of cardiovascular stress may be useful in the surveillance of asymptomatic patients and may serve as a trigger for valve replacement even before symptoms have developed and when natriuretic peptide levels are low. Additionally, those with a higher number of elevated biomarkers may benefit from more intensive follow-up and medical therapy after valve replacement to reduce rehospitalizations.
This is the largest multimarker study of patients with AS to date. It was restricted to patients with severe AS treated with surgical AVR, providing more uniformity to the patients included in the cohort, and included very long-term follow-up of both vital status and repeat hospitalization. We also adjusted biomarker levels to account for the confounding influence of age, sex, renal function, and body size, before evaluating their association with clinical outcomes. This cohort is very similar to the average patient in the Society of Thoracic Surgeons (STS) database undergoing AVR in terms of age, sex, LVEF, and NYHA class distribution (15). Interestingly, the most common trigger for referral for AVR—symptoms—was not associated with mortality after AVR in this study, which is consistent with several other reports (16).
To date, almost all biomarker studies in patients with AS have evaluated a single biomarker, most commonly a natriuretic peptide (BNP or NTproBNP). Among asymptomatic patients with moderate or severe AS, a higher natriuretic peptide level predicts symptom onset or a clinical composite that is usually dominated by the performance of AVR (17). Some studies show that a higher natriuretic peptide level is associated with increased mortality among symptomatic patients treated with valve replacement (18). Additional studies have evaluated high sensitivity troponin, GDF15, and soluble ST2, among others (5,6,19). One study included an evaluation of multiple biomarkers in combination and showed that an increased number of elevated biomarkers (GDF15, sST2, and NTproBNP) was associated with a higher hazard of mortality after transcatheter or surgical aortic valve replacement (7). Across the board, these analyses have not accounted for the confounding influence of age, sex, renal function, and BMI on the association between biomarker level and outcomes.
Biomarkers—a window into the biology of the left ventricle?
Chronic pressure overload from valve obstruction leads to hypertrophic remodeling of the LV characterized by myocyte hypertrophy, myocardial fibrosis, and apoptosis, yielding diastolic and systolic impairment (20). Cardiac hypertrophy can be both adaptive and maladaptive, but simply measuring the LV mass does little to distinguish these processes in an individual patient (21). Furthermore, systolic function is usually measured crudely with ejection fraction, which can provide misleading reassurance of normal function, particularly in the hypertrophied heart. The adverse effects of sustained pressure overload on the LV may occur at a molecular and tissue level well before they manifest on echocardiography or symptoms develop (22). As such, circulating biomarkers that reflect biological processes in the LV may provide important insights—as a complement to clinical and echocardiographic assessment—to inform clinical decision making on the timing of valve replacement.
Clinical implications
Many of the recommendations for valve repair or replacement for various valve lesions are made based on data like those presented herein, showing that a certain factor (e.g. reduced LVEF, excessive chamber dilation) is associated with a worse clinical outcome after surgery. In line with this, we showed that an increasing number of elevated biomarkers is associated with higher mortality and rehospitalization rates after AVR. This relationship was observed in patients who are often not referred for valve replacement, including minimally symptomatic patients and those with a preserved LVEF. While the guidelines recommend that patients with any heart failure symptoms (i.e. NYHA II) be referred for valve replacement, the reality is that the majority of patients are not sent for valve replacement until they have more severe heart failure symptoms (NYHA III or IV) (15). Notably, 42% of patients with no or minimal heart failure symptoms had 4 or more biomarkers elevated and these patients had an increased risk for mortality and hospitalization. As such, these data suggest that patients that are often not referred for valve replacement (those in NYHA functional class I or II) may benefit from earlier valve replacement, perhaps by preventing further damage to the pressure-overloaded ventricle, thereby optimizing long-term cardiac performance and freedom from heart failure.
