This systematic review and meta-analysis uses standardized criteria and prognostic factors to examine the clinical hair regrowth outcomes of contact immunotherapy with diphenylcyclopropenone or squaric acid dibutyl ester for patients with alopecia areata.
Key Points
Question
At what rate is contact immunotherapy associated with satisfactory hair regrowth in patients with alopecia areata?
Findings
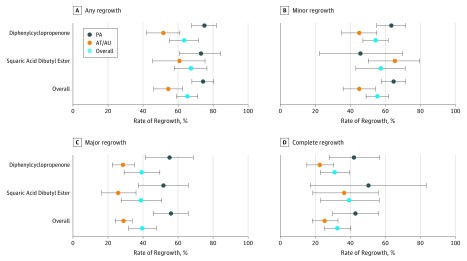
In this meta-analysis of 45 studies that included 2227 patients, any hair regrowth was observed in 74.6%, minor regrowth in 64.9%, major regrowth in 56.1%, and complete regrowth in 42.6% of patients with patchy alopecia. Any regrowth was observed in 54.5%, minor regrowth in 45.0%, major regrowth in 28.7%, and complete regrowth in 24.9% of patients with alopecia totalis/universalis.
Meaning
Hair regrowth outcomes of contact immunotherapy may be associated with various factors, and quantitative summarization may improve patient education and lead to better therapeutic adherence and hair regrowth.
Abstract
Importance
Contact immunotherapy with diphenylcyclopropenone or squaric acid dibutyl ester is a preferred treatment for severe alopecia areata; however, the defined criteria for therapeutic hair regrowth and regrowth rate have been highly heterogeneous across studies.
Objective
To summarize the clinical outcomes of contact immunotherapy for alopecia areata according to standardized criteria for therapeutic hair regrowth and several prognostic factors.
Data Source
A database search of MEDLINE, Embase, and Cochrane Library was performed for articles published before November 20, 2017, using the search terms areata, totalis, universalis, sensitizer, sensitization, immunotherapy, DPCP, diphenylcyclopropenone, diphencyprone, SADBE, and squaric.
Study Selection
Clinical trials or observational studies that investigated contact immunotherapy for alopecia areata and subgrouped the disease into patchy alopecia or alopecia totalis/universalis and reported their hair regrowth rates were included, whereas studies that investigated combination therapy or nonconventional protocol and case series or reviews were excluded.
Data Extraction and Synthesis
The following data were extracted from each of the studies included in this meta-analysis: study year and setting, sensitizer type, study population, study population composition by disease subtype, defined criteria for therapeutic hair regrowth, and regrowth rate of contact immunotherapy. The incidence of adverse effects and recurrence rate were also recorded. A random effects model was used for data synthesis because of the expected high heterogeneity of the included studies.
Main Outcomes and Measures
The main outcome was therapeutic hair regrowth rate according to the 4-grade criteria for therapeutic regrowth. Secondary outcomes included incidence of treatment-related adverse effects and recurrence rate.
Results
Forty-five studies comprising 2227 patients were analyzed. The overall rate of any hair regrowth was 65.5% among patients with alopecia areata (74.6% in the patchy alopecia and 54.5% in the alopecia totalis/universalis subgroups). However, the complete regrowth rate was 32.3% (24.9% in the patchy alopecia and 32.3% in the alopecia totalis/universalis subgroups). Disease extent of 50% or greater (odds ratio [OR], 3.05; 95% CI, 2.26-4.12), atopic history (OR, 1.61; 95% CI, 1.03-2.50), and nail involvement (OR, 2.06; 95% CI. 1.26-3.36) were associated with poorer therapeutic outcome. Recurrence rates were 38.3% among patients receiving maintenance treatment and 49.0% among those not receiving maintenance treatment.
Conclusions and Relevance
Various factors were associated with the clinical outcomes of contact immunotherapy for alopecia areata, with significant differences in hair regrowth rates according to the level of expected therapeutic regrowth. Quantitative summarization may improve patient education and lead to better therapeutic adherence and outcomes.
