Abstract
This randomized clinical trial assesses whether treatment with the neurokinin 1 receptor antagonist aprepitant decreases pruritus vs placebo in patients with Sézary syndrome.
Pruritus may impair quality of life in patients with Sézary syndrome, the leukemic variant of cutaneous T-cell lymphomas. Management of pruritus is challenging and often unsatisfactory. Retrospective reports have found aprepitant, a neurokinin 1 receptor antagonist approved for the treatment of chemotherapy-induced or postoperative emesis, to show some efficacy in managing pruritus in patients with cutaneous T-cell lymphoma.1,2,3,4,5
We conducted a randomized, double-blind, placebo-controlled crossover study in patients with Sézary syndrome to test the hypothesis that treatment with the neurokinin 1 receptor antagonist aprepitant would decrease pruritus.
Methods
Patients seen in the Vanderbilt University Cutaneous Lymphoma clinic meeting the International Society for Cutaneous Lymphomas–European Organisation for Research and Treatment of Cancer criteria6 for Sézary syndrome were eligible. Patients with Sézary syndrome with uncontrolled pruritus and a baseline visual analog scale for pruritus of greater than 40 mm were eligible. Participants were also required to be on a stable medication regimen for Sézary syndrome and a stable antipruritic medication regimen for 3 months prior to the study. Written informed consent was obtained and the protocol was approved by the Vanderbilt Institutional Review Board and carried out according to the Declaration of Helsinki. The study protocol is available in the Supplement. Placebo or aprepitant (125 mg on day 1, followed by 80 mg on days 2-7) was ingested daily for 7 days followed by a 1-week washout period before taking the other treatment. The primary outcome measure was severity of pruritus measured using a visual analog scale of 0 to 100 mm (worst pruritus imaginable). The secondary outcome measure was quality of life using the Dermatology Life Quality Index instrument.
Mixed-effect models were used to analyze the data with a random subject effect and with treatment (aprepitant vs placebo) and time as fixed effects; baseline pruritus by visual analog scale was included as a covariate. Paired comparisons at specific time points or between time points were made using a Wilcoxon signed-rank test. Hypotheses were tested at the level of α = .05.
Results
Five patients were randomized to therapy and completed the study. The Table provides characteristics of the patients. All of the patients had been treated with more than 1 medication, and all were undergoing photopheresis.
Table. Characteristics of 5 Patients With Sézary Syndrome.
| Characteristic | Value |
|---|---|
| Age, mean (SD), y | 62.4 (12.8) |
| Sex, No. | |
| Male | 2 |
| Female | 3 |
| Race, European descent, No. | 5 |
| Time since diagnosis, mean (SD), mo | 31.4 (28.2) |
| Treatment, No. | |
| Photopheresis | 5 |
| Bexarotene | 3 |
| Antihistamine | 3 |
| Gabapentin | 4 |
| Histone deacetylase inhibitor | 2 |
| Triamcinolone cream | 3 |
| Benzodiazepine | 1 |
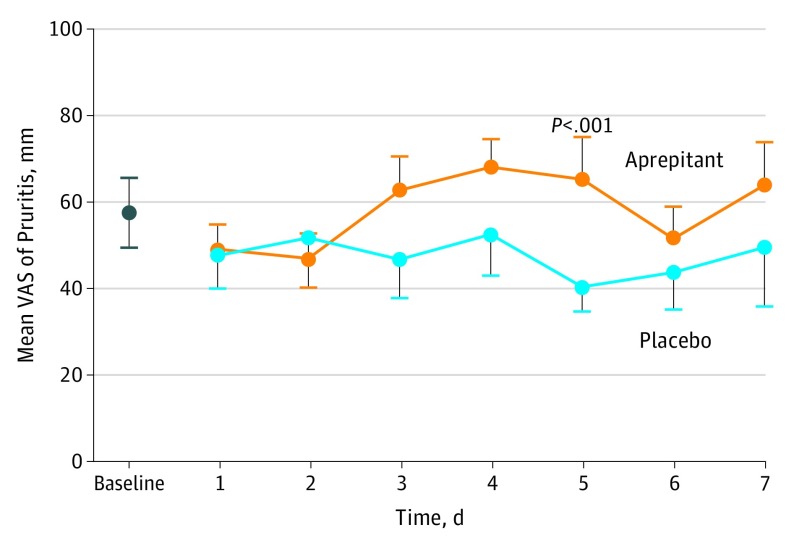
There were no differences in quality-of-life measures between the 2 interventions. Pruritus did not change over 7 days of treatment in the placebo arm but increased significantly during the aprepitant treatment (Figure). In multivariable analysis, baseline pruritus (every 10 unit increment, 7.20; 95% CI, 5.98-8.44; P < .001) and treatment (10.63; 95% CI, 3.49-17.77; P = .004) had a significant effect on pruritus over the 7-day treatment period.
