Abstract
The field of tissue engineering has produced new therapies for the repair of damaged tissues and organs, utilizing biomimetic scaffolds that mirror the mechanical and biological properties of host tissue. The emergence of three-dimensional printing (3DP) technologies has enabled the fabrication of highly complex scaffolds which offer a more accurate replication of native tissue properties and architecture than previously possible. Of strong interest to tissue engineers is the construction of multilayered scaffolds that target distinct regions of complex tissues. Musculoskeletal and dental tissues in particular, such as the osteochondral unit and periodontal complex, are composed of multiple interfacing tissue types, and thus benefit from the usage of multilayered scaffold fabrication. Traditional 3DP technologies such as extrusion printing and selective laser sintering have been used for the construction of scaffolds with gradient architectures and mixed material compositions. Additionally, emerging bioprinting strategies have been used for the direct printing and spatial patterning of cells and chemical factors, capturing the complex organization found in the body. To better replicate the varied and gradated properties of larger tissues, researchers have created scaffolds composed of multiple materials spanning natural polymers, synthetic polymers, and ceramics. By utilizing high precision 3DP techniques and judicious material selection, scaffolds can thus be designed to address the regeneration of previously challenging musculoskeletal, dental, and other heterogeneous target tissues. These multilayered 3DP strategies show great promise in the future of tissue engineering.
Graphical Abstract

1. Introduction
Tissue engineering has emerged as a promising method to address a variety of clinical needs. Strategies used in this field encompass tissue and organ regeneration through the use of cells, bioactive molecules, and scaffolds.(1,2) The development of implantable scaffolds that accurately reflect native tissue architecture and biological function is critical to the success of these strategies.(3–5) To ensure these scaffolds achieve proper tissue regeneration, truly biomimetic constructs must address the vasculature, homo- and heterotypic cell-cell interactions, and mechanical properties unique to each tissue. Additionally, more intricate phenomena, such as synergistic effects on growth between adjacent tissue layers and reconstruction of the interface between these layers, must be incorporated into implanted scaffolds.
Incorporating these properties into developed scaffolds as they appear in native tissue directly affects the growth and development of new tissue. For example, mechanical and architectural properties can vary across tissue types and also spatially within a tissue. These properties may include compressive and tensile strength and porosity. They can affect biological cues such as cell differentiation, expansion, and growth. Thus, incorporating spatiotemporal gradients to mimic tissue organization within heterogeneous multilayered scaffolds can better support and guide proper tissue regeneration. Such properties are necessary to advance beyond engineering thin, hollow organs and into more complex tissues.(6) Musculoskeletal and dental tissue, in particular, are frequently comprised of a heterogeneous collection of tissues working in unison. The osteochondral unit is one such tissue that contains bone, cartilage, and transitional layers with gradated mechanical and biological properties.(7) Thus, gradient-based scaffold design is essential for recapitulating the properties of musculoskeletal and other heterogeneous tissues.
Three-dimensional printing (3DP) and related techniques are powerful alternatives to traditional scaffold fabrication methods, offering significant advantages in construct size, complexity of internal architecture, and material choice. Of particular interest are modifications to 3DP techniques for fabricating biologically inspired materials and scaffolds. These methods can be used to print multiple materials simultaneously, print localized and distributed cells and bioactive molecules without loss of function, and create gradients within scaffolds. Such abilities impart unparalleled control over internal microarchitecture and physical and chemical properties. This offers the ability to create models and implantable scaffolds that better mimic complex native tissues. By using natural architecture and organization to inspire biomaterials design, tissue engineers can better facilitate tissue growth and integration.
In this review, we discuss the major 3DP techniques and classes of materials used for tissue engineering applications. We also describe how 3DP has been applied to the fabrication of multilayered, complex tissues, with an emphasis on applications in musculoskeletal and dental tissue. Finally, we highlight several studies from primary literature describing novel modifications and combinations of these technologies to improve scaffold fabrication.
2. Commonly-Used Fabrication Methods
With the significant rise in additive manufacturing research over the last 20–25 years, a variety of different 3DP and rapid prototyping technologies have been adapted for medical applications. As noted by Trachtenberg et al., the recent developments in open source, low cost 3DP technologies have allowed for much more widespread use of additive manufacturing in biomedical research.(8) The most common 3DP techniques are extrusion printing/bioprinting, stereolithography (SLA), powder fusion printing (PFP), laser-assisted bioprinting (LAB), and inkjet bioprinting. Detailed reviews of each of these 3DP techniques have been conducted and can be found elsewhere; a summary of the primary advantages and limitations of these methods is given in Table 1.(3,4,6,9–12) This section serves to highlight examples of applying each method to multilayered scaffold design, with some additional specific examples of musculoskeletal tissue applications in recent literature.
Table 1.
Advantages and Limitations of Common 3DP Methods
| 3DP Method | Advantages | Limitations | References |
|---|---|---|---|
| Extrusion printing/bioprinting | +Precise positioning system +Precise control over printing conditions +Wide variety of materials +Can print physical and compositional gradients +Can directly print cells and bioactive factors |
-Cannot readily print single cells -Support for overhanging parts increases build time, material usage |
(4,6,13–23) |
| Stereolithography (SLA) | +High resolution +Can print complex internal architectures +Can easily print overhanging parts +No shear forces on print stock |
-Requires photocrosslinkable polymer -Unreacted monomer, photoinitiator can be toxic -Low mechanical strength -Resolution limited by light source, photomask -Horizontal mechanical property gradients are difficult to produce -Material gradients are difficult to produce with good fidelity |
(9,20,24–32) |
| Powder fusion printing (PFP)/Selective laser sintering (SLS) | +Wide variety of materials, including metals +High mechanical strength +Can print complex shapes +Vertical property gradients |
-Microfractures/voids caused by powder microstructure -Horizontal property gradients are difficult to produce -Cracking caused by multiple fusion steps |
(3,26,33–37) |
| Laser-assisted bioprinting (LAB) | +Prints at ambient conditions with high precision +Patterns single cells within scaffold +Uses a variety of bioactive materials +Prints multiple solutions simultaneously +Can print nanoliter scale volumes |
-Cost prohibitive -Limited height of scaffolds |
(6,16,38–46) |
| Inkjet bioprinting | +Very low cost +High-speed printing +Easily creates compositional gradients +Specific co-printing of multiple solutions +Bioactive material printing |
-Material choice is limited by printing speed and low viscosity requirements -Piezoelectric printers may be harmful to printed cells |
(4,13,16,38,47–59) |
a. Extrusion printing/Solid freeform/Fusion deposition
In extrusion printing, a premixed polymer feedstock is heated and continuously deposited onto the printing platform through a computer-controlled nozzle via motor-driven plungers or pneumatic pressure. Extrusion printing is an attractive vehicle for the fabrication of complex, multilayered scaffolds such as for tissue engineering applications. The scaffold heterogeneity and spatial organization are mainly limited only by the user’s skill in designing the CAD model and in the number of different material dispensers available. Many extrusion-based printers offer the ability to assign concurrent or independent printing instructions to different materials and easily switch between them. In this way, scaffolds with both vertical and lateral heterogeneity can be produced. For example, Lee et al. carried out an extrusion-based bioprinting proof of concept study for the fabrication of human skin.(60) In their study, collagen, keratinocytes, and fibroblasts were deposited sequentially to produce a layer-by-layer construct that mimics, albeit simply, the differences in composition in the dermal and epidermal layers of human skin. Similarly, Trachtenberg et al. produced uniform, two-material bilayer, and up to four-material gradient scaffolds using poly(propylene fumarate) (PPF) with varying hydroxyapatite (HAp) content.(23) Previous studies from this laboratory and others have shown that mechanical properties can also be altered through changing the internal pore morphology as well as the materials printed.(21,23,61) Finally, Liu et al. and Schuurman et al. displayed the ability to print lateral material gradients within scaffolds by depositing different materials adjacent to one another within the same layer in addition to channels, in addition to vertical gradients as described in other studies.(56,62) In each case, the mechanical properties of the scaffolds were tunable based on the incorporation of one or more printed materials and the relative composition of each within the construct.
In addition to general multilayer scaffolds, extrusion printing has been used extensively for musculoskeletal tissue applications. In a recent example, Kundu et al. used a multi-head system to produce biodegradable PCL-alginate scaffolds for tissue-engineered cartilage.(14) In the study, they successfully patterned both chondrocytes and TGF-β into the scaffolds, which retained bioactivity and improved cartilage formation in vivo. As a clinically-involved example, Woodfield et al. created poly(ethylene glycol)-terephthalate/poly(butylene terephthalate) (PEGT/PBT) scaffolds seeded with autologous chondrocytes which were developed from rabbit tibia and femur CT scans.(63) After six weeks in vivo, the constructs integrated well into the host tissue, and it was observed that all subjects regained limited load-bearing and function in the operated limbs. However, the authors noted that soft tissue formation was largely fibrocartilage rather than true articular cartilage, which may lead to movement and load-bearing limitations and pain long-term, whereas new bone tissue completely filled the scaffold space.(63) In another case, Zopf et al. created a patient-specific resorbable PCL-based airway splint for implantation in an infant with tracheobronchomalacia, a soft tissue condition which can cause narrowing or collapse of airway walls.(64) Ventilator support was totally discontinued 21 days after implantation, and after a one-year follow up, replacement of the construct with native tissue had begun and the patient did not present with complications. Although high temperature printing usually prohibits inclusion of cells and other biologically relevant components, one alternative solution has been cold/room temperature 3D “plotting”, as demonstrated by Ahlfeld et al. and others, avoiding the temperature barrier to biological molecules.(65–67) The former study demonstrated fabrication of multilayered constructs from calcium phosphate cement (CPC) paste and an alginate-based hydrogel. Using this room temperature plotting method, the authors were able to both create biphasic scaffold layers of CPC/alginate gel and incorporate VEGF within the alginate strands for extended release, which would not be possible in high-temperature printing. This group also demonstrated room temperature plotting of nanosilicate clay Laponite/alginate/methylcellulose scaffolds.(68) This experiment demonstrated that Laponite incorporation led to a sustained release of bovine serum albumin and VEGF, as well as that cold plotting parameters supported cellular printing with 70–75% viability.
b. Stereolithography
In stereolithography (SLA), a projected light source, such as a UV bulb or laser, is used to polymerize a polymer solution into a specified pattern.(4,20,27,32) SLA operates via a layer-by-layer process, where each 2D layer is cured in its entirety before moving to the next layer of the construct. SLA methods are useful in creating multilayered constructs with varying mechanical properties; specifically, varying the amount of solution exposed to light from layer to layer, the user can create vertical and lateral architectural gradients in the scaffold, which has been shown in several recent studies.(25,29,30) However, creating scaffolds with material heterogeneity using SLA methods has been previously challenging, as the solution platform must be emptied and refilled or interchanged with another fluid bed at each layer. However, Zhou et al., Choi et al., and Han et al. have all successfully demonstrated adaptation of SLA equipment for automatic material replacement, which, while potentially time-consuming, allows for the fabrication of scaffolds with material heterogeneity from layer to layer.(25,69,70) In particular, Zhou et al. presented one of the first examples of material heterogeneity within the same layer.(25) They displayed several test cases where separate and composite objects were printed with two different materials simultaneously without cross-contamination of the solutions. One area of improvement for future experiments would be further variability in materials; although the authors used different materials, all were from the same class of resins and as a result had similar bonding and exposure requirements. However, as initial test examples their experiments showed promise for creating multilayered heterogeneous scaffolds using lithography-based printing.(25) SLA also better accommodates overhanging/unsupported parts, enabling more complex bulk architectures, and the improved resolution enables printing of complicated internal architectures within enclosed scaffolds.(9,25,26,29) In this way, constructs with heterogeneous pore morphology and interconnectedness can be fabricated in an attempt to address the architectural complexity of a native tissue, which is particularly appropriate for developing multilayered scaffolds and multi-material interfaces present in tissues such as the osteochondral unit. SLA methods also represent an alternative for cell printing, as the shear forces observed in extrusion are absent.(30,31) For example, Elomaa et al. successfully developed a biodegradable poly(ethylene glycol-co-depsipeptide) hydrogel with branching vascular tubes and encapsulated human umbilical vein endothelial cells (HUVECs).(28)
c. Powder fusion printing/selective laser sintering
In powder fusion printing (PFP) and selective laser sintering (SLS), a bed of granular particles is selectively fused together using a binder solution (PFP) or a high-energy laser (SLS), creating a two-dimensional cross section of the desired construct.(9,33,37) Additional layers of powder are added on top of the previous print and sintered, generating a three-dimensional scaffold.(20,26,35) The use of SLS and PFP for creating single-material multilayered scaffolds has been extensively studied, and these techniques are relevant for hard tissues such as bone, because the fusion process can create rigid constructs.(71–77) The ease of creating structural gradients is useful for the fabrication of clinical size space fillers and bone substitutes, such as those shown by Klammert et al. and Bergmann et al.(71,75) These methods have struggled, however, to fabricate multi-material constructs, for largely the same reasons as SLA printing. To fabricate constructs with interlayer variability, the powder bed must be cleared and powder for the new material added, which may be time consuming and may risk contamination of the new layer. Likewise, fabricating constructs with intralayer heterogeneity is limited by adding the new powder layer all at once, which may result in mixing of the particles. In order to address these challenges, Du et al. presented a method for presetting layers of powder and manually adding each layer, as discussed in Section 4 of this paper.(76) Their method enabled the fabrication of PCL and PCL/HAp composite scaffolds with layers of varying HAp content to mimic the gradient structure of the osteochondral unit. In a different approach, Taylor et al. used a combination of extrusion printing and hydrogen sintering in order to fabricate metallic and metal oxide-based constructs.(72) This approach incorporated the control offered by extrusion printing to build constructs of varying architectures that were then sintered and reduced to form the final metallic structure. Inzana et al. demonstrated a room temperature powder fusion method using phosphoric acid-based binder solutions for calcium phosphate powder scaffolds.(78) In their experiment, the authors varied the composition of the binder solutions in order to alter the mechanical and biochemical properties of the scaffolds; in vitro, it was shown that the incorporation of dermal type I collagen into the binder solution or as a coating on the CP scaffold led to improved cell viability after 72 hours, and in vivo it was shown that new bone formation after 9 weeks in a murine femoral defect was similar between CP and collagen-binder CP scaffolds and allograft bone tissue, although no scaffolds achieved host-host bridging after 9 weeks.
d. Laser-assisted bioprinting
Laser-assisted bioprinting (LAB) uses laser pulses to heat and vaporize a solution containing bioactive contents (e.g. growth factors, cells, etc.), depositing the contents onto the scaffold without damage from the laser, which could have a destructive effect on these factors.(4,40,46) Although scaling limitations imply that LAB would be inappropriate for large, heterogeneous multilayer scaffolds such as those for bone or cartilage, the technique remains powerful for creating spatially heterogeneous constructs which may be needed in thin connective tissues such as skin, as well as specific cell and bioactive molecule depositing within a larger scaffold.(6,42) Several research groups have also attempted to improve the ability to scale. As early as 2004, Barron et al. printed stacks of mammalian cells onto a hydrogel substrate of 50–100μm in height, while maintaining near 100% viability.(79) More recently, Michael et al. generated full-thickness replacement tissues for 6mm dorsal skin fold chamber wounds in nude mice.(80) The authors fabricated simple skin-like constructs by printing 20 layers of fibroblasts and then 20 layers of keratinocytes on top of a Matriderm® substrate. The transplants were placed into full-thickness skin wounds and were fully connected to the surrounding tissue when explanted after 11 days.(80) Although it will be necessary to continue increasing the scale of fabrication possible using LAB, their experiment was among the first to demonstrate the possibility of using these methods to create multilayered, heterogeneous scaffolds for use in tissue engineering applications.
e. Inkjet bioprinting
In inkjet bioprinting, small droplets of biological “ink” solutions (bioinks) are deposited onto a substrate using either thermal of piezoelectric print heads.(4,16,54) Inkjet printers have been used in a variety of biological applications, including cell, DNA, and growth factor printing.(4,6,50). This technique is well-suited for fabricating complex scaffolds due to the ability to print multiple materials into relevant-sizes constructs with high fidelity, as has been shown previously.(47,58,81,82) In a 2009 study, Cooper et al. demonstrated spatially-controlled printing of a BMP-2 biological ink solution onto a DermaMatrix scaffold and observing the penetration depth of the solution as well as creating scaffolds for in vitro and in vivo cell differentiation and tissue formation studies.(47) Their example more closely resembles a 2.5D structure, as the biological solution was printed atop a separate 3D scaffold and penetrated into the matrix. More recently, Li et al. and Arai et al. each demonstrated the development of true multilayered scaffolds developed using inkjet printing.(81,82) In the former example, the authors developed two polypeptide-DNA hydrogels that could be separately printed into individual objects or printed together to create heterogeneous composites.(81) These solutions rapidly crosslinked, lending mechanical strength to the construct, as indicated by the ability to handle the constructs almost immediately after printing. Additionally, the authors demonstrated that AtT-20 cells could be printed within a polypeptide-DNA hydrogel solution with greater than 98% viability post printing.(81) In the latter study, Arai et al. fabricated several different multilayered hydrogel prints from sodium alginate solutions that were crosslinked using CaCl2.(82) The two most prominent examples displayed the ability of their inkjet printer to a) build significant scaffold height, and b) print cell-encapsulating hydrogels into high resolution patterns. In the first case, the authors constructed cell-free pyramids of up to 95 layers (5mm tall) by stacking images of progressively smaller squares as each 2D layer. In the second case, the authors printed
HeLa cell-encapsulating hydrogels up to 13 layers into the logo of their university. The features of this logo were recreated with good fidelity, and fluorescence staining indicated high viability of the cells after printing. Despite creating homogeneous scaffolds only, this study displayed that inkjet printing is a valuable technique for the bioprinting of scaffolds of clinically relevant size. Further modifications to their printing system, such as additional printheads for multiple materials, would be necessary to accommodate heterogeneous scaffold printing. Taken together, these examples present a strong case for the use of inkjet printing in developing multilayered scaffolds.
3. Materials and Biomaterial Inks
Regenerating more complex tissues requires heterogeneous, multilayered scaffold design. Such designs require composite materials and material gradients which can more accurately replicate the mechanical and biological properties of complex tissue units. In these multilayered constructs, each material is chosen for its mechanical properties and/or induced biological response, which should ultimately mimic those of the target tissue. The following discussion covers some commonly used materials and provides an assessment of their most prominent advantages and pitfalls in the context of multilayered scaffold design.
a. Synthetic Polymers
Synthetic polymers are compatible with a variety of 3DP techniques and are particularly advantageous for the fabrication of multilayered scaffolds due to their high tunability. The usage of 3DP processing offers a new selection of parameters to be tweaked as needed to generate gradients or defined separations of material properties. In one demonstration of this, Woodfield et al. fabricated poly(ethylene glycol)-terephthalate–poly(butylene terephthalate) (PEGT/PBT) scaffolds for cartilage with highly defined pore size gradients, by controlling fiber spacing during printing. The resulting scaffolds mimicked the zonal organization seen in layers of cartilage ECM with a degree of fidelity not achievable using traditional scaffold fabrication methods.(83) Alternatively, a mixture of multiple polymers can be deposited layer-by-layer with varying compositions of the polymers to create such distinct regions. In one study, Luca et al. printed a mixture of poly(lactic acid) (PLA), PCL, and poly(ethylene oxide terephthalate)/poly(butylene terephthalate) (PEOT/PBT) copolymers in varying ratios to create a stiffness gradient within the scaffold.(84) This gradient design produced differential amounts of glycosaminoglycan (GAG) production by seeded mesenchymal stem cells, mimetic of the non-uniform GAG distribution seen in heterogeneous tissues such as the osteochondral unit. Thus, synthetic polymer gradients can even produce biological responses that more accurately reflect the milieu of multilayered tissues.
Table 2 summarizes some of the most commonly applied polymers for the printing of tissue engineering constructs, along with the benefits and drawbacks in the context of multilayered scaffold fabrication.
Table 2.
Advantages and Limitations of Common Synthetic Polymers
| Polymer | Fabrication Methods | Advantages | Limitations | References |
|---|---|---|---|---|
| Poly(ε-caprolactone ) (PCL) |
|
+Provides hydrolysable regions with long lifespan +Allows co-polymerization with polyesters for gradation of properties |
-Produces regions with slow degradation | (84–87) |
| Poly(glycoli c acid) (PGA), Poly(lactic acid) (PLA), and Poly(lactic-co-glycolic acid) (PLGA) |
|
+Provides hydrolysable regions with short lifespan +Allows co-polymerization with polyesters for gradation of properties |
-Produces regions with fast degradation, accompanied by release of acidic factors | (61,84,88 90) |
| Poly(ethylen e glycol) (PEG) Derivatives |
|
+Provides highly hydrated (i.e. gel-like) regions – useful for tissues like cartilage | -Introduces non-hydrolyzable sections -Limits fabrication methods compared to other synthetic polymers |
(28,91,92) |
In the context of bioprinting and cell deposition, many synthetic polymers – including those shown in Table 2 – are effectively bioinert, allowing for the patterning of secondary biomolecules to generate regions of tissue specificity.(56) However, many of these synthetic polymers present relatively high glass transition and melting temperatures, requiring and polymer flow and printing to be performed above the 35–40 °C threshold tolerated by most cells.(16) The usage of cytotoxic crosslinking agents and initiators, as in SLA printing, presents a similar issue.(16) Thus, cells are typically encapsulated within natural polymers and protein materials instead, which can then be printed layer-by-layer with synthetic polymers.(62) For instance, Merceron et al. co-printed polyurethane with a cell-laden natural polymer bioink to generate elastic muscle tissue in one region, while co-printing PCL with a similar bioink to generate stiff tendon tissue in another region, ultimately creating a stratified muscle-tendon unit.(93) Ongoing work in the field should thus focus on the development of multi-material printing systems that can process both synthetic and natural polymer “inks” at their respective printing conditions. Multi-cartridge/printhead systems such as that developed by Kang et al. show promise in this regard by enabling the simultaneous printing but separate processing of several print solutions.(94)
b. Natural Polymers and Proteins
Natural polymers and proteins provide inherently bioactive properties but far less tunable material properties compared to synthetic polymers.(11,95) Thus, they are often printed as co-materials and provide areas of specific bioactivity for the intended tissue phenotype of a specific layer or microregion of the scaffold. In one such approach, the layer-by-layer deposition of decellularized ECM and vascular endothelial growth factor (VEGF) in between PCL fibers was used to generate patterned vasculature, as shown by Jang et al.(96) Creating defined vasculature as shown here is crucial for the viability of larger, multilayered tissues that require thorough vascularization. Biological polymers are typically printed by inkjet printing or lithography, due to their sensitivity to the high temperatures or organic solvents used in many traditional 3DP techniques.(11,97)
Table 3 summarizes a few of the most common biological polymers that have been used in the printing of multilayer scaffolds.