Introduction
Alopecia areata (AA) is a chronic and relapsing hair follicle–specific autoimmune disease that leads to nonscarring hair loss.1 The deterioration in the quality of life caused by the disease and the temporal and financial burden of treatment are the major issues among patients with AA.2,3 The preferred treatments for AA include therapeutic agents, such as topical or intralesional corticosteroid, and contact immunotherapy.4,5 Other treatments, including systemic corticosteroids, anthralin, excimer laser, and novel therapeutics, such as Janus kinase inhibitors, have also been used. Despite the numerous treatment options, the overall prognosis of AA is not favorable, involving relapse and recalcitrant progression. Specifically, patients with severe subtypes, such as alopecia totalis (AT) and alopecia universalis (AU), which involve complete scalp hair loss, have a lower likelihood of hair regrowth than those with patchy alopecia (PA).1
Diphenylcyclopropenone and squaric acid dibutyl ester have been commonly used as contact allergens for treatment, whereas dinitrochlorobenzene is no longer used because of its mutagenicity.6 As potent contact allergens, these agents exert their therapeutic effects by inducing allergic contact dermatitis on the scalp.4 As a result, adverse effects, such as erythema, pruritus, eczema, and lymphadenopathy, frequently accompany the treatment, and careful monitoring of patients is needed throughout treatment. Despite the frequent and sometimes serious adverse effects of contact immunotherapy, it remains one of the most commonly used and effective treatments for severe and chronic AA.
Numerous studies3,4,5 have examined the effects of contact immunotherapy with diphenylcyclopropenone or squaric acid dibutyl ester in patients with AA; however, most were not placebo-controlled or randomized. According to an evidence-based review,3 although contact immunotherapy is considered to be effective in half of patients with AA, the hair regrowth rate was diverse and heterogeneous across the studies. This variability may have resulted from the different compositions of the study population and the nonstandardized criteria for therapeutic regrowth used in each study. As a result, the heterogeneity in the reported treatment-related outcomes causes confusion among practitioners and patients and makes the disease further unpredictable. This study primarily aimed to quantitatively summarize the regrowth rates of contact immunotherapy with diphenylcyclopropenone or squaric acid dibutyl ester among patients with AA, according to the standardized criteria for therapeutic regrowth and several prognostic factors.
Methods
Search Strategy
We conducted a comprehensive literature search to identify articles written in English or Korean because of our language proficiency. The studies were identified by searching the MEDLINE, Embase, and Cochrane Library databases for articles published before November 20, 2017, using the following search terms: areata, totalis, universalis, sensitizer, sensitization, immunotherapy, DPCP, diphenylcyclopropenone, diphencyprone, SADBE, and squaric. The detailed search strategy used for each database is described in the eAppendix in the Supplement.
Study Selection
Two main reviewers (S.L. and B.J.K.) independently evaluated the titles and abstracts of the retrieved studies, and a final decision was made through consensus discussion with the other 2 reviewers (Y.B.L. and W.-S.L.). Clinical trials or observational studies that investigated contact immunotherapy with diphenylcyclopropenone or squaric acid dibutyl ester for AA and subgrouped the disease according to extent (PA or AT/AU) and reported their regrowth rates were included. Studies that investigated combination therapy (eg, diphenylcyclopropenone plus anthralin) or nonconventional protocol (eg, minimal or no sensitization) and case series or review articles were excluded. If the abstract did not provide sufficient information for inclusion or exclusion to this study, a full-text evaluation was performed to determine eligibility. Forty-five studies were identified. The PRISMA flow diagram is presented in the eFigure in the Supplement.
Quality Assessment
Quality assessment was based on whether the study population or criteria for successful therapeutic hair regrowth were clearly defined and whether the regrowth rate was assessed according to the following prognostic factors: disease subtype (PA or AT/AU), extent of hair loss (Severity of Alopecia Tool [SALT]7 score <50 or ≥50, where higher scores indicate greater hair loss), acute or chronic disease (duration of <1 year or ≥1 year), atopic disease (personal history of atopic dermatitis, allergic rhinitis, or asthma), and nail involvement. Clarity on the reporting of incidence of adverse effects or recurrence rate was also assessed.
Data Extraction
The data extracted from each study were study year, study setting, sensitizer type (diphenylcyclopropenone or squaric acid dibutyl ester), study population, sex ratio, mean age, proportion of AT and AU in the total study population, defined criteria for therapeutic hair regrowth, and regrowth rate of contact immunotherapy. The main outcome for ascertainment was the therapeutic regrowth rate according to disease subtype (PA or AT/AU). Moreover, the recurrence rate and treatment status at the time of relapse, as well as the incidence of adverse effects, were recorded. Only adverse effects reported by at least 2 studies were included, and mild to moderate eczema, erythema, and pruritus were excluded because these are usually expected to appear in most patients.
