This preclinical in vitro study identifies novel small molecules that induce extrinsic apoptosis and serve as an alternative treatment for cutaneous T-cell lymphoma.
Key Points
Question
Are there cost-effective alternatives to therapies currently available for early-stage cutaneous T-cell lymphoma?
Findings
Using in vitro and ex vivo methods, this study performed high-throughput screening of 1710 compounds and identified gentian violet as an anti–cutaneous T-cell lymphoma agent that induces high levels of tumor cell death and blocks tumor cell growth.
Meaning
These preclinical data suggest that gentian violet (a well-known topical antimicrobial agent) has potential as a novel topical therapy for cutaneous T-cell lymphoma that is inexpensive and available worldwide.
Abstract
Importance
Triggering the extrinsic apoptotic pathway is an effective way to kill cutaneous T-cell lymphoma (CTCL) cells in vitro and ex vivo.
Objective
To compare small molecules that induce extrinsic apoptosis in CTCL to identify and analyze compounds that induce high levels of tumor cell death and block tumor cell growth.
Design, Setting, and Participants
From November 5, 2014, to January 30, 2018, this study performed high-throughput small molecule screening of 1710 compounds followed by detailed analysis of the ability of gentian violet (GV) to promote apoptosis and inhibit proliferation of CTCL cells.
Exposures
In vitro and ex vivo analyses using enzyme-linked immunosorbent assays, flow cytometry, and immunoblotting.
Main Outcomes and Measures
Apoptosis, cleaved caspases, extrinsic apoptotic death receptors and ligands, cell proliferation, nuclear factor–κB expression, and other factors.
Results
This study used high-throughput screening to detect cleaved caspase 8 induced in CTCL cells by 1710 unique compounds. The nonprescription, topical antimicrobial remedy GV induced more total apoptosis than did nitrogen mustard (mechlorethamine). Furthermore, GV induced 4 to 6 times greater apoptosis in CTCL lines than in normal keratinocytes, suggesting a favorable topical toxicity profile. In addition to increasing caspase 8, GV also upregulated death receptors 4 and 5, tumor necrosis factor (TNF)–related apoptosis-inducing ligand, and Fas ligand but not the Fas receptor, TNF receptor, or TNF-α ligand. These results are consistent with induction of extrinsic apoptosis via the Fas and TNF-related apoptosis-inducing ligand pathways. Increased phosphorylation of phospholipase C–γ1, Ca2+ influx, and reactive oxygen species were also detected, indicating that the mechanism of Fas ligand upregulation involves key elements of the activation-induced cell death pathway. In ex vivo studies, 1-μmol/L GV induced up to 90% CTCL apoptosis in Sézary blood cells. In addition, GV reduced expression of antiapoptotic myeloid cell leukemia 1 and proproliferative nuclear factor–κB components and increased inhibitory κB levels. This finding was associated with cell cycle arrest and reduced CTCL tumor cell proliferation. Furthermore, the CTCL killing associated with GV was augmented when used in combination with methotrexate.
Conclusions and Relevance
This study found that GV attacked tumor viability and growth in CTCL. Although purple at neutral pH, GV can be rendered colorless by altering its pH. These preclinical findings may help to broaden knowledge of the antineoplastic features of GV and provide a rationale for clinical studies of its use as a novel, inexpensive, topical therapy for CTCL that is available worldwide.
Introduction
Mycosis fungoides and Sézary syndrome (SS) are 2 major forms of cutaneous T-cell lymphoma (CTCL). In this study, we use the term CTCL to refer specifically to mycosis fungoides and SS. On the basis of a variety of phenotypic, genetic, and functional investigations, there is ample evidence that CTCL is characterized by resistance to apoptosis.1,2,3,4,5,6,7,8,9,10 Often, CTCL cells express only low levels of extrinsic apoptotic pathway death receptors (DRs), such as Fas (CD95), and are less responsive to apoptotic triggers than are normal T cells. Activation-induced cell death (AICD) is a key pathway for apoptosis among CD4+/CD45RO+ memory T cells, the subset from which both mycosis fungoides (effector memory differentiation) and SS (central memory differentiation) are derived. Triggered through the T-cell receptor (TCR), AICD causes apoptosis through extrinsic (DR-mediated) pathways. It has been known for more than a decade that TCR signaling is defective in CTCL; however, the underlying mechanism was not apparent.1,5,11,12,13 A previous study14 found that the c-CBL E3 ubiquitin ligase is highly expressed in many cases of CTCL and that c-CBL knockdown using small interfering RNA technology restores the TCR signaling cascade that leads to up-regulation of extrinsic pathway apoptosis in CTCL cells. We also found that some extrinsic apoptotic pathway factors, such as the Fas (CD95) DR, can be upregulated epigenetically by inhibition of promoter methylation using methotrexate or other folate analogues.15 In aggregate, our findings provide proof of principle that by enhancing extrinsic pathway apoptosis CTCL cells can be killed effectively.