Table 3.
Advantages and Limitations of Common Biological Polymers
| Biological Polymer | Advantages | Disadvantages | References |
|---|---|---|---|
| Agar/Agarose | +Very inexpensive | -Not tissue-specific | (98,99) |
| Alginate | +Very inexpensive | -Not tissue-specific, and poorly attractive to serum proteins | (100,101) |
| Collagen/Gelatin | +Extractable from specific tissue of interest | -Variable in composition/quality from batch to batch | (38,97,102,103) |
| Decellularized ECM | +Extractable from specific tissue of interest +Most similar composition to native tissue matrix |
-Variable in composition/quality from batch to batch | (96,104) |
| Hyaluronic Acid (HA), Glycosaminoglycans (GAGs) | +Can be selected based on tissue of interest, e.g. chondroitin sulfate for cartilage +Especially useful for hydrated tissue types |
-Variable in composition/quality from batch to batch | (102,105) |
Bioactive proteins – e.g. growth factors – can also be distributed, essentially as additives, within a defined gradient to induce differentiation of seeded cells into defined and stratified tissue phenotypes. The most beneficial approach is to deposit tissue-specific growth factors in a spatial layout that corresponds to the distribution of tissue layers in vivo. In one instance, Castro et al. mimicked the organization of bone and cartilage layers within an osteochondral unit by delivering chondrogenic transforming growth-factor β1 (TGF-β1) in a gradient with osteogenic HAp.(91) In another approach, Ker et al. combined fiber orientation with the patterning of bone morphogenetic protein-2 (BMP-2) and tendon-promoting fibroblast growth factor-2 to produce stratified bone and tendon-like cell differentiation.(106) As evidenced by these and other approaches,(107,108) 3DP techniques have been used for the patterning of growth factors with higher fidelity and spatial resolution than traditional growth factor delivery.
However, biological factors, similarly to cells, are sensitive to the high temperatures often required for 3D printing. Thus, growth factors must be either printed within natural polymer-composed layers that are printed below 60 °C, or alternatively, encapsulated within some type of protective “shell” – such as a polymeric microparticle – during printing.(109) Additionally, challenges remain in mimicking the complex, multi-factor expression profiles seen in native tissue development.(108,110) In bone fracture healing, for instance, BMP-2 serves as an early osteogenic signal, while different bone morphogenetic proteins continue osteogenic development at later timepoints.(111) Future research must be done to translate studies of native growth factor expression and knowledge from 2D physicochemical gradient work to new applications in 3D constructs. Time-controlled release of growth factors must then be accomplished with a combination of new materials, novel strategies for the stimulation of growth factor release, and suitable 3DP structural designs. One straightforward approach is to encapsulate two different growth factors inside materials with distinct degradation kinetics, producing differential release profiles of the two factors.(112,113) Further development of such strategies will account for multiple growth factors of interest and could utilize external physical stimuli – such as light stimulation of multiple wavelengths – to regulate differential growth factor release.(114)
c. Ceramics
Ceramic materials have been popularly utilized in the reconstruction of hard tissue – such as bone – due to their rigidity, hardness, overall good mechanical strength, and similarity to the inorganic phase of bone and other mineralized tissue.(3) Given these properties, ceramics can be used as an additive material to create mechanical property gradients or mineralized layers in heterogeneous tissues that include bone. Jiang et al. demonstrated this approach by designing a multiphasic scaffold in which bioactive glass containing microspheres are used both as the primary material for a bone-mimetic layer as well as a mineralized additive for interfacial cartilage.(115) By doing so, they were able to replicate the unique transitional properties at the osteochondral interface more effectively than with any type of monophasic design. 3D printing techniques in particular have improved the ability of researchers to pattern these ceramic additives, and furthermore, to print them together with living cells of the desired phenotype – offering a powerful means of directing the growth of mineralized tissue layers.(116) In one case, Catros et al. utilized laser-assisted bioprinting to generate 3D patterns of mineralized tissue from the co-deposition of hydroxyapatite (HAp) with human osteopregenitors. The brittle mechanical properties and poor biodegradation of these ceramic materials, however, requires that they be co-printed with polymers.(110) For instance, Taboas et al. fabricated biphasic polymer/ceramic constructs of a controlled internal architecture containing discrete HAp layers.(117) Shim et al., on the other hand, printed incorporated TCP as a powder additive into molten polycaprolactone (PCL) and poly(lactide-co-glycolide) (PLGA) to print a hybrid scaffold with osteoconductive properties.(118) Given their osteoconductive properties, these ceramic materials are most beneficial when localized to the scaffold regions that target tissues such as the subchondral bone found in osteochondral tissue.(115)
4. Bioprinting and Cell-Based Inks
a. Current Approaches
Stratification of tissue phenotypes is crucial in multilayer tissue constructs, and one major approach towards this is to deliver or print tissue-specific cells within the 3DP ink(s) themselves, a technique known as bioprinting. Unlike the implantation of printed acellular scaffolds which require the recruitment cells adjacent to the construct, a bioprinting approach produces a scaffold with pre-encapsulated cells of the desired phenotypes.(119) For instance, by printing distinct layers of pre-osteogenic and pre-vascular cell types, Xu et al. generated osseous tissue interspersed with vasculature.(58) All encapsulated cells, however, must remain viable and phenotypically stable within the 3DP ink, so it is crucial that the scaffold incorporates either biological polymers and/or the appropriate growth factors for each cell type. Park et al. demonstrated this by showing that chondrocytes and osteoblasts proliferate more effectively and migrate towards bioprinted ECM materials that match their native ECM components – i.e. HA and collagen I, respectively.(120) These results have significant implications for osteochondral tissue engineering in particular, by revealing a means of localizing distinct cell types within their respective layers and maintaining tissue stratification. Additionally, the patterning of tissue-specific growth factors in coordination with the appropriate stem or precursor cells can provide the pre-stratification of tissue development. Jang et al. exemplified such an approach by patterning mesenchymal stem cells (MSCs) together with VEGF to generate vasculature within a heterogeneous tissue construct.(96) Miller et al. presented another highly informative study in which gradients of immobilized bone morphogenetic protein-2 (BMP-2) and insulin-like growth factor-II (IGF-II) were printed to characterize the effects of relative growth factor concentrations on cell fate.(121) Interestingly, the promotion of osteogenesis by BMP-2 was inhibited with increasing presence of IGF-II.(121) Studies such as these will elucidate the ideal growth factor combinations for different types of tissue, and are a precursor for optimal multi-tissue bioprinting.
b. Challenges and Future Directions
Challenges still remain in maintaining cell viability for more sensitive cell types, such as MSCs, during processing by 3DP techniques, which often require conditions harsher than physiological conditions.(119) If all tissue types are to be eventually printable, 3DP techniques must account for a wide variety of cell types with varying degrees of tolerance for these conditions. Future development of 3DP technologies should thus improve upon temperature control and sensitivity of print processes to minimize the thermal and mechanical stresses placed upon cells during printing. The harvest of certain cell types is another outstanding challenge for bioprinting techniques. Primary cell types tend to be present in smaller numbers in native tissue, presenting challenges with acquiring sufficient cell numbers from autologous and allogeneic sources when the tissue is scarce, as in the case of dental and periodontal tissues, or when the tissue is largely acellular, as in the case of cartilage.(119,122) Stem cell sources can alleviate some issues associated with scarcity, but in the case of heterogeneous tissues, require careful stratification of microenvironmental cues to ensure that cell phenotypes develop accordingly.(119) For smaller scale tissues such as the periodontal ligament, this will require high resolution bioprinting capabilities and the ability to co-print multiple growth factor formulations.(123)
Additionally, tissue engineers will have to identify the optimal growth factor gradients for inducing development of specific tissue types – an especially crucial task for the mimicry of interfaces between two or more tissue types. The osteochondral unit, for example, contains an interfacial layer of calcified cartilage with a steady transition between calcified, bone-like tissue and non-mineralized cartilage that could be a highly suitable target for the printing of a growth factor gradient.(124) While researchers have utilized ceramic additives and multiphasic scaffold design to provide a closer level of mimicry of this transitional tissue, designing truly mimetic gradients of growth factors or other biomolecules is still an ongoing effort.(107,115) Combinatorial studies with multiple growth factors have begun to shed light on this – for instance on the interactions of BMP-2 and IGF-II on bone development.(121)
One last point of importance is the spatiotemporal control of growth factor release from printed scaffolds. As discussed earlier, the maturation or repair of any tissue is a temporally complex process involving different growth factors at different stages of tissue development. Cell phenotypes shift in coordination with the expression of these growth factors, and current studies are only beginning to elucidate the growth factor expression profiles for single tissues such as bone.(109) For complex, heterogeneous tissues, the distribution of growth factors and interplay between cell and tissue layers become even more challenging to characterize. In the osteochondral unit, for instance, the development of subchondral bone provides a synergistic effect on the growth of cartilage tissue.(124) Ultimately, the field of bioprinting will benefit greatly from biological studies of temporal growth factor expression and the interactions between tissues as they develop. Once these developmental pathways become clearer, tissue engineers could utilize novel strategies from drug delivery, such as light or magnetic stimuli-induced release, to control the release of growth factors from printed scaffolds.(114,125)
5. Target Tissues
As discussed previously, multilayer scaffold design has enabled the repair of thick, heterogeneous tissues that have previously been challenging for single layer or homogenous scaffolds. Complex tissues such cartilage and bone benefit greatly from a multilayer scaffold approach. For thicker tissues in particular, 3DP techniques have been used to create vascular networks that help resolve the challenge of poor oxygen diffusion and waste exchange within the core of these constructs.(126) The following discussion will showcase a few of the complex and vascularized tissue types that have been reconstructed using a 3DP multilayered scaffold approach.