Data Synthesis and Outcomes
The overall therapeutic hair regrowth rate was synthesized in the meta-analysis. Because each study used heterogeneous criteria for successful therapeutic regrowth, which was quantitatively or qualitatively measured, it was classified using 4-grade criteria (eTable 1 in the Supplement). This hierarchy was based on the review of included studies in which both types of criteria were simultaneously used. If therapeutic regrowth could not be definitively classified according to this scale, it was assigned to the inferior category (eg, partial or cosmetically acceptable regrowth was categorized as minor regrowth, whereas 70%-100% regrowth was considered to be major regrowth). Subgroup analyses were performed by stratifying the data according to sensitizer type (diphenylcyclopropenone or squaric acid dibutyl ester) and the 4 categories of therapeutic regrowth. For applicable studies, the meta-analyzed odds ratios of poorer therapeutic outcomes in patients with several prognostic factors were calculated. A random effects model was used for data synthesis because the study population, study setting, and treatment duration in the included studies were expected to be highly heterogeneous. The heterogeneity of the included studies was calculated using the I2 statistic for inconsistency. Publication bias was evaluated using the Egger regression test. Because the quantitative and qualitative criteria were merged in the meta-analysis, a sensitivity analysis was conducted excluding studies with qualitative criteria for therapeutic regrowth. Statistical analysis was performed using the Comprehensive Meta-analysis software, version 3.3.0 (Biostat).
Results
Study Characteristics and Quality Assessment
Forty-five studies that included 2227 patients were analyzed. The characteristics and quality assessment of these studies are summarized in eTable 2 in the Supplement. None of the studies were randomized or placebo controlled. Overall, 31 studies investigated diphenylcyclopropenone and 15 investigated squaric acid dibutyl ester; only 1 study assessed both diphenylcyclopropenone and squaric acid dibutyl ester. All studies except for 1 pediatric study were not age restricted; 20 studies reported qualitative criteria and 29 reported quantitative criteria for therapeutic regrowth. With these various criteria, the rates of any regrowth were calculated from 16 studies, minor regrowth from 22 studies, major regrowth from 19 studies, and complete regrowth from 18 studies. Twenty-nine studies reported the incidence of treatment-related adverse effects, and 23 studies reported the recurrence rate. A total of 1638 diphenylcyclopropenone-treated and 589 squaric acid dibutyl ester–treated patients were included in the meta-analysis.
Overall Therapeutic Hair Regrowth Rate of Contact Immunotherapy
The results of the meta-analyzed therapeutic regrowth rate are summarized in Figure 1 and Table 1. The overall rate of any regrowth was 65.5% among patients with AA (74.6% in the PA subgroup and 54.5% in the AT/AU subgroup); however, complete regrowth was observed in only 32.3% of patients with AA (42.6% in the PA subgroup and 24.9% in the AT/AU subgroup). As expected, the heterogeneity of the studies was estimated to be high (I2 = 68.94%). However, it was partially resolved in the serial subgroup analyses. In the Egger regression test, the risk of publication bias was estimated to be not statistically significant (P = .20). In the sensitivity analysis in which only quantitative studies were analyzed, most estimates showed minimal difference (<5%) compared with those in the original analysis (eTable 3 in the Supplement).
Figure 1. Therapeutic Hair Regrowth Rate of Contact Immunotherapy in Patients With Alopecia Areata.
Any regrowth was less than 10% of hair loss; minor regrowth, 10% to 59%; major regrowth, 60% to 89%; and complete regrowth, 90% to 100%. Error bars indicate 95% CIs. AT indicates alopecia totalis; AU, alopecia universalis; and PA, patchy alopecia.
Table 1. Therapeutic Hair Regrowth Outcomes of Contact Immunotherapy in Patients With Alopecia Areataa.
| Sensitizer and AA Subtype | Any Regrowth | Minor Regrowth | Major Regrowth | Complete Regrowth | ||||
|---|---|---|---|---|---|---|---|---|
| Response Rate, % (95% CI) | No. of Studies (No. of Patients) | Response Rate, % (95% CI) | No. of Studies (No. of Patients) | Response Rate, % (95% CI) | No. of Studies (No. of Patients) | Response Rate, % (95% CI) | No. of Studies (No. of Patients) | |
| Diphenylcyclopropenone | ||||||||
| PA | 75.5 (67.6-82.0) | 8 (248) | 63.7 (55.1-71.6) | 15 (315) | 55.4 (41.4-68.7) | 12 (322) | 41.6 (27.9-56.8) | 13 (311) |
| AT/AU | 51.6 (42.0-61.1) | 9 (193) | 45.0 (35.1-55.2) | 14 (279) | 28.3 (22.4-35.0) | 12 (326) | 22.0 (15.2-30.6) | 13 (366) |
| Overall | 63.8 (55.0-71.7) | 9 (441) | 54.5 (47.2-61.6) | 16 (594) | 38.9 (29.2-49.6) | 13 (648) | 30.7 (22.9-39.6) | 13 (677) |
| Squaric acid dibutyl ester | ||||||||
| PA | 74.2 (60.8-84.3) | 7 (211) | 66.5 (50.2-79.6) | 6 (106) | 51.8 (37.4-65.8) | 6 (120) | 50.5 (17.2-83.4) | 4 (60) |
| AT/AU | 61.5 (45.5-75.4) | 7 (92) | 45.0 (22.4-69.9) | 6 (78) | 25.0 (16.5-36.0) | 6 (79) | 35.0 (18.6-55.9) | 5 (77) |
| Overall | 67.8 (57.8-76.5) | 7 (303) | 57.9 (43.2-71.3) | 7 (184) | 38.4 (27.5-50.6) | 7 (199) | 38.4 (23.1-56.5) | 5 (137) |
| Overall | ||||||||
| PA | 74.6 (67.9-80.3) | 15 (459) | 64.9 (57.7-71.6) | 20 (409) | 56.1 (45.9-65.8) | 18 (430) | 42.6 (29.9-56.3) | 17 (371) |
| AT/AU | 54.5 (46.2-62.6) | 16 (285) | 45.0 (35.9-54.4) | 20 (357) | 28.7 (24.1-33.8) | 18 (405) | 24.9 (18.2-33.0) | 18 (443) |
| Overall | 65.5 (59.1-71.3) | 16 (744) | 55.5 (49.0-61.9) | 22 (766) | 39.4 (31.8-47.6) | 19 (835) | 32.3 (25.3-40.2) | 18 (814) |
Abbreviations: AA, alopecia areata; AT, alopecia totalis; AU, alopecia universalis; PA, patchy alopecia.
Any regrowth was less than 10% of hair loss; minor regrowth, 10% to 59%; major regrowth, 60% to 89%; and complete regrowth, 90% to 100%.
Prognostic Factors Associated With Therapeutic Hair Regrowth
The differences in the therapeutic outcomes stratified according to various prognostic factors were analyzed (Table 2). Patients with SALT scores of 50 or greater, atopic disease (personal history of atopic dermatitis, allergic rhinitis, or asthma), and nail involvement were more likely to have poorer therapeutic outcomes after contact immunotherapy than were patients without these characteristics. In addition, disease duration of 1 year or more was also associated with poorer therapeutic outcomes, although without statistical significance.
Table 2. Prognostic Factors Associated With Poorer Therapeutic Hair Regrowth Outcomes.
| Prognostic Factor | No. of Studies | No. of Patients | No. of Controls | Odds Ratio (95% CI) |
|---|---|---|---|---|
| SALT score ≥50 | 35 | 1134 | 810 | 3.05 (2.26-4.11) |
| Disease duration ≥1 y | 7 | 299 | 143 | 1.56 (0.95-2.55) |
| Atopic diseasea | 14 | 191 | 415 | 1.61 (1.03-2.50) |
| Nail involvement | 10 | 251 | 353 | 2.06 (1.26-3.36) |
Abbreviation: SALT, Severity of Alopecia Tool (where higher scores indicate greater hair loss).
Personal history of atopic dermatitis, allergic rhinitis, or asthma.
Adverse Effects
The incidence of adverse effects reported in at least 2 studies is given in eTable 4 in the Supplement. Except for mild to moderate eczema, erythema, and pruritus, the most frequent adverse effect was severe eczema (30.8%) (eg, blistering, scaling, or exudation) followed by lymphadenopathy (25.7%), although its incidence was heterogeneous across the studies. Generalized eczema (15.8%), hyperpigmentation (12.7%), and influenza-like symptoms (11.1%) were also reported as common adverse effects of contact immunotherapy.