Although c-CBL knockdown using small interfering RNA technology is an effective method for increasing CTCL apoptosis in vitro, it is not suitable for use in patients. Unfortunately, there are few other agents known to increase extrinsic apoptosis. Therefore, we performed a small molecule screen of more than 1700 compounds to search for those capable of inducing cleaved caspase 8 (a major indicator of extrinsic apoptosis). Nitrogen mustard (mechlorethamine, a US Food and Drug Administration–approved topical therapy for CTCL) was among the highest generators of cleaved caspase 8. Gentian violet (GV; also known as crystal violet) was also identified as a compound that, when compared with nitrogen mustard, induced similar or greater levels of cleaved caspase 8 in CTCL cells without additional toxic effects on normal keratinocytes. Gentian violet is a triarylmethane dye that has been used for more than a century worldwide as an antimicrobial agent, cytologic stain, and the gram-positive stain for classifying bacteria.16,17 In this study, we used in vitro and ex vivo models to analyze the association of this ubiquitous, inexpensive compound with activation of extrinsic pathway apoptosis that involves the Fas and tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) DRs and levels of nuclear factor (NF)–κB components that are typically overexpressed in advanced-stage CTCL and drive tumor cell proliferation.
Methods
CTCL Cell Lines and SS Blood Cells
Human CTCL cell lines MyLa, HH, SZ4, Hut-78, and SeAx and their culture conditions were described previously.6,15 The acute T-cell lymphoblastic leukemia cell line Jurkat was obtained from the American Type Culture Collection. It was included in some studies for comparative purposes. Blood samples from patients with SS samples were obtained. The mononuclear cells were separated from the whole blood and cultured in the same conditions as the SZ4 cell line. Most cells were assessed to be tumor cells as described previously.8 Cells were treated with GV (Sigma-Aldrich) or dimethyl sulfoxide (DMSO) diluted to final concentrations of 1 to 100 μmol/L in culture medium. In some experiments, cells were treated with methotrexate (MP Biomedicals) dissolved in DMSO and diluted to 10 μmol/L in culture medium. This study was approved by the institutional review board of University of Wisconsin, Madison, and all patients provided written informed consent. All data were deidentified.
High-Throughput Screening
We used the Small Molecule Screening Facility at the University of Wisconsin Comprehensive Cancer Center to identify small molecules that induce extrinsic apoptosis in CTCL. We used the HH CTCL cell line in an enzyme-linked immunosorbent assay to quantitatively detect the level of cleaved caspase 8 induced by 1710 unique compounds contained in the National Institutes of Health Clinical Collection, Selleck Kinase Inhibitor Library, and Prestwick Chemical Library.
Flow Cytometry
Percentage of apoptotic cells; levels of cleaved caspases (8, 9, 3, and 2); expression of Fas, Fas ligand (FasL), DR4, DR5, TRAIL, TNF receptor 1 (TNF-R1), and TNF-α; and levels of phosphorylated phospholipase C–γ1 (PLC-γ1), calcium mobilization, and reactive oxygen species (ROS) were assessed by flow cytometry and analyzed as described previously.18 Phycoerythrin-conjugated anticleaved caspases (8, 9, 3, and 2), anti-DR4 (TRAIL-R1), anti-DR5 (TRAIL-R2), and anti-TRAIL were obtained from PD Biosciences. Anti–TNF-R1 primary antibody was obtained from Santa Cruz Biotechnology, and fluorescein isothiocyanate conjugated anti–TNF-α was obtained from Molecular Probes. Secondary antibodies were described previously.18 For analysis of apoptosis, the total numbers of both annexin V single-positive cells (early apoptosis) and annexin V and propidium iodide double-positive cells (late apoptosis) were interpreted as apoptotic cells.
Western Blotting
Western blotting was performed as described previously.18 Anticleaved caspases 8, 9, and 3, anti–myeloid cell leukemia 1 (MCL-1), anti–NF-κB1, anti-RelA, anti–NF-κB2, and anti-RelB primary antibodies were obtained from Cell Signaling. Horseradish peroxidase–conjugated appropriate secondary antibodies and loading control anti–β-actin antibody were obtained from Cell Signaling. The blots were analyzed as described previously.18
Statistical Analysis
Statistical analysis was performed using the 2-tailed, paired t test and analysis of variance. A 2-sided P < .05 was considered to be statistically significant.