a. Cartilage and the Osteochondral Unit
Tissue engineering of cartilage and osteochondral joint defects and degeneration remains a challenge. Over 20% of the adult population in the United States suffers from cartilage-related conditions, including over 25 million adults with osteoarthritis.(129–133) These conditions collectively represent the most common cause of disability in adults over 30. Native cartilage is avascular and has a low cell density of chondrocytes, leading to a limited capacity for self-renewal compared to other tissues. The relatively simple makeup of cartilage tissue (extracellular matrix, GAGs, chondrocytes, and collagen fibers) is deceptive. Articular cartilage has an extremely heterogeneous gradient structure and a number of different biological cues. An inability to fabricate constructs matching this level of complexity has prevented the progress of restorative solutions.(29,124,134–136) The complexity of osteochondral lesions is even more pronounced, with an upper cartilage layer, characteristic cartilage-bone transition region, and a lower bone layer. Defects of the osteochondral joint are typically caused by acute traumatic injuries rather than progressive deterioration; these lesions are usually associated with great instability and will worsen over time if left untreated.(7,133)
The complex structures of these tissues, with physical and chemical gradients, require a highly organized heterogeneous scaffold to best facilitate regeneration. Engineering at the interface requires an accurate recreation of load-bearing and stress distribution mechanical properties as well as of the spatial orientation of chemical factors. Previous reports from our laboratory and others have shown that the development of bilayered hydrogel composites with cell and growth factor gradients can have a significant positive impact on cartilage and bone healing, showing increased expression of chondrogenic and osteogenic factors and improved tissue formation.(94,112,124,134,136–143) Many research groups have turned to the various 3DP techniques described in order to fabricate multilayered, heterogeneous constructs.(14,29,144–146)
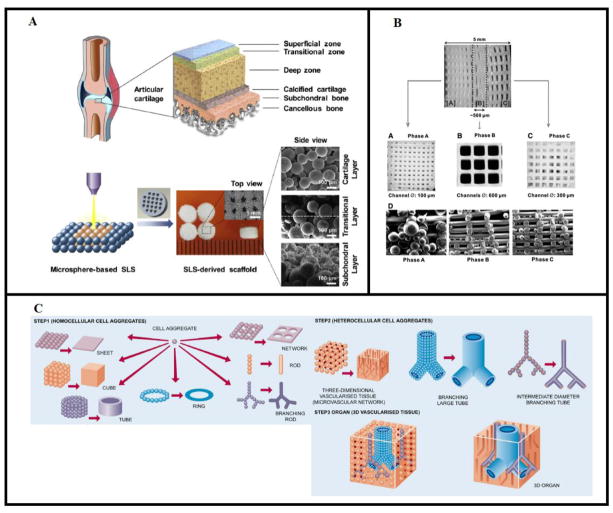
As an example, Du et al. created multilayered scaffolds from PCL and composite hydroxyapatite(HA)/PCL microspheres to address the heterogeneity of the osteochondral unit using SLS methods (Figure 1A).(76) The mechanical gradient and interconnected porosity of the scaffold, which more accurately represent the cartilage-bone interface, were shown to facilitate cell migration and new tissue formation. Specifically, after 6 and 12 weeks in vivo, the implanted scaffolds were shown to significantly improve chondrogenesis and subchondral bone formation. Additionally, the newly formed cartilage was of a comparable thickness and maturity to native cartilage, and the regenerated tissues integrated well into the host tissue. By contrast, Kundu et al. used a multi-head extrusion-based printing system to design constructs made of alternating layers of PCL and an alginate hydrogel with encapsulated chondrocytes.(14) The group also explored the ability to pattern transforming growth factor-β (TGF-β) within the gels. As expected, PCL incorporation led to improved stiffness and mechanical strength of the gels as they degraded. After 4 weeks in vivo, it was shown that 3DP scaffolds containing chondrocytes and chondrocytes+TGF-β had superior collagen II formation compared to PCL/alginate scaffolds alone, and in particular the addition of TGF-β led to more mature cartilage formation than in chondrocyte-only gels.(14) Tarafer et al. also used an extrusion printing system to facilitate spatial delivery of connective tissue growth factor (CTGF)- and TGF-β-filled PLGA microspheres within a PCL matrix. Microsphere-encapsulated growth factors maintained bioactivity as well as continued release over 42 days. In vitro bioactivity of these delivered factors was shown via directed differentiation of human bone marrow MSCs. CTGF and TGF-β-exposed MSCs matured into fibrogenic and chondrogenic cells respectively. Additionally, rabbit temporomandibular joint scaffolds created with CTGF and TGF-β3 microspheres led to heterogeneous fibrocartilage formation after 6 weeks in culture. In vivo, 4-week TMJ disc explants displayed recovery of the defect site, being mechanically sound with a highly-organized fibrocartilage structure mirroring native tissue.(145) In contrast, constructs printed without growth factors were observed to have significant tissue breakdown. Collectively, the results of these experiments indicate the ability of a multilayered, heterogeneous construct to better mimic the complexity of native cartilage tissue.
Figure 1.
Multilayered scaffold approaches to various complex tissue types. A) Using SLS, PCL and HA/PCL microspheres were used to create heterogeneous scaffolds with mechanical property gradients in order to model the cartilage, interface, and subchondral bone layers of the osteochondral unit. Reproduced with permission from Du et al.(76) B) Multiphasic scaffold design to target the cementum/dentin interface, periodontal ligament, and alveolar bone in phases A, B, C, respectively. Includes differential microchannel diameter across three phases as well as the delivery of amelogenin, connective tissue growth factor, and bone morphogenetic protein-2 to phases A, B, and C, respectively, via encapsulation in poly(lactic-co-glycolic acid) microspheres. Reproduced with permission from Lee et al.(127) C) Homogeneous and heterogeneous tissue constructs can be constructed from the spontaneous fusion of homocellular and heterocellular spheroids, respectively. The goal of whole organ printing is to eventually develop fully vascularized, 3D organs from the printing and subsequent fusion of these spheroids. Reproduced with permission from Mironov et al.(128)
b. Dental Tissue and the Periodontal Complex
Dental tissue is a collection of hard and soft tissues that includes the teeth and the various supporting tissues that comprise the periodontium. The periodontium, which consists of the alveolar bone, cementum, gingiva, and periodontal ligament (PDL) is subject to injury from trauma as well as a host of degenerative diseases which can comprise integrity of the dental unit and impair function.(123) The regeneration of any portion of the periodontium, similarly to the osteochondral unit, is a highly coordinated process between its components, so tissue engineering approaches must account for the biological and mechanical properties of these multiple tissue types.(127) While the bone and cementum are hard tissues, the gingiva and PDL are soft tissues, necessitating strong compartmentalization of mechanical properties in any multiphasic scaffold that targets the periodontium.(123)
One crucial target in periodontal repair is the PDL that bridges alveolar bone and cementum, a challenge considering that the bone and cementum layers should grow compartmentally.(123) 3DP scaffolds for the peridontium will often utilize geometric features such as differential pore size and architecture to direct the orientation of PDL fiber growth or compartmentalize these tissue layers. Park et al. fabricated such a composite scaffold in which a bone scaffold layer was printed with uniform porosity while an adjacent, fused PDL layer was printed with columnar pores to direct perpendicular PDL fiber growth.(147) The cell-seeded constructs then produced not only formation of PDL fibers perpendicular to bone, but also the beginning of PDL fiber ingrowth into its adjacent tissue layers.(147) Lee et al. combined the printing of three scaffold phases of distinct microchannel size with the delivery of three different growth factors and cell types in each phase to generate a construct with three distinct tissue phenotypes: a cementum/dentin interface, the PDL, and alveolar bone (Figure 1B).(127)
However, many of these 3DP approaches face a major translational challenge in their requirement for progenitor or germ cells, which cannot be acquired from the dental tissue of an adult patient and can present issues of immunogenicity if acquired from sources such as non-human animals.(122) Thus, some tissue engineers have printed scaffolds with no cell delivery but simply with incorporation of the appropriate growth factors for inducing development of multiple tissue types. Kim et al. applied such an approach to a rat model by printing anatomically shaped rat incisor scaffolds with microchannels for the perfusion and coating of two growth factors: bone morphogenetic protein-7 for its effects on mineralized tissues and stromal-derived factor-1 for its interactions with various progenitor cells.(122) Interestingly, their approach produced not only a high degree of tissue ingrowth but also the development of compartmentalized bone tissue and PDL structures. In one clinical case, Rasperini et al. fabricated an acellular, patient-customized scaffold with dip coating of platelet-derived growth factor BB.(148) They used a computed tomography scan of the patient’s periodontal bone defect to print a composite scaffold modeled after the shape of defect with additional struts to guide PDL formation perpendicular to the bone. While the resulting scaffold generated only minimal repair of bone and connective tissue, the printing of a patient-customized scaffold for both bone and PDL repair shows promise for future clinical applications.(148)
c. Vascularized Tissue
Vascularization, or the formation of blood vessels, is crucial for the function of nearly all tissue types.(126) The generation of vascularized scaffolds is a critical concern for tissues thicker than 100–200 μm – a category which includes many musculoskeletal tissue types – given that these thicker tissues suffer from poor diffusion of oxygen to their interior.(149) 3DP strategies have enabled the patterning of vascular channels within other tissues with high spatial resolution, producing scaffolds with vessel organization similar to that of native tissue. To this end, several groups have presented noteworthy strategies in which defined carbohydrate or synthetic polymer lattices are printed and then dissolved to create vascular channels for endothelial cell seeding.(150,151) These methods have been used in conjunction with the printing of cell-laden osteogenic inks as well to generate hybrid scaffolds with both vasculature and bone-like tissue development. Kolesky et al., in one case, co-printed a fugitive Pluronic ink with an MSC-laden gelatin/fibrinogen matrix, with subsequent dissolution of the fugitive ink to produce channels for endothelialization and exposure to osteogenic factors to produce bone-like tissue.(152) While these approaches have effectively generated uniform, interconnected vascular networks, native vasculature is usually organized with a more irregular distribution.(149) Thus, future 3DP approaches must include the capability to develop non-uniform, user-defined networks resembling the vascular “trees” characteristic of different tissue types. Improvements in the precision of bioprinting techniques will improve the resolution with which complex vascular “trees” can be printed – a critical measure given that vasculature is a particularly thin tissue that can have a diameter as low as 2–5 μm at its smallest.(153)
A different approach is to directly bioprint layers of endothelial cell-laden ink within a scaffold, as demonstrated by Cui et al. with the bioprinting of endothelial cell-laden fibrin channels.(154) Some bioinks have also utilized non-endothelial cell populations to avoid the potential immunogenicity and short lifespan of endothelial cell-derived vasculature.(86,96) One interesting approach taken recently by Jang et al. is to bioprint layers of MSCs and VEGF to induce MSC-derived vasculogenesis in addition to MSC-induced trophic effects on neighboring cell layers.(96)
d. Whole Organs
Whole organ printing is an emerging area of tissue engineering research that aims to fabricate tissues and organs via the bioprinting of heterogeneous cell distributions or cell spheroids. The shape and structure of entire organs may be replicated by the patterned deposition and subsequent self-assembly of relevant cell types into macroscale tissue, taking inspiration from embryonic organ development (Figure 1C).(119,155) Given the critical importance of cell viability, inkjet printing, low-temperature extrusion, or stereolithography of cell-containing hydrogels are often utilized in organ printing.(156) Kang et al., in a landmark study, demonstrated the printing of ear-shaped cartilage, mandibular bone, and other tissue of physiological size/shape by the patterning of cell-laden hydrogels with a PCL support and sacrificial Pluronic F-127.(94)
One approach involves the printing of spheroids containing homogeneous or heterogeneous cell populations. These spheroids, after printing, undergo spontaneous fusion to generate spatially organized tissue, as shown by Alajati et al. as they generated organized vascular networks from endothelial cell spheroids.(128,157) Challenges remain in this area of research with regards to the harvest of large cell quantities necessary for spheroid fabrication and the slow speed of spheroid fusion.(119,155) The speed of spheroid fusion in particular varies depending on cell type and maturity, and in the case of model fibroblast spheroids, has been found by Hajdu et al. to occur within the span of approximately 72 hours.(158) Tissue maturation from fused spheroids, however, can take as long as months.(156,158) In addition, spheroid fusion does not always occur as spontaneously as expected, and the resulting fused tissue can suffer from loss of the intended phenotype.(128) Thus, additional studies are needed to investigate how the introduction of appropriate biomolecules or the mechanical and chemical microenvironments of these spheroids influences their fusion and phenotypic fate. Future work may utilize low-cost model systems such as those developed by Hajdu et al. to investigate delivery of “maturogenic” factors which hasten the speed of spheroid fusion and subsequent maturation.(158) Nonetheless, spheroid-based 3DP strategies, both with and without polymeric support, have shown promise for the generation of organized layered, macroscale tissue.(159–161)
6. Novel Techniques from Recent Literature
3DP research for tissue engineering has begun transitioning to using a combination of 3DP and other fabrication techniques in order to achieve heterogenous multi-layered scaffolds suitable for eventual clinical application. This section discusses a few notable experiments in recent literature.