Disease Relapse
In 23 studies, the recurrence rate after hair regrowth was reported to be approximately 48.5% (95% CI, 39.7%-57.3%; n = 637) among patients with AA. Of these studies, 17 reported the treatment status at the time of relapse (with or without maintenance treatment). The recurrence rate was 38.3% (95% CI, 18.4%-58.2%; n = 98) among patients receiving maintenance treatment and 49.0% (95% CI, 35.5%-62.6%; n = 310) among those not receiving maintenance treatment. However, most studies did not reveal the detailed protocol used for maintenance treatment or the mean time to recurrence.
Discussion
Diphenylcyclopropenone or squaric acid dibutyl ester are topical sensitizers that cause contact dermatitis through hapten binding.4 Their exact mechanism for hair regrowth in AA is not clearly elucidated, although many therapeutic mechanisms have been suggested, including immunomodulatory effect,8 changes in the CD4+/CD8+ lymphocyte ratio,9 lesional T-lymphocyte inhibition induced by TH2-related cytokine, lymphocyte apoptosis,10 and antigenic competition.11
The therapeutic hair regrowth benefits of contact immunotherapy have been reported to be variable in the literature. In the 45 studies included in this analysis, the regrowth rate ranged from 4% to 81%, according to the composition of the study population and the criteria for therapeutic regrowth used.12,13 In this study, we analyzed the overall regrowth rate across the studies, applying the 4-grade and standardized criteria for successful therapeutic regrowth. In addition, we investigated the difference in therapeutic outcomes according to disease subtype and several prognostic factors using subgroup analysis.
Unlike other treatments, contact immunotherapy is necessarily accompanied by the process of sensitization and concentration control throughout the treatment. Although this meta-analysis included only studies that investigated conventional therapeutic protocol based on the predefined inclusion and exclusion criteria, few studies were excluded during the literature search owing to their use of nonconventional or atypical protocols. A few studies14,15 on modified protocols for the reduction of eczematous reactions during sensitization and treatment recently reported their noninferior association with hair regrowth and lower incidence of adverse effects compared with conventional protocols. Nevertheless, additional comparative studies will be required to evaluate efficacy. Overall, the conventional protocol for contact immunotherapy with 2% diphenylcyclopropenone or 2% to 3% squaric acid dibutyl ester for sensitization is still the preferred method for treatment of patients with AA.
Our results indicate that the mean therapeutic hair regrowth rate of contact immunotherapy, which ranged from 24.9% to 74.6%, was associated with disease subtype and level of expected therapeutic regrowth. Moreover, several prognostic factors, including SALT score of 50 or greater, disease duration of 1 year or more, atopic disease, and nail involvement, were associated with poor therapeutic outcomes after contact immunotherapy. Successful hair regrowth was expected to occur in only approximately one-half to one-third of patients with these negative prognostic factors. Therefore, a tailored approach considering patient factors and expectation for regrowth is needed to improve therapeutic adherence and outcome satisfaction. In the subgroup analysis for each sensitizer, the mean regrowth rate was similar between squaric acid dibutyl ester–treated and diphenylcyclopropenone-treated patients or slightly higher among the squaric acid dibutyl ester–treated patients than among the diphenylcyclopropenone-treated patients. However, direct comparisons between both were not performed in most studies, and the number and sample size in studies of squaric acid dibutyl ester were limited compared with those in studies of diphenylcyclopropenone. Although we could not determine whether one sensitizer is superior to the other in this study, it is likely that the use of diphenylcyclopropenone is preferred according to recent published studies.3,4
Except for mild to moderate eczematous reactions, the most common adverse effect was severe eczema, including scaling, exudation, and blistering. Furthermore, generalized eczema in nontreated and treated sites was common. This result suggests that at least approximately one-third of patients will require supportive care with systemic and/or topical corticosteroids and antihistamines during the treatment. Systemic symptoms, including lymphadenopathy and influenza-like symptoms, were also relatively common. Pigmentary changes were frequently reported in approximately one-fifth of patients. Other unexpected events, such as anaphylactoid reaction16 and hair curling,17 were also noted. Compared with other therapeutic agents used for AA, contact immunotherapy had various and frequent treatment-related adverse effects, occasionally with fatal or irreversible consequences. Thus, sufficient education and supportive care for various adverse effects should be provided to patients. Moreover, many physicians experience allergic contact dermatitis during the procedure, although the exact incidence cannot be assessed owing to underreporting. Self-sensitization in physicians should never be ignored because they will be repeatedly exposed to diphenylcyclopropenone or squaric acid dibutyl ester for years. To minimize this, physicians should have appropriate protective equipment throughout the treatment process.