Looking ahead, more definitive data would need to come from a strategy trial comparing early valve replacement with clinical surveillance and deferred valve replacement in patients with severe asymptomatic AS. The current data would be strengthened by evaluating whether multiple elevated biomarkers identify a sub-group of patients for whom early valve replacement would yield better clinical outcomes. Further work is also needed to identify which and how many biomarkers are helpful to measure to identify these patients. While a multimarker approach is likely to be more fruitful than measurement of a single biomarker, a more parsimonious list is likely to be useful.
Limitations
The 665 patients included in this analysis represent only a subset of the patients treated with AVR at this center over the 12-year period. Due to logistical issues and case urgency, it was not always feasible to collect biospecimens. For some patients included in the biobank, there were no more samples available for our analysis. Accordingly, there may have been a selection bias. Nonetheless, this cohort was similar to those undergoing AVR at our center during this period without banked blood available and is similar in characteristics to other large AVR cohorts from this period, including those in the STS database (15). While the seven biomarkers included in this analysis have each been associated with cardiovascular pathophysiology and demonstrated to have prognostic utility in AS or non-AS heart failure population, due to limited sample volumes we were unable to include additional promising biomarkers of cardiovascular stress that may also or better identify increased risk in patients with AS. We included patients who underwent isolated AVR and those who underwent a concomitant procedure, however our primary findings were similar in these two sub-groups (Online Table 4). We included only patients referred for AVR and our symptom-based sub-group analysis focused on the presence or absence of heart failure symptoms. Although dyspnea is the most common symptom of patients with AS, we did not analyze additional sub-groups based the presence of angina or syncope. This is proof-of-concept study to show the utility of a multimarker approach to identify patients who may benefit from earlier valve replacement. The multi-biomarker approach now needs to be validated in asymptomatic patients with severe AS and no indication for AVR at baseline, but our sub-group analysis of patients with NYHA functional class I/II is helpful in this regard. Some of these biomarkers are not yet commercially available and further work is needed to clarify optimal cut-points for these biomarkers. As performed in this study, one would need an app to determine what the expected biomarker level would be for a specific patient based on their age, sex, BMI, and renal function.
CONCLUSION
An increasing number of circulating biomarkers of cardiovascular stress identifies patients with severe AS undergoing AVR who have higher mortality and rehospitalization rates after surgery. Notably, an increased number of biomarkers elevated was associated with higher mortality in the sub-groups of patients with no to minimal heart failure symptoms or a low natriuretic peptide level. These findings suggest that a multimarker approach may be useful in the surveillance of asymptomatic patients with severe AS and may serve as a trigger for valve replacement even before symptoms have developed and when natriuretic peptide levels are low.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN?
The society valve guidelines include accepted triggers for AVR in patients with severe asymptomatic AS, but circulating biomarkers do not have a clear role.
WHAT IS NEW?
An increasing number of elevated circulating biomarkers of cardiovascular stress is associated with increased all-cause and cardiovascular mortality and hospitalization rates after aortic valve replacement for severe AS. This association was observed among those with minimal symptoms (NYHA I/II) and those with a low NTproBNP. Among patients with no or minimal symptoms, 42% had ≥4 (out of 7) biomarkers elevated.
WHAT IS NEXT?
Biomarker cut-points and the type and number of biomarkers in the multimarker panel need to be clarified. Incorporating biomarkers into a strategy trial testing early versus deferred valve replacement for patients with severe asymptomatic AS could further clarify whether the sub-group of patients with elevated biomarkers benefits, perhaps selectively, from earlier valve replacement.
Acknowledgments
Funding:
Dr. Lindman was supported by K23 HL116660. Dr. Pibarot was supported by research grant # FDN-143225 from Canadian Institutes of Health research (CIHR), Ottawa, Ontario, Canada. Dr. Pibarot is the Canada Research Chair in Valvular Heart Disease (CIHR). Additional support was provided by a research grant from Roche Diagnostics.