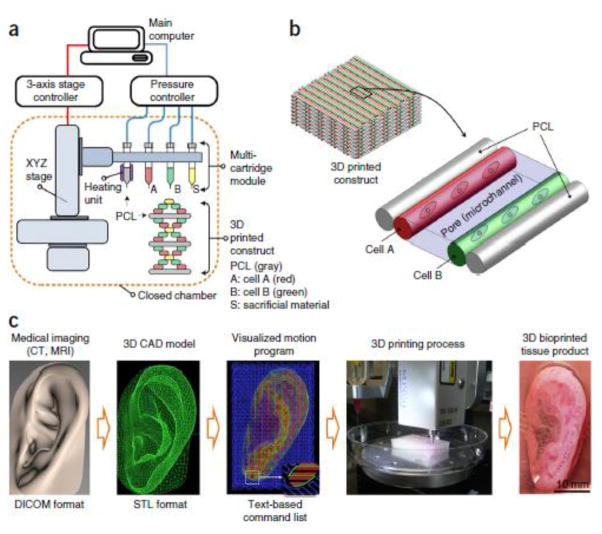
In a particularly noteworthy study, Kang et al.(94) used a multi-head integrated tissue-organ printing (ITOP) system to print cell-laden hydrogel-polymer constructs within a Pluronic F-127 sacrificial mold, similar to previous works by Gelber et al. and Miller et al.(162,163) The authors created hydrogels with varying concentrations of gelatin, fibrinogen, HA, and glycerol to mimic the native soft tissue environment. Mechanical strength was improved via incorporation of PCL, and the scaffolds were printed within an external sacrificial mold to maintain the desired shape. Constructs with appropriate mechanical and chemical properties were printed for use in the reconstruction of mandibular and calvarial bone, ear cartilage, and skeletal muscle, demonstrating the versatility and effectiveness of the ITOP system, shown in Figure 2.(94) The calvarial bone and ear cartilage reconstruction testing were of particular interest due to their relevance to current clinical challenges. In bone reconstruction, circular rat calvarial bone constructs were printed and cultured in osteogenic media prior to implantation within a defect site. The ITOP-developed constructs demonstrated mature bone formation with vascularization throughout the scaffold after 5 months in vivo, whereas the untreated defects and positive control showed fibrotic tissue growth and minimal new bone and vascular tissue formation. In the cartilage reconstruction study, new cartilage tissue was formed both in vitro and in vivo and mirrored the compositional gradients found in native cartilage. Cells within the constructs also exhibited a similar morphology to those of native tissue. Finally, in vivo implantation and maturation of ear constructs was shown to significantly improve the load bearing and resilience of the scaffold compared to non-implanted constructs.(94)
Figure 2.
Integrated Tissue-Organ Printing system used by Kang et. al. a) A computer-controlled 3-axis stage and multi-cartridge print head are used to print heterogeneous structures with high fidelity. b) Within a 3DP construct, different cell-laden hydrogels are patterned within a PCL framework. c) Medical images, such as MRIs, can be used to create 3D CAD models that give specific xyz locations and deposition instructions to the stage and print heads respectively to produce a 3DP construct. Reproduced with permission from Kang et al.(94)
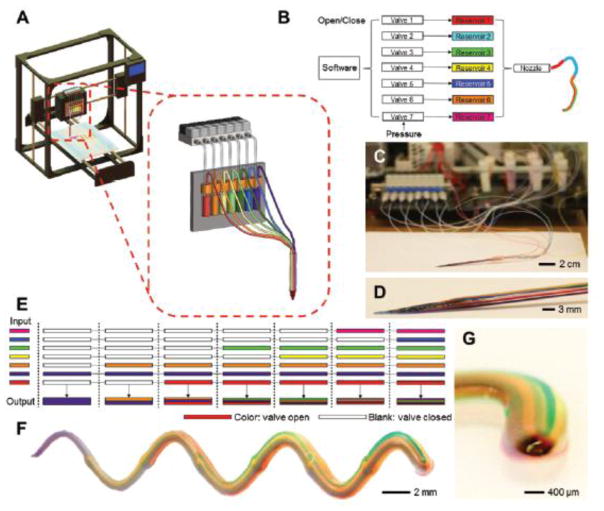
In another study, Liu et al. developed a multi-head extrusion printing system with simultaneous control over seven different printing solutions, as shown in Figure 3.(62) While the different reservoirs lead to and are deposited via the same nozzle, the user retains individual pneumatic control over the printing of each solution. Their system would be incredibly powerful for use in multilayered tissue scaffolds, as the different inks can be deposited separately in homogeneous layers or mixed in any composition to develop scaffolds with spatially-varying physical and chemical properties to better mimic the non-uniformity found in many native tissues. In their study, the authors successfully printed cell-laden constructs mimicking heart and bone tissue amongst others and developed a continuous circuit for use in bioelectronics applications by using a conductive alginate/carbon nanotube (CNT) bioink.(62)
Figure 3.
Multi-head extrusion system used by Liu et al. a, b) Schematic of the printer and pneumatic controls. The multi-head system contains up to 7 different cartridges, but individual pneumatic control is retained for each cartridge during printing. c, d) Photographs of the cartridges and print head described. e) Sample schematic showing how different bioinks can be mixed through pneumatic control. f,g) Printed microfiber of a multi-bioink extrusion material. Reproduced with permission from Liu et al.(62)
Xu et al. and Mellor et al. investigated hybrid 3DP/electrospinning techniques.(146,164) In the former study, the authors used a combined electrospinning/inkjet printing apparatus for use in cartilage tissue engineering. Five alternating layers of electrospun PCL and printed fibrin/collagen hydrogel were deposited to mimic the heterogeneous structure of native cartilage. The printed hydrogel was used to encapsulate rabbit articular chondrocytes, which remained viable after printing and retained functionality in the hybrid scaffold. Addition of electrospun PCL led to significantly improved mechanical properties compared to inkjet-printed constructs alone. In vitro and in vivo, new tissue formation was characterized by type II collagen and GAG deposition. In vitro, this deposition was largely observed at the periphery of the construct, indicating insufficient porosity; however, in vivo explants after 8 weeks displayed dense collagen II formation, ECM deposition, and chondrocyte phenotype representative of native articular cartilage.(146) Mellor et al. conducted a similar study, using extrusion-printed PCL for the top and bottom of the construct and hybrid electrospun PCL/collgen I gel for the middle layers, on which human adipose-derived stem cells were seeded.(164) The authors generated larger constructs via this combined method than with 3DP or electrospinning alone, creating a scaffold of relevant size that addresses the heterogeneity of the osteochondral joint.(164) In vitro, the authors noted that cell proliferation increased in 3DP-only, electrospun-only, and hybrid scaffolds, although the hybrid case displayed lower proliferation than the individual methods. Acellular scaffolds were also implanted into a cadaveric porcine knee defect in order to observe handling ability of each construct during surgery. The authors observed that although the electrospun-only scaffolds quickly delaminated upon implantation, the 3DP-only and combined 3DP/electrospun scaffolds were implanted with no structural limitations.(164)
Finally, Jakus et. al have demonstrated success in fabricating multilayered scaffolds for both osteoregeneration and neuroregeneration as well as composite scaffolds for complex tissue restoration.(165–167) In their 2017 study, the authors examined the ability to create composite osteogenic and neurogenic scaffolds via extrusion 3DP using two particle-based inks (named hyperelastic bone and 3D-graphene respectively) mixed into a single printable solution.(167) Although the osteogenic and neurogenic biomaterial inks were dry mixed in a 1:1 ratio prior to printing, the properties of the printed constructs could not be modeled as a linear average of the starting materials. The composite ink microstructure, elastic moduli, and failure strain were very similar to 3D-graphene rather than hyperelastic bone; however, the compressive properties varied between those of the individual inks depending on strain.(167) At low strain, the compressive properties matched that of 3D-graphene, with load increasing linearly with strain. Between 28–45% strain, however, the compressive properties resembled hyperelastic bone. The electrical conduction of the composite material was significantly less than the 3D-graphene and similar to hyperelastic bone, despite the 3D-graphene-dominated structure. Finally, the authors evaluated the scaffolds in vitro, and it was observed that seeded hMSCs displayed somewhat mixed morphology between those seeded on hyperelastic bone or 3D-graphene alone. Neuro- and osteogenic gene expression was also observed, with seeded cells demonstrating upregulation of both components over two weeks.(167) Their results provide strong support for the use of mixed biomaterial ink solutions in fabricating heterogeneous scaffolds with tunable mechanical and biological properties.
7. Conclusions and Future Directions
Three-dimensional printing strategies have shown great promise for the fabrication of multilayered scaffolds with heterogeneous properties mimicking those of native tissue. 3DP techniques range from the traditional strategies, such as selective laser sintering and powder fusion printing, to more recent developments such as bioprinting and deposition of tissue-specific cell spheroids. Further improvement in these techniques can be achieved by broadening the scope of printable fiber diameter, pore sizes, and other parameters relevant to tissue engineering scaffolds. Additionally, tissue engineers and materials scientists will need to develop means of maintaining structural integrity during the printing of complex and less conventionally shaped scaffolds, in order to diversify the range of printable geometries and tissue structures.
An exciting development in recent years has been the deposition of distinct, tissue-specific cell populations via bioprinting. These bioprinting strategies have enabled the spatial patterning of vascular networks, addressing a longstanding issue in the reconstruction of thicker tissues. Macroscale tissues are more reproducible than before due to the spatial control of cells and materials afforded by 3DP techniques. Future studies will improve on maintenance of cell viability, printing of biomolecule gradients, and spatiotemporal control of growth factor release.
Acknowledgments
We acknowledge support by the National Institutes of Health in the preparation of this work (P41 EB023833 and R01 AR068073). SMB also acknowledges support from the National Science Foundation Graduate Research Fellowship Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher JP, Mikos AG, Bronzino JD, Peterson DR. Tissue Engineering: Principles and Practices [Internet] Taylor&Francis Group; 2012. [cited 2017 Jul 11]. Available from: https://www.crcpress.com/Tissue-Engineering-Principles-and-Practices/Fisher-Mikos-Bronzino-Peterson/p/book/9781138077867. [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue engineering. Science. 1993 May 14;260(5110):920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013 Dec;16(12):496–504. [Google Scholar]
- 4.Sears NA, Seshadri DR, Dhavalikar PS, Cosgriff-Hernandez E. A Review of Three-Dimensional Printing in Tissue Engineering. Tissue Eng Part B Rev. 2016 Feb 9;22(4):298–310. doi: 10.1089/ten.TEB.2015.0464. [DOI] [PubMed] [Google Scholar]
- 5.Shim J-H, Lee J-S, Kim JY, Cho D-W. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J Micromechanics Microengineering. 2012;22(8):085014. [Google Scholar]
- 6.Gao G, Cui X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol Lett. 2016 Feb 1;38(2):203–11. doi: 10.1007/s10529-015-1975-1. [DOI] [PubMed] [Google Scholar]
- 7.Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. J Biomech. 2007;40(4):750–65. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Trachtenberg JE, Mountziaris PM, Miller JS, Wettergreen M, Kasper FK, Mikos AG. Open-source three-dimensional printing of biodegradable polymer scaffolds for tissue engineering. J Biomed Mater Res A. 2014 Dec 1;102(12):4326–35. doi: 10.1002/jbm.a.35108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bracaglia LG, Smith BT, Watson E, Arumugasaamy N, Mikos AG, Fisher JP. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater. 2017 Jul 1;56:3–13. doi: 10.1016/j.actbio.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakus AE, Rutz AL, Shah RN. Advancing the field of 3D biomaterial printing. Biomed Mater. 2016;11(1):014102. doi: 10.1088/1748-6041/11/1/014102. [DOI] [PubMed] [Google Scholar]
- 11.Mandrycky C, Wang Z, Kim K, Kim D-H. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016 Jul;34(4):422–34. doi: 10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016 Jan;76:321–43. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 13.Dababneh AB, Ozbolat IT. Bioprinting Technology: A Current State-of-the-Art Review. J Manuf Sci Eng. 2014 Oct 24;136(6):061016–11. [Google Scholar]
- 14.Kundu J, Shim J-H, Jang J, Kim S-W, Cho D-W. An additive manufacturing-based PCL alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med. 2015 Nov 1;9(11):1286–97. doi: 10.1002/term.1682. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed OA, Masood SH, Bhowmik JL. Optimization of fused deposition modeling process parameters: a review of current research and future prospects. Adv Manuf. 2015 Mar 1;3(1):42–53. [Google Scholar]
- 16.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014 Aug;32(8):773–85. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 17.Seol Y-J, Kang H-W, Lee SJ, Atala A, Yoo JJ. Bioprinting technology and its applications. Eur J Cardiothorac Surg. 2014 Sep 1;46(3):342–8. doi: 10.1093/ejcts/ezu148. [DOI] [PubMed] [Google Scholar]
- 18.Zein I, Hutmacher DW, Tan KC, Teoh SH. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 2002 Feb 15;23(4):1169–85. doi: 10.1016/s0142-9612(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D Biofabrication Strategies for Tissue Engineering and Regenerative Medicine. Annu Rev Biomed Eng. 2014;16(1):247–76. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik HH, Darwood ARJ, Shaunak S, Kulatilake P, El-Hilly AA, Mulki O, et al. Three-dimensional printing in surgery: a review of current surgical applications. J Surg Res. 2015 Dec;199(2):512–22. doi: 10.1016/j.jss.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 21.Trachtenberg JE, Placone JK, Smith BT, Piard CM, Santoro M, Scott DW, et al. Extrusion-Based 3D Printing of Poly(propylene fumarate) in a Full-Factorial Design. ACS Biomater Sci Eng. 2016 Oct 10;2(10):1771–80. doi: 10.1021/acsbiomaterials.6b00026. [DOI] [PubMed] [Google Scholar]
- 22.Trachtenberg JE, Santoro M, Williams C, Piard CM, Smith BT, Placone JK, et al. Effects of Shear Stress Gradients on Ewing Sarcoma Cells Using 3D Printed Scaffolds and Flow Perfusion. ACS Biomater Sci Eng [Internet] 2017 Feb 1; doi: 10.1021/acsbiomaterials.6b00641. Available from: [DOI] [PubMed]
- 23.Trachtenberg JE, Placone JK, Smith BT, Fisher JP, Mikos AG. Extrusion-based 3D printing of poly(propylene fumarate) scaffolds with hydroxyapatite gradients. J Biomater Sci Polym Ed. 2017 Apr 13;28(6):532–54. doi: 10.1080/09205063.2017.1286184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albritton JL, Miller JS. 3D bioprinting: improving in vitro models of metastasis with heterogeneous tumor microenvironments. Dis Model Mech. 2017 Jan 1;10(1):3–14. doi: 10.1242/dmm.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Chi, Chen Yong, Yang Zhigang, Khoshnevis Behrokh. Digital material fabrication using mask−image−projection−based stereolithography. Rapid Prototyp J. 2013 Apr 19;19(3):153–65. [Google Scholar]
- 26.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015 Mar 1;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooke MN, Fisher JP, Dean D, Rimnac C, Mikos AG. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J Biomed Mater Res B Appl Biomater. 2003 Feb 15;64B(2):65–9. doi: 10.1002/jbm.b.10485. [DOI] [PubMed] [Google Scholar]
- 28.Elomaa L, Pan C-C, Shanjani Y, Malkovskiy AV, Seppälä J, Yang Y. Three-dimensional fabrication of cell-laden biodegradable poly(ethylene glycol- co -depsipeptide) hydrogels by visible light stereolithography. J Mater Chem B. 2015;3(42):8348–58. doi: 10.1039/c5tb01468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro JN, O’Brien J, Grace Zhang L. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale. 2015;7(33):14010–22. doi: 10.1039/c5nr03425f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H, Zhang D, Alexander PG, Yang G, Tan J, Cheng AW-M, et al. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials. 2013 Jan 1;34(2):331–9. doi: 10.1016/j.biomaterials.2012.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinstlinger SI, Miller SJ. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip. 2016;16(11):2025–43. doi: 10.1039/c6lc00193a. [DOI] [PubMed] [Google Scholar]
- 32.Christenson EM, Soofi W, Holm JL, Cameron NR, Mikos AG. Biodegradable Fumarate-Based PolyHIPEs as Tissue Engineering Scaffolds. Biomacromolecules. 2007 Dec 1;8(12):3806–14. doi: 10.1021/bm7007235. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S. Selective laser sintering: A qualitative and objective approach. JOM. 2003 Oct 1;55(10):43–7. [Google Scholar]
- 34.Leong KF, Cheah CM, Chua CK. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 2003 Jun 1;24(13):2363–78. doi: 10.1016/s0142-9612(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 35.Mazzoli A. Selective laser sintering in biomedical engineering. Med Biol Eng Comput. 2013 Mar 1;51(3):245–56. doi: 10.1007/s11517-012-1001-x. [DOI] [PubMed] [Google Scholar]
- 36.Sachs EM, Haggerty JS, Cima MJ, Williams PA. Three-dimensional printing techniques [Internet] 5340656 A. US. 1994 Available from: http://www.google.com/patents/US5340656.
- 37.Yang S, Leong K-F, Du Z, Chua C-K. The Design of Scaffolds for Use in Tissue Engineering. Part II. Rapid Prototyping Techniques. Tissue Eng. 2002 Feb 1;8(1):1–11. doi: 10.1089/107632702753503009. [DOI] [PubMed] [Google Scholar]
- 38.Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014 Jan;35(1):49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 39.Devillard R, Pagès E, Correa MM, Kériquel V, Rémy M, Kalisky J, et al. Cell Patterning by Laser-Assisted Bioprinting. Methods Cell Biol. 2014 Jan 1;119:159–74. doi: 10.1016/B978-0-12-416742-1.00009-3. [DOI] [PubMed] [Google Scholar]
- 40.Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010 Oct 1;31(28):7250–6. doi: 10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 41.Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, Gaebel R, et al. Laser Printing of Skin Cells and Human Stem Cells. Tissue Eng Part C Methods. 2009 Nov 2;16(5):847–54. doi: 10.1089/ten.TEC.2009.0397. [DOI] [PubMed] [Google Scholar]
- 42.Odde DJ, Renn MJ. Laser-guided direct writing of living cells. Biotechnol Bioeng. 2000 Feb 5;67(3):312–8. doi: 10.1002/(sici)1097-0290(20000205)67:3<312::aid-bit7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 43.Ringeisen BR, Wu PK, Kim H, Piqué A, Auyeung RYC, Young HD, et al. Picoliter-Scale Protein Microarrays by Laser Direct Write. Biotechnol Prog. 2002 Jan 1;18(5):1126–9. doi: 10.1021/bp015516g. [DOI] [PubMed] [Google Scholar]
- 44.Ringeisen BR, Kim H, Barron JA, Krizman DB, Chrisey DB, Jackman S, et al. Laser Printing of Pluripotent Embryonal Carcinoma Cells. Tissue Eng. 2004 Mar 1;10(3–4):483–91. doi: 10.1089/107632704323061843. [DOI] [PubMed] [Google Scholar]
- 45.Unger C, Gruene M, Koch L, Koch J, Chichkov BN. Time-resolved imaging of hydrogel printing via laser-induced forward transfer. Appl Phys A. 2011 May 1;103(2):271–7. [Google Scholar]
- 46.Zhang Z, Xiong R, Mei R, Huang Y, Chrisey DB. Time-Resolved Imaging Study of Jetting Dynamics during Laser Printing of Viscoelastic Alginate Solutions. Langmuir. 2015 Jun 16;31(23):6447–56. doi: 10.1021/acs.langmuir.5b00919. [DOI] [PubMed] [Google Scholar]
- 47.Cooper GM, Miller ED, DeCesare GE, Usas A, Lensie EL, Bykowski MR, et al. Inkjet-Based Biopatterning of Bone Morphogenetic Protein-2 to Spatially Control Calvarial Bone Formation. Tissue Eng Part A. 2009 Dec 22;16(5):1749–59. doi: 10.1089/ten.tea.2009.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui X, Dean D, Ruggeri ZM, Boland T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol Bioeng. 2010 Aug 15;106(6):963–9. doi: 10.1002/bit.22762. [DOI] [PubMed] [Google Scholar]
- 49.Cui X, Boland TD, D’Lima DK, Lotz M. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent Pat Drug Deliv Formul. 2012 Aug 1;6(2):149–55. doi: 10.2174/187221112800672949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldmann T, Gonzalez JS. DNA-printing: utilization of a standard inkjet printer for the transfer of nucleic acids to solid supports. J Biochem Biophys Methods. 2000 Mar 16;42(3):105–10. doi: 10.1016/s0165-022x(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 51.de Jong J, de Bruin G, Reinten H, van den Berg M, Wijshoff H, Versluis M, et al. Air entrapment in piezo-driven inkjet printheads. J Acoust Soc Am. 2006 Sep 1;120(3):1257–65. [Google Scholar]
- 52.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv Mater. 2014 May 1;26(19):3124–30. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 53.Lorber B, Hsiao W-K, Hutchings IM, Martin KR. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication. 2014;6(1):015001. doi: 10.1088/1758-5082/6/1/015001. [DOI] [PubMed] [Google Scholar]
- 54.Mohebi MM, Evans JRG. A Drop-on-Demand Ink-Jet Printer for Combinatorial Libraries and Functionally Graded Ceramics. J Comb Chem. 2002 Jul 1;4(4):267–74. doi: 10.1021/cc010075e. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura M, Kobayashi A, Takagi F, Watanabe A, Hiruma Y, Ohuchi K, et al. Biocompatible Inkjet Printing Technique for Designed Seeding of Individual Living Cells. Tissue Eng. 2005 Nov 1;11(11–12):1658–66. doi: 10.1089/ten.2005.11.1658. [DOI] [PubMed] [Google Scholar]
- 56.Schuurman W, Khristov V, Pot MW, van Weeren PR, Dhert WJA, Malda J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication. 2011;3(2):021001. doi: 10.1088/1758-5082/3/2/021001. [DOI] [PubMed] [Google Scholar]
- 57.Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005 Jan 1;26(1):93–9. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 58.Xu T, Zhao W, Zhu J-M, Albanna MZ, Yoo JJ, Atala A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013 Jan;34(1):130–9. doi: 10.1016/j.biomaterials.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 59.Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A. 2013 Jan 1;101A(1):272–84. doi: 10.1002/jbm.a.34326. [DOI] [PubMed] [Google Scholar]
- 60.Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, et al. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng Part C Methods. 2013 Nov 4;20(6):473–84. doi: 10.1089/ten.tec.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo T, Holzberg TR, Lim CG, Gao F, Gargava A, Trachtenberg JE, et al. 3D printing PLGA: a quantitative examination of the effects of polymer composition and printing parameters on print resolution. Biofabrication. 2017;9(2):024101. doi: 10.1088/1758-5090/aa6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu W, Zhang YS, Heinrich MA, De Ferrari F, Jang HL, Bakht SM, et al. Rapid Continuous Multimaterial Extrusion Bioprinting. Adv Mater. 2016 Nov 17;29(3) doi: 10.1002/adma.201604630. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodfield TBF, Guggenheim M, von Rechenberg B, Riesle J, van Blitterswijk CA, Wedler V. Rapid prototyping of anatomically shaped, tissue-engineered implants for restoring congruent articulating surfaces in small joints. Cell Prolif. 2009;42:485–97. doi: 10.1111/j.1365-2184.2009.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. Bioresorbable Airway Splint Created with a Three-Dimensional Printer. N Engl J Med. 2013 May 23;368(21):2043–5. doi: 10.1056/NEJMc1206319. [DOI] [PubMed] [Google Scholar]
- 65.Ahlfeld T, Akkineni AR, Förster Y, Köhler T, Knaack S, Gelinsky M, et al. Design and Fabrication of Complex Scaffolds for Bone Defect Healing: Combined 3D Plotting of a Calcium Phosphate Cement and a Growth Factor-Loaded Hydrogel. Ann Biomed Eng. 2017 Jan 1;45(1):224–36. doi: 10.1007/s10439-016-1685-4. [DOI] [PubMed] [Google Scholar]
- 66.Bergmann CJD, Odekerken JCE, Welting TJM, Jungwirth F, Devine D, Bouré L, et al. Calcium Phosphate Based Three-Dimensional Cold Plotted Bone Scaffolds for Critical Size Bone Defects [Internet] BioMed Research International. 2014 doi: 10.1155/2014/852610. [cited 2018 Feb 5]. Available from: https://www-hindawi-com.ezproxy.rice.edu/journals/bmri/2014/852610/ [DOI] [PMC free article] [PubMed]
- 67.Lode A, Meissner K, Luo Y, Sonntag F, Glorius S, Nies B, et al. Fabrication of porous scaffolds by three-dimensional plotting of a pasty calcium phosphate bone cement under mild conditions. J Tissue Eng Regen Med. 2014 Sep 1;8(9):682–93. doi: 10.1002/term.1563. [DOI] [PubMed] [Google Scholar]
- 68.Ahlfeld T, Cidonio G, Kilian D, Duin S, Akkineni AR, Dawson JI, et al. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication. 2017;9(3):034103. doi: 10.1088/1758-5090/aa7e96. [DOI] [PubMed] [Google Scholar]
- 69.Choi J-W, MacDonald E, Wicker R. Multi-material microstereolithography. Int J Adv Manuf Technol. 2010 Jul 1;49(5–8):543–51. [Google Scholar]
- 70.Han L-H, Suri S, Schmidt CE, Chen S. Fabrication of three-dimensional scaffolds for heterogeneous tissue engineering. Biomed Microdevices. 2010 Aug 1;12(4):721–5. doi: 10.1007/s10544-010-9425-2. [DOI] [PubMed] [Google Scholar]
- 71.Klammert U, Gbureck U, Vorndran E, Rödiger J, Meyer-Marcotty P, Kübler AC. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J Cranio-Maxillofac Surg. 2010 Dec;38(8):565–70. doi: 10.1016/j.jcms.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 72.Taylor SL, Jakus AE, Shah RN, Dunand DC. Iron and Nickel Cellular Structures by Sintering of 3D-Printed Oxide or Metallic Particle Inks. Adv Eng Mater. 2016 Sep 1; n/a-n/a. [Google Scholar]
- 73.Jakus AE, Taylor SL, Geisendorfer NR, Dunand DC, Shah RN. Metallic Architectures from 3D-Printed Powder-Based Liquid Inks. Adv Funct Mater. 2015 Dec 1;25(45):6985–95. [Google Scholar]
- 74.Zhou Z, Buchanan F, Mitchell C, Dunne N. Printability of calcium phosphate: Calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Mater Sci Eng C. 2014 May 1;38:1–10. doi: 10.1016/j.msec.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 75.Bergmann C, Lindner M, Zhang W, Koczur K, Kirsten A, Telle R, et al. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J Eur Ceram Soc. 2010 Sep;30(12):2563–7. [Google Scholar]
- 76.Du Y, Liu H, Yang Q, Wang S, Wang J, Ma J, et al. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials. 2017 Aug 1;137(Supplement C):37–48. doi: 10.1016/j.biomaterials.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duan B, Wang M, Zhou WY, Cheung WL, Li ZY, Lu WW. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010 Dec 1;6(12):4495–505. doi: 10.1016/j.actbio.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 78.Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014 Apr;35(13):4026–34. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barron JA, Ringeisen BR, Kim H, Spargo BJ, Chrisey DB. Application of laser printing to mammalian cells. Thin Solid Films. 2004 Apr 1;453–454(Supplement C):383–7. [Google Scholar]
- 80.Michael S, Sorg H, Peck C-T, Koch L, Deiwick A, Chichkov B, et al. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PloS One. 2013;8(3):e57741. doi: 10.1371/journal.pone.0057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li C, Faulkner-Jones A, Dun AR, Jin J, Chen P, Xing Y, et al. Rapid Formation of a Supramolecular Polypeptide–DNA Hydrogel for In Situ Three-Dimensional Multilayer Bioprinting. Angew Chem Int Ed. 2015 Mar 23;54(13):3957–61. doi: 10.1002/anie.201411383. [DOI] [PubMed] [Google Scholar]
- 82.Arai K, Iwanaga S, Toda H, Genci C, Nishiyama Y, Nakamura M. Three-dimensional inkjet biofabrication based on designed images. Biofabrication. 2011;3(3):034113. doi: 10.1088/1758-5082/3/3/034113. [DOI] [PubMed] [Google Scholar]
- 83.Woodfield TBF, Malda J, de Wijn J, Péters F, Riesle J, van Blitterswijk CA. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials. 2004 Aug 1;25(18):4149–61. doi: 10.1016/j.biomaterials.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 84.Di Luca A, Longoni A, Criscenti G, Lorenzo-Moldero I, Klein-Gunnewiek M, Vancso J, et al. Surface energy and stiffness discrete gradients in additive manufactured scaffolds for osteochondral regeneration. Biofabrication. 2016;8(1):015014. doi: 10.1088/1758-5090/8/1/015014. [DOI] [PubMed] [Google Scholar]
- 85.Cao T, Ho K-H, Teoh S-H. Scaffold design and in vitro study of osteochondral coculture in a three-dimensional porous polycaprolactone scaffold fabricated by fused deposition modeling. Tissue Eng. 2003;9(4, Supplement 1):103–12. doi: 10.1089/10763270360697012. [DOI] [PubMed] [Google Scholar]
- 86.Temple JP, Hutton DL, Hung BP, Huri PY, Cook CA, Kondragunta R, et al. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D−printed PCL scaffolds. J Biomed Mater Res A. 2014;102(12):4317–25. doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 87.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26(23):4817–27. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 88.Heymer A, Bradica G, Eulert J, Nöth U. Multiphasic collagen fibre–PLA composites seeded with human mesenchymal stem cells for osteochondral defect repair: an in vitro study. J Tissue Eng Regen Med. 2009;3(5):389–97. doi: 10.1002/term.175. [DOI] [PubMed] [Google Scholar]
- 89.Ghosh S, Viana JC, Reis RL, Mano JF. Bi-layered constructs based on poly(l-lactic acid) and starch for tissue engineering of osteochondral defects. Mater Sci Eng C. 2008 Jan 10;28(1):80–6. [Google Scholar]
- 90.Zhou WY, Lee SH, Wang M, Cheung WL, Ip WY. Selective laser sintering of porous tissue engineering scaffolds from poly (L-lactide)/carbonated hydroxyapatite nanocomposite microspheres. J Mater Sci Mater Med. 2008;19(7):2535–40. doi: 10.1007/s10856-007-3089-3. [DOI] [PubMed] [Google Scholar]
- 91.Castro NJ, O’Brien J, Zhang LG. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale. 2015;7(33):14010–22. doi: 10.1039/c5nr03425f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang W, Lian Q, Li D, Wang K, Hao D, Bian W, et al. The effect of interface microstructure on interfacial shear strength for osteochondral scaffolds based on biomimetic design and 3D printing. Mater Sci Eng C. 2015;46:10–5. doi: 10.1016/j.msec.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 93.Merceron TK, Burt M, Seol Y-J, Kang H-W, Lee SJ, Yoo JJ, et al. A 3D bioprinted complex structure for engineering the muscle–tendon unit. Biofabrication. 2015;7(3):035003. doi: 10.1088/1758-5090/7/3/035003. [DOI] [PubMed] [Google Scholar]
- 94.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016 Mar;34(3):312–9. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 95.Lee EJ, Kasper FK, Mikos AG. Biomaterials for Tissue Engineering. Ann Biomed Eng. 2014 Feb;42(2):323–37. doi: 10.1007/s10439-013-0859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jang J, Park H-J, Kim S-W, Kim H, Park JY, Na SJ, et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017 Jan;112:264–74. doi: 10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 97.Lam CX, Mo X, Teoh S, Hutmacher D. Scaffold development using 3D printing with a starch-based polymer. Mater Sci Eng C. 2002 May 31;20(1–2):49–56. [Google Scholar]
- 98.Diridollou S, Patat F, Gens F, Vaillant L, Black D, Lagarde JM, et al. In vivo model of the mechanical properties of the human skin under suction. Skin Res Technol Off J Int Soc Bioeng Skin ISBS Int Soc Digit Imaging Skin ISDIS Int Soc Skin Imaging ISSI. 2000 Nov;6(4):214–21. doi: 10.1034/j.1600-0846.2000.006004214.x. [DOI] [PubMed] [Google Scholar]
- 99.Landers R, Hübner U, Schmelzeisen R, Mülhaupt R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials. 2002;23(23):4437–47. doi: 10.1016/s0142-9612(02)00139-4. [DOI] [PubMed] [Google Scholar]
- 100.Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O, et al. Nanowired three-dimensional cardiac patches. Nat Nanotechnol. 2011;6(11):720–5. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Griffith LG, Wu BEN, Cima MJ, Powers MJ, Chaignaud B, Vacanti JP. In vitro organogenesis of liver tissue. Ann N Y Acad Sci. 1997;831(1):382–97. doi: 10.1111/j.1749-6632.1997.tb52212.x. [DOI] [PubMed] [Google Scholar]
- 102.Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339–48. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 103.Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2012;5(1):015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 104.Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014 Mar;32(2):462–84. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Collins MN, Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr Polym. 2013;92(2):1262–79. doi: 10.1016/j.carbpol.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 106.Ker ED, Nain AS, Weiss LE, Wang J, Suhan J, Amon CH, et al. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials. 2011;32(32):8097–107. doi: 10.1016/j.biomaterials.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 107.Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Eng Part A. 2011 Nov;17(21–22):2845–55. doi: 10.1089/ten.tea.2011.0135. [DOI] [PubMed] [Google Scholar]
- 108.Singh M, Berkland C, Detamore MS. Strategies and applications for incorporating physical and chemical signal gradients in tissue engineering. Tissue Eng Part B Rev. 2008;14(4):341–66. doi: 10.1089/ten.teb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8(55):153–70. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Gaalen S, Kruyt M, Meijer G, Mistry A, Mikos A, van den Beucken J, et al. Tissue engineering. Elsevier Inc; 2008. Tissue engineering of bone. [Google Scholar]
- 111.Cho T-J, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J Bone Miner Res. 2002;17(3):513–20. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 112.Lam J, Lu S, Kasper FK, Mikos AG. Strategies for controlled delivery of biologics for cartilage repair. Adv Drug Deliv Rev. 2015 Apr 1;84:123–34. doi: 10.1016/j.addr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park JY, Shim J-H, Choi S-A, Jang J, Kim M, Lee SH, et al. 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J Mater Chem B. 2015;3(27):5415–25. doi: 10.1039/c5tb00637f. [DOI] [PubMed] [Google Scholar]
- 114.Azagarsamy MA, Anseth KS. Light wavelengths to regulate the release of multiple growth factors. Soc Biomater. 2014 [Google Scholar]
- 115.Jiang J, Tang A, Ateshian GA, Guo XE, Hung CT, Lu HH. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng. 2010 Jun;38(6):2183–96. doi: 10.1007/s10439-010-0038-y. [DOI] [PubMed] [Google Scholar]
- 116.Catros S, Fricain J-C, Guillotin B, Pippenger B, Bareille R, Remy M, et al. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication. 2011;3(2):025001. doi: 10.1088/1758-5082/3/2/025001. [DOI] [PubMed] [Google Scholar]
- 117.Taboas JM, Maddox RD, Krebsbach PH, Hollister SJ. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials. 2003;24(1):181–94. doi: 10.1016/s0142-9612(02)00276-4. [DOI] [PubMed] [Google Scholar]
- 118.Shim J-H, Yoon M-C, Jeong C-M, Jang J, Jeong S-I, Cho D-W, et al. Efficacy of rhBMP-2 loaded PCL/PLGA/β-TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomed Mater. 2014;9(6):065006. doi: 10.1088/1748-6041/9/6/065006. [DOI] [PubMed] [Google Scholar]
- 119.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–85. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 120.Park JY, Choi J-C, Shim J-H, Lee J-S, Park H, Kim SW, et al. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication. 2014;6(3):035004. doi: 10.1088/1758-5082/6/3/035004. [DOI] [PubMed] [Google Scholar]
- 121.Miller ED, Phillippi JA, Fisher GW, Campbell PG, Walker LM, Weiss LE. Inkjet printing of growth factor concentration gradients and combinatorial arrays immobilized on biologically-relevant substrates. Comb Chem High Throughput Screen. 2009;12(6):604–18. doi: 10.2174/138620709788681907. [DOI] [PubMed] [Google Scholar]
- 122.Kim K, Lee CH, Kim BK, Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010;89(8):842–7. doi: 10.1177/0022034510370803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ivanovski S, Vaquette C, Gronthos S, Hutmacher DW, Bartold PM. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res. 2014;93(12):1212–21. doi: 10.1177/0022034514544301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lam J, Lu S, Meretoja VV, Tabata Y, Mikos AG, Kasper FK. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomater. 2014 Mar 1;10(3):1112–23. doi: 10.1016/j.actbio.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ulbrich K, Hola K, Subr V, Bakandritsos A, Tucek J, Zboril R. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem Rev. 2016;116(9):5338–431. doi: 10.1021/acs.chemrev.5b00589. [DOI] [PubMed] [Google Scholar]
- 126.Nerem RM, Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3(1):225–43. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 127.Lee CH, Hajibandeh J, Suzuki T, Fan A, Shang P, Mao JJ. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A. 2014;20(7–8):1342–51. doi: 10.1089/ten.tea.2013.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30(12):2164–74. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Csaki C, Schneider PRA, Shakibaei M. Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann Anat - Anat Anz. 2008 Nov 20;190(5):395–412. doi: 10.1016/j.aanat.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 130.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008 Jan 1;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 131.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008 Jan 1;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murphy L, Helmick CG. The Impact of Osteoarthritis in the United States: A... : Orthopaedic Nursing [Internet] doi: 10.1097/NOR.0b013e31824fcd42. [cited 2017 Jul 6]. Available from: http://journals.lww.com/orthopaedicnursing/Fulltext/2012/03000/The_Impact_of_Osteoarthritis_in_the_United_States_.6.aspx. [DOI] [PubMed]
- 133.Yang J, Shrike Zhang Y, Yue K, Khademhosseini A. Cell-Laden Hydrogels for Osteochondral and Cartilage Tissue Engineering. Acta Biomater [Internet] 2017 doi: 10.1016/j.actbio.2017.01.036. [cited 2017 May 17]; Available from: http://www.sciencedirect.com/science/article/pii/S1742706117300363. [DOI] [PMC free article] [PubMed]
- 134.Lam J, Lu S, Lee EJ, Trachtenberg JE, Meretoja VV, Dahlin RL, et al. Osteochondral defect repair using bilayered hydrogels encapsulating both chondrogenically and osteogenically pre-differentiated mesenchymal stem cells in a rabbit model. Osteoarthritis Cartilage. 2014 Sep 1;22(9):1291–300. doi: 10.1016/j.joca.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee J-S, Hong JM, Jung JW, Shim J-H, Oh J-H, Cho D-W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6(2):024103. doi: 10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- 136.Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, van den Beucken JJJP, et al. Technical Report: Correlation Between the Repair of Cartilage and Subchondral Bone in an Osteochondral Defect Using Bilayered, Biodegradable Hydrogel Composites. Tissue Eng Part C Methods. 2015 Jul 15;21(12):1216–25. doi: 10.1089/ten.tec.2015.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guo X, Park H, Liu G, Liu W, Cao Y, Tabata Y, et al. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials. 2009 May 1;30(14):2741–52. doi: 10.1016/j.biomaterials.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo X, Liao J, Park H, Saraf A, Raphael RM, Tabata Y, et al. Effects of TGF-β3 and preculture period of osteogenic cells on the chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in a bilayered hydrogel composite. Acta Biomater. 2010 Aug 1;6(8):2920–31. doi: 10.1016/j.actbio.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guo X, Park H, Young S, Kretlow JD, van den Beucken JJ, Baggett LS, et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010 Jan 1;6(1):39–47. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim K, Lam J, Lu S, Spicer PP, Lueckgen A, Tabata Y, et al. Osteochondral tissue regeneration using a bilayered composite hydrogel with modulating dual growth factor release kinetics in a rabbit model. J Controlled Release. 2013 Jun 10;168(2):166–78. doi: 10.1016/j.jconrel.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lam J, Clark EC, Fong ELS, Lee EJ, Lu S, Tabata Y, et al. Evaluation of cell-laden polyelectrolyte hydrogels incorporating poly(l-Lysine) for applications in cartilage tissue engineering. Biomaterials. 2016 Mar 1;83:332–46. doi: 10.1016/j.biomaterials.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, van den Beucken JJJP, et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials. 2014 Oct 1;35(31):8829–39. doi: 10.1016/j.biomaterials.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rodrigues MT, Gomes ME, Reis RL. Current strategies for osteochondral regeneration: from stem cells to pre-clinical approaches. Curr Opin Biotechnol. 2011 Oct;22(5):726–33. doi: 10.1016/j.copbio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 144.Fedorovich NE, Schuurman W, Wijnberg HM, Prins H-J, van Weeren PR, Malda J, et al. Biofabrication of Osteochondral Tissue Equivalents by Printing Topologically Defined, Cell-Laden Hydrogel Scaffolds. Tissue Eng Part C Methods. 2011 Aug 20;18(1):33–44. doi: 10.1089/ten.tec.2011.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tarafder S, Koch A, Jun Y, Chou C, Awadallah MR, Lee CH. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 2016;8(2):025003. doi: 10.1088/1758-5090/8/2/025003. [DOI] [PubMed] [Google Scholar]
- 146.Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5(1):015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 147.Park CH, Rios HF, Jin Q, Bland ME, Flanagan CL, Hollister SJ, et al. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 2010;31(23):5945–52. doi: 10.1016/j.biomaterials.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rasperini G, Pilipchuk SP, Flanagan CL, Park CH, Pagni G, Hollister SJ, et al. 3D-printed bioresorbable scaffold for periodontal repair. J Dent Res. 2015;94(9_suppl):153S–157S. doi: 10.1177/0022034515588303. [DOI] [PubMed] [Google Scholar]
- 149.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23(7):821–3. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 150.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012 Sep;11(9):768–74. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv Mater. 2014 May 1;26(19):3124–30. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 152.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci. 2016;113(12):3179–84. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Olefirowicz TM, Ewing AG. Capillary electrophoresis in 2 and 5 mu. m diameter capillaries: application to cytoplasmic analysis. Anal Chem. 1990;62(17):1872–6. doi: 10.1021/ac00216a026. [DOI] [PubMed] [Google Scholar]
- 154.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30(31):6221–7. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 155.Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. TRENDS Biotechnol. 2003;21(4):157–61. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 156.Mironov V, Kasyanov V, Drake C, Markwald RR. Organ printing: promises and challenges. Regen Med. 2008;3(1):93–103. doi: 10.2217/17460751.3.1.93. [DOI] [PubMed] [Google Scholar]
- 157.Alajati A, Laib AM, Weber H, Boos AM, Bartol A, Ikenberg K, et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods. 2008;5(5):439–45. doi: 10.1038/nmeth.1198. [DOI] [PubMed] [Google Scholar]
- 158.Hajdu Z, Mironov V, Mehesz AN, Norris RA, Markwald RR, Visconti RP. Tissue spheroid fusion−based in vitro screening assays for analysis of tissue maturation. J Tissue Eng Regen Med. 2010;4(8):659–64. doi: 10.1002/term.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Laschke MW, Menger MD. Life is 3D: boosting spheroid function for tissue engineering. Trends Biotechnol. 2017;35(2):133–44. doi: 10.1016/j.tibtech.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 160.Lin H, Li Q, Lei Y. Three-dimensional tissues using human pluripotent stem cell spheroids as biofabrication building blocks. Biofabrication. 2017;9(2):025007. doi: 10.1088/1758-5090/aa663b. [DOI] [PubMed] [Google Scholar]
- 161.Mironov V, Khesuani YD, Bulanova EA, Koudan EV, Parfenov VA, Knyazeva AD, et al. Patterning of tissue spheroids biofabricated from human fibroblasts on the surface of electrospun polyurethane matrix using 3D bioprinter. Int J Bioprinting. 2016;2(1) [Google Scholar]
- 162.Gelber KM, Bhargava R. Monolithic multilayer microfluidics via sacrificial molding of 3D-printed isomalt. Lab Chip. 2015;15(7):1736–41. doi: 10.1039/c4lc01392a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012 Sep;11(9):768–74. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mellor LF, Huebner P, Cai S, Mohiti-Asli M, Taylor MA, Spang J, et al. Fabrication and Evaluation of Electrospun, 3D-Bioplotted, and Combination of Electrospun/3D-Bioplotted Scaffolds for Tissue Engineering Applications. BioMed Res Int. 2017 Apr 27;2017:e6956794. doi: 10.1155/2017/6956794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jakus A, Secor E, Rutz A, Jordan S, Hersam M, Shah RN. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano. 2015;(9):4636–4648. doi: 10.1021/acsnano.5b01179. [DOI] [PubMed] [Google Scholar]
- 166.Jakus AE, Rutz AL, Jordan SW, Kannan A, Mitchell SM, Yun C, et al. Hyperelastic “bone”: A highly versatile, growth factor–free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci Transl Med. 2016 Sep 28;8(358):358ra127–358ra127. doi: 10.1126/scitranslmed.aaf7704. [DOI] [PubMed] [Google Scholar]
- 167.Jakus AE, Shah RN. Multi and mixed 3D-printing of graphene-hydroxyapatite hybrid materials for complex tissue engineering. J Biomed Mater Res A. 2017 Jan 1;105(1):274–83. doi: 10.1002/jbm.a.35684. [DOI] [PubMed] [Google Scholar]