Results
GV as a CTCL Killer
Among the 1710 unique compounds screened, nitrogen mustard proved to be among the best inducers of cleaved caspase 8; however, GV was identified as another compound of interest that also induced high levels of cleaved caspase 8 in the HH CTCL cell line.
Apoptosis in CTCL Cell Lines and SS Blood Samples
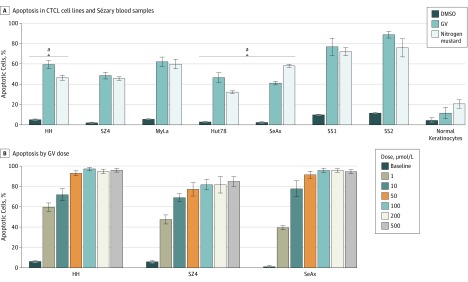
In vitro experiments of annexin V flow cytometry demonstrated that GV (1 μmol/L) induced 40% to 70% apoptosis at 24 hours among 5 CTCL lines (Figure 1A). Gentian violet is also associated with the T-cell acute lymphoblastic leukemia line Jurkat, which was included for comparison. Ex vivo experiments showed even greater apoptosis of 75% to 90% for GV among SS blood samples. In some cases, apoptosis was significantly greater than that observed using nitrogen mustard at the same concentration. Apoptosis of normal keratinocytes was less than that of nitrogen mustard, consistent with the clinical observation that GV is well tolerated as a topical agent. As shown by dose titration experiments in Figure 1B, at 24 hours, significant apoptosis was induced by GV at 1 μmol/L, and apoptosis did not increase much beyond the 50-μmol/L concentration.
Figure 1. Gentian Violet (GV)–Induced Apoptosis in Cutaneous T-Cell Lymphoma Cells.
Cells were treated with GV, nitrogen mustard (mechlorethamine), or dimethyl sulfoxide (DMSO) at 1 μmol/L for 24 hours; apoptosis was detected by flow cytometry. The Jurkat T-cell acute lymphoblastic luekemia cell line line was included for comparison. A, P < .01 for GV compared with DMSO for all. B, P < .01 between each GV-treated column with the DMSO-treated column. SS1 indicates Sézary syndrome 1; SS2, Sézary syndrome 2.
aP < .01 compared with nitrogen mustard.
Extrinsic and Intrinsic Apoptotic Pathways
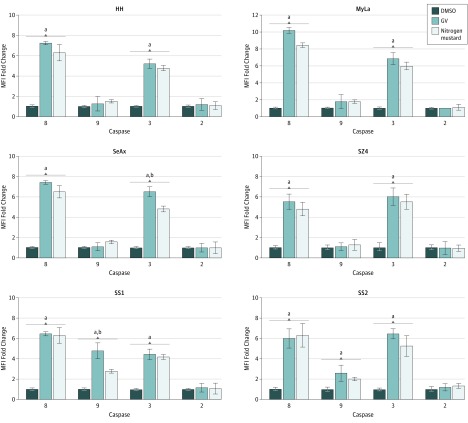
As shown in Figure 2, we examined changes in the activation of various caspases in response to GV. Cleavage of caspase 8 and caspase 9 is indicative of extrinsic (DR) and intrinsic (mitochondrial) apoptotic pathways, respectively. Caspase 2 involves an alternative upstream pathway, and cleavage of caspase 3 is a downstream indicator of total apoptosis. Among CTCL cell lines, GV primarily induced extrinsic pathway activation (cleaved caspase 8), whereas SS blood cells showed activation of the extrinsic and intrinsic pathways. Caspase 2 was not affected. In some instances, the GV-induced findings were significantly greater than those of nitrogen mustard. These flow cytometric alterations were confirmed by immunoblot analysis as shown in eFigure 1 in the Supplement, which also shows that the antiapoptotic factor MCL-1 was reduced by GV in CTCL lines and SS blood cells.
Figure 2. Gentian Violet (GV)–Induced Apoptosis Involving the Extrinsic and Intrinsic Pathways.
Cells were treated with GV, nitrogen mustard (mechlorethamine), or dimethyl sulfoxide (DMSO) at 1 μmol/L for 24 hours, and cleaved caspases 8, 9, 3, and 2 were measured by flow cytometry. MFI indicates mean fluorescence intensity; SS1, Sézary syndrome 1; SS2, Sézary syndrome 2.
aP < .01 compared with DMSO.
bP < .01 compared with nitrogen mustard.
FasL, TRAIL Pathway Factors, and FasL-Related Signaling Components