ABBREVIATIONS AND ACRONYMS
- AS
aortic stenosis
- AVR
aortic valve replacement
- CA125
cancer antigen 125
- GDF15
growth differentiation factor 15
- HE4
human epididymis protein
- hsCRP
high sensitivity C-reactive protein
- hs-cTnT
high sensitivity cardiac troponin T
- LV
left ventricle or left ventricular
- LVEF
left ventricular ejection fraction
- NTproBNP
aminoterminal prohormone of B-type natriuretic peptide
- NYHA
New York Heart Association
- sST2
soluble ST2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Lindman serves on the scientific advisory board for Roche Diagnostics, has received research grants from Edwards Lifesciences and Roche Diagnostics, and has consulted for Medtronic.
Dr. Pibarot has received research contracts from Edwards Lifesciences and Medtronic.
The other authors report no potential conflicts of interest.
REFERENCES
- 1.Nishimura RA, Otto CM, Bonow RO et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–88. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner H, Falk V, Bax JJ et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 3.Clavel MA, Malouf J, Michelena HI et al. B-type natriuretic peptide clinical activation in aortic stenosis: impact on long-term survival. J Am Coll Cardiol 2014;63:2016–25. [DOI] [PubMed] [Google Scholar]
- 4.Bergler-Klein J, Klaar U, Heger M et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004;109:2302–8. [DOI] [PubMed] [Google Scholar]
- 5.Chin CW, Shah AS, McAllister DA et al. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J 2014;35:2312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinning JM, Wollert KC, Sedaghat A et al. Risk scores and biomarkers for the prediction of 1-year outcome after transcatheter aortic valve replacement. Am Heart J 2015;170:821–9. [DOI] [PubMed] [Google Scholar]
- 7.Lindman BR, Breyley JG, Schilling JD et al. Prognostic utility of novel biomarkers of cardiovascular stress in patients with aortic stenosis undergoing valve replacement. Heart 2015;101:1382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempf T, von Haehling S, Peter T et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol 2007;50:1054–60. [DOI] [PubMed] [Google Scholar]
- 9.Ky B, French B, McCloskey K et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail 2011;4:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettencourt P, Azevedo A, Pimenta J, Frioes F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation 2004;110:2168–74. [DOI] [PubMed] [Google Scholar]
- 11.Braunwald E Biomarkers in heart failure. N Engl J Med 2008;358:2148–59. [DOI] [PubMed] [Google Scholar]
- 12.de Boer RA, Cao Q, Postmus D et al. The WAP four-disulfide core domain protein HE4: a novel biomarker for heart failure. JACC Heart failure 2013;1:164–9. [DOI] [PubMed] [Google Scholar]
- 13.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343–6. [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 1999;94:496–509. [Google Scholar]
- 15.Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82–90. [DOI] [PubMed] [Google Scholar]
- 16.Tjang YS, van Hees Y, Korfer R, Grobbee DE, van der Heijden GJ. Predictors of mortality after aortic valve replacement. Eur J Cardiothorac Surg 2007;32:469–74. [DOI] [PubMed] [Google Scholar]
- 17.Monin JL, Lancellotti P, Monchi M et al. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation 2009;120:69–75. [DOI] [PubMed] [Google Scholar]
- 18.Abramowitz Y, Chakravarty T, Jilaihawi H et al. Impact of Preprocedural B-Type Natriuretic Peptide Levels on the Outcomes After Transcatheter Aortic Valve Implantation. Am J Cardiol 2015;116:1904–9. [DOI] [PubMed] [Google Scholar]
- 19.Lancellotti P, Dulgheru R, Magne J et al. Elevated Plasma Soluble ST2 Is Associated with Heart Failure Symptoms and Outcome in Aortic Stenosis. PLoS One 2015;10:e0138940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindman BR, Clavel M-A, Mathieu P et al. Calcific aortic stenosis. Nature Reviews Disease Primers 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carabello BA. Is cardiac hypertrophy good or bad? The answer, of course, is yes. JACC Cardiovasc Imaging 2014;7:1081–3. [DOI] [PubMed] [Google Scholar]
- 22.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol 2012;60:1854–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