Approximately half of patients experienced disease relapse after hair regrowth. The recurrence rate was lower with maintenance treatment; however, most studies did not directly compare the recurrence rate between patients receiving maintenance treatment and those not receiving maintenance treatment. Moreover, they did not reveal or discuss the detailed treatment interval or protocol used for maintenance treatment. Nevertheless, patients not receiving maintenance treatment were reported to have a higher risk of early disease relapse (hazard ratio, 4.87) in a recent study.18 Therefore, even after the desired regrowth has been achieved, maintenance treatment may help reduce disease relapse. Although to our knowledge there has never been consensus on the optimal interval for maintenance treatment, at least once every 4 weeks or less than 2 weeks if possible can be recommended.18,19 Home-based contact immunotherapy is as effective and safe as clinic-based treatment and can be helpful in reducing the temporal and financial burden on patients who require long-term maintenance treatment.20
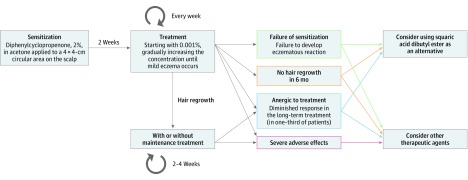
On the basis of the literature reviewed in this study, we suggest a schematic treatment protocol for contact immunotherapy in patients with AA (Figure 2). Although diphenylcyclopropenone or squaric acid dibutyl ester are not approved by the US Food and Drug Administration and the European Medicines Agency for their use in AA, both are still considered to be the main therapeutic agents for severe diseases in many countries. Moreover, in recent years, several attempts have been made to expand the application of contact immunotherapy among pediatric patients. In these studies,21,22,23,24 the efficacy and safety of contact immunotherapy among pediatric patients with AA were similar to those among adult patients. Despite various safety and regulatory issues, the need for contact immunotherapy is increasing, and it is still performed for both adult and pediatric patients in many health care facilities.25 In addition, biologic agents, including Janus kinase inhibitors, which have recently emerged as promising agents, have not always been effective according to recent reports.26,27,28 Institutional improvement, including manufacturing, handling, and application of diphenylcyclopropenone or squaric acid dibutyl ester, should be made so that patients with AA can be legally and safely treated using contact immunotherapy, ideally with medical insurance coverage.
Figure 2. Suggested Schematic Treatment Protocol for Contact Immunotherapy for Alopecia Areata.
Treatment begins with application of 2% diphenylcyclopropenone to a 4 × 4-cm circular area on the scalp. After 2 weeks of sensitization, 0.001% diphenylcyclopropenone is applied to the scalp on a weekly basis, and the concentration of diphenylcyclopropenone is gradually increased until a mild eczematous reaction occurs. If a satisfactory hair regrowth is achieved, maintenance treatment may be used to reduce the recurrence rate. Contact immunotherapy with squaric acid dibutyl ester or other therapeutic agents can be considered when treatment failure or severe adverse effects occur.
Limitations
A major limitation of this study is that most studies included in the analysis reported low-quality evidence. Most reports described noncomparative studies that only allowed the calculation of the estimated mean therapeutic regrowth rate. Thus, we could not calculate comparative estimates, such as odds ratios or relative risks, to evaluate the efficacy of contact immunotherapy on hair regrowth compared with that of placebo or other therapeutic agents. In addition, special considerations for some specific subtypes (eg, alopecia ophiasis, acute diffuse and total alopecia) were not given because of limited studies of these subtypes in the literature. Nonetheless, this meta-analysis quantitatively summarized the therapeutic regrowth rate of contact immunotherapy according to the standardized criteria and several prognostic factors. This study will enable practitioners to provide patients with detailed information about their treatment-related prognosis and expected hair regrowth rates.
Conclusions
The therapeutic hair regrowth outcomes of contact immunotherapy with diphenylcyclopropenone or squaric acid dibutyl ester for AA were associated with various factors in the evaluated studies, and there was a significant variability in the criteria used for each therapeutic end point. Patients with AA should be individually provided with accurate information based on personal prognostic factors and level of expected therapeutic outcomes. Nevertheless, to our knowledge, no treatment has been able to modify the long-term prognosis of AA. Therefore, an accurate understanding of the disease and treatment-related prognosis is needed, and education should be provided to improve the patient’s therapeutic adherence and outcomes.
eText 1. Search strategy
eFigure 1. Literature search
eTable 1. Definition of therapeutic regrowth
eTable 2. Study characteristics and quality assessment
eTable 3. Sensitivity analysis
eTable 4. Treatment-related adverse effects
eReferences
References
- 1.Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1-12. doi: 10.1016/j.jaad.2017.04.1141 [DOI] [PubMed] [Google Scholar]
- 2.Liu LY, Craiglow BG, King BA. Successful treatment of moderate-to-severe alopecia areata improves health-related quality of life. J Am Acad Dermatol. 2018;78(3):597-599.e2. doi: 10.1016/j.jaad.2017.10.046 [DOI] [PubMed] [Google Scholar]
- 3.Kuin RA, Spuls PI, Limpens J, van Zuuren EJ. Diphenylcyclopropenone in patients with alopecia areata: a critically appraised topic. Br J Dermatol. 2015;173(4):896-909. doi: 10.1111/bjd.14040 [DOI] [PubMed] [Google Scholar]
- 4.Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78(1):15-24. doi: 10.1016/j.jaad.2017.04.1142 [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Lee WS. Management of alopecia areata: updates and algorithmic approach. J Dermatol. 2017;44(11):1199-1211. doi: 10.1111/1346-8138.13933 [DOI] [PubMed] [Google Scholar]
- 6.Wilkerson MG, Connor TH, Wilkin JK. Dinitrochlorobenzene is inherently mutagenic in the presence of trace mutagenic contaminants. Arch Dermatol. 1988;124(3):396-398. doi: 10.1001/archderm.1988.01670030062023 [DOI] [PubMed] [Google Scholar]
- 7.Olsen EA, Hordinsky MK, Price VH, et al. ; National Alopecia Areata Foundation . Alopecia areata investigational assessment guidelines, part II. J Am Acad Dermatol. 2004;51(3):440-447. doi: 10.1016/j.jaad.2003.09.032 [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann R, Wenzel E, Huth A, et al. Cytokine mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol. 1994;103(4):530-533. doi: 10.1111/1523-1747.ep12395722 [DOI] [PubMed] [Google Scholar]
- 9.Wasyłyszyn T, Kozłowski W, Zabielski SL. Changes in distribution pattern of CD8 lymphocytes in the scalp in alopecia areata during treatment with diphencyprone. Arch Dermatol Res. 2007;299(5-6):231-237. doi: 10.1007/s00403-007-0759-4 [DOI] [PubMed] [Google Scholar]
- 10.Herbst V, Zöller M, Kissling S, Wenzel E, Stutz N, Freyschmidt-Paul P. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16(5):537-542. [PubMed] [Google Scholar]
- 11.Happle R. Antigenic competition as a therapeutic concept for alopecia areata. Arch Dermatol Res. 1980;267(1):109-114. doi: 10.1007/BF00416931 [DOI] [PubMed] [Google Scholar]
- 12.Orecchia G, Rabbiosi G. Treatment of alopecia areata with diphencyprone. Dermatologica. 1985;171(3):193-196. doi: 10.1159/000249418 [DOI] [PubMed] [Google Scholar]
- 13.Iijima S, Otsuka F. Prognostic factors for clinical response of alopecia areata to topical immunotherapy with squaric acid dibutylester. Arch Dermatol. 1997;133(4):539-540. doi: 10.1001/archderm.133.4.539 [DOI] [PubMed] [Google Scholar]
- 14.Choe SJ, Lee S, Pi LQ, et al. Subclinical sensitization with diphenylcyclopropenone is sufficient for the treatment of alopecia areata: retrospective analysis of 159 cases. J Am Acad Dermatol. 2018;78(3):515-521.e4. doi: 10.1016/j.jaad.2017.10.042 [DOI] [PubMed] [Google Scholar]
- 15.Vedak P, Kroshinsky D. Squaric acid sensitization is not required for response in the treatment of alopecia areata. J Am Acad Dermatol. 2015;73(3):471-476. doi: 10.1016/j.jaad.2015.04.064 [DOI] [PubMed] [Google Scholar]
- 16.Hatzis J, Georgiotouo K, Kostakis P, et al. Treatment of alopecia areata with diphencyprone. Australas J Dermatol. 1988;29(1):33-36. doi: 10.1111/j.1440-0960.1988.tb01223.x [DOI] [PubMed] [Google Scholar]
- 17.Choe SJ, Kim BJ, Choi J, Lee WS. Acquired hair curling after diphenylcyclopropenone immunotherapy in alopecia areata patient. J Eur Acad Dermatol Venereol. 2017;31(8):e371-e372. doi: 10.1111/jdv.14169 [DOI] [PubMed] [Google Scholar]
- 18.Choe SJ, Lee S, Lee H, Choi J, Lee WS. Efficacy of topical diphenylcyclopropenone maintenance treatment for patients with alopecia areata: a retrospective study. J Am Acad Dermatol. 2018;78(1):205-207.e1. doi: 10.1016/j.jaad.2017.07.028 [DOI] [PubMed] [Google Scholar]
- 19.El-Zawahry BM, Bassiouny DA, Khella A, Zaki NS. Five-year experience in the treatment of alopecia areata with DPC. J Eur Acad Dermatol Venereol. 2010;24(3):264-269. doi: 10.1111/j.1468-3083.2009.03401.x [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Lee WS. Home-based contact immunotherapy with diphenylcyclopropenone for alopecia areata is as effective and safe as clinic-based treatment in patients with stable disease: a retrospective study of 40 patients. J Am Acad Dermatol. 2018;78(3):599-601.e1. doi: 10.1016/j.jaad.2017.09.037 [DOI] [PubMed] [Google Scholar]
- 21.Hull SM, Pepall L, Cunliffe WJ. Alopecia areata in children: response to treatment with diphencyprone. Br J Dermatol. 1991;125(2):164-168. doi: 10.1111/j.1365-2133.1991.tb06064.x [DOI] [PubMed] [Google Scholar]
- 22.Orecchia G, Malagoli P. Topical immunotherapy in children with alopecia areata. J Invest Dermatol. 1995;104(5)(suppl):35S-36S. doi: 10.1038/jid.1995.53 [DOI] [PubMed] [Google Scholar]
- 23.Salsberg JM, Donovan J. The safety and efficacy of diphencyprone for the treatment of alopecia areata in children. Arch Dermatol. 2012;148(9):1084-1085. doi: 10.1001/archdermatol.2012.1622 [DOI] [PubMed] [Google Scholar]
- 24.Schuttelaar ML, Hamstra JJ, Plinck EP, et al. Alopecia areata in children: treatment with diphencyprone. Br J Dermatol. 1996;135(4):581-585. doi: 10.1111/j.1365-2133.1996.tb03835.x [DOI] [PubMed] [Google Scholar]
- 25.Karanovic S, Harries M, Kaur MR. Diphencyclopropenone (DPCP) for alopecia areata: a U.K. survey [published online February 26, 2018]. Br J Dermatol. doi: 10.1111/bjd.16489 [DOI] [PubMed] [Google Scholar]
- 26.Salman A, Sarac G, Ergun T. Alopecia universalis unresponsive to treatment with tofacitinib: report of a case with a brief review of the literature. Dermatol Online J. 2017;23(7):13030/qt224878kb. [PubMed] [Google Scholar]
- 27.Deeb M, Beach RA. A case of topical ruxolitinib treatment failure in alopecia areata. J Cutan Med Surg. 2017;21(6):562-563. doi: 10.1177/1203475417716363 [DOI] [PubMed] [Google Scholar]
- 28.Anzengruber F, Maul JT, Kamarachev J, Trüeb RM, French LE, Navarini AA. Transient efficacy of tofacitinib in alopecia areata universalis. Case Rep Dermatol. 2016;8(1):102-106. doi: 10.1159/000445182 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eText 1. Search strategy
eFigure 1. Literature search
eTable 1. Definition of therapeutic regrowth
eTable 2. Study characteristics and quality assessment
eTable 3. Sensitivity analysis
eTable 4. Treatment-related adverse effects
eReferences