Figure. Self-reported Visual Analog Scale (VAS) Assessment of Pruritus at Baseline Screening and on Each Day of Study Medication Use.
A score of 100 mm indicated the worst pruritus imaginable, and a score of 0 indicated no pruritus. Orange circles indicate the aprepitant study arm; blue circles indicate the placebo study arm. Error bars indicate standard error of the mean. There was a significant difference at day 5 of P < .001 vs baseline.
Discussion
To our knowledge, we report herein the results of the first randomized, double-blind, placebo-controlled crossover study of aprepitant for the treatment of pruritus in patients with Sézary syndrome. The data do not support the efficacy of aprepitant as an antipruritic agent in patients with Sézary syndrome. This is in contrast to at least 7 case series that have reported an improvement in symptoms in a total of 17 patients with Sézary syndrome or mycosis fungoides.1,2,3,4,5
This study has limitations. Although the study was randomized, blinded, and placebo controlled, the sample size was small including only 5 patients. Because pruritus can vary in patients with Sézary syndrome due to changes in disease activity and external factors such as ambient temperature and humidity, we cannot exclude the impact of these factors on the scoring of pruritus by visual analog scale. We dosed aprepitant daily for 1 week, and we cannot exclude the possibility that the results would have been different if we had used intermittent dosing.
In conclusion, in patients with Sézary syndrome, aprepitant treatment may not improve pruritus as reported in previous retrospective observational studies. The unexpected observation of worsened pruritus in patients receiving aprepitant vs placebo warrants larger prospective studies with a similar design to confirm our findings.
Study Protocol
References
- 1.Booken N, Heck M, Nicolay JP, Klemke CD, Goerdt S, Utikal J. Oral aprepitant in the therapy of refractory pruritus in erythrodermic cutaneous T-cell lymphoma. Br J Dermatol. 2011;164(3):665-667. [DOI] [PubMed] [Google Scholar]
- 2.Ladizinski B, Bazakas A, Olsen EA. Aprepitant: a novel neurokinin-1 receptor/substance P antagonist as antipruritic therapy in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2012;67(5):e198-e199. doi: 10.1016/j.jaad.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 3.Jiménez Gallo D, Albarrán Planelles C, Linares Barrios M, Fernández Anguita MJ, Márquez Enríquez J, Rodríguez Mateos ME. Treatment of pruritus in early-stage hypopigmented mycosis fungoides with aprepitant. Dermatol Ther. 2014;27(3):178-182. doi: 10.1111/dth.12113 [DOI] [PubMed] [Google Scholar]
- 4.Borja-Consigliere HA, López-Pestaña A, Vidal-Manceñido MJ, Tuneu-Valls A. Aprepitant in the treatment of refractory pruritus secondary to cutaneous T-cell lymphoma. Actas Dermosifiliogr. 2014;105(7):716-718. doi: 10.1016/j.ad.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 5.Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017;17(1):200. doi: 10.1186/s12885-017-3194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devata S, Wilcox RA. Cutaneous T-cell lymphoma: a review with a focus on targeted agents. Am J Clin Dermatol. 2016;17(3):225-237. doi: 10.1007/s40257-016-0177-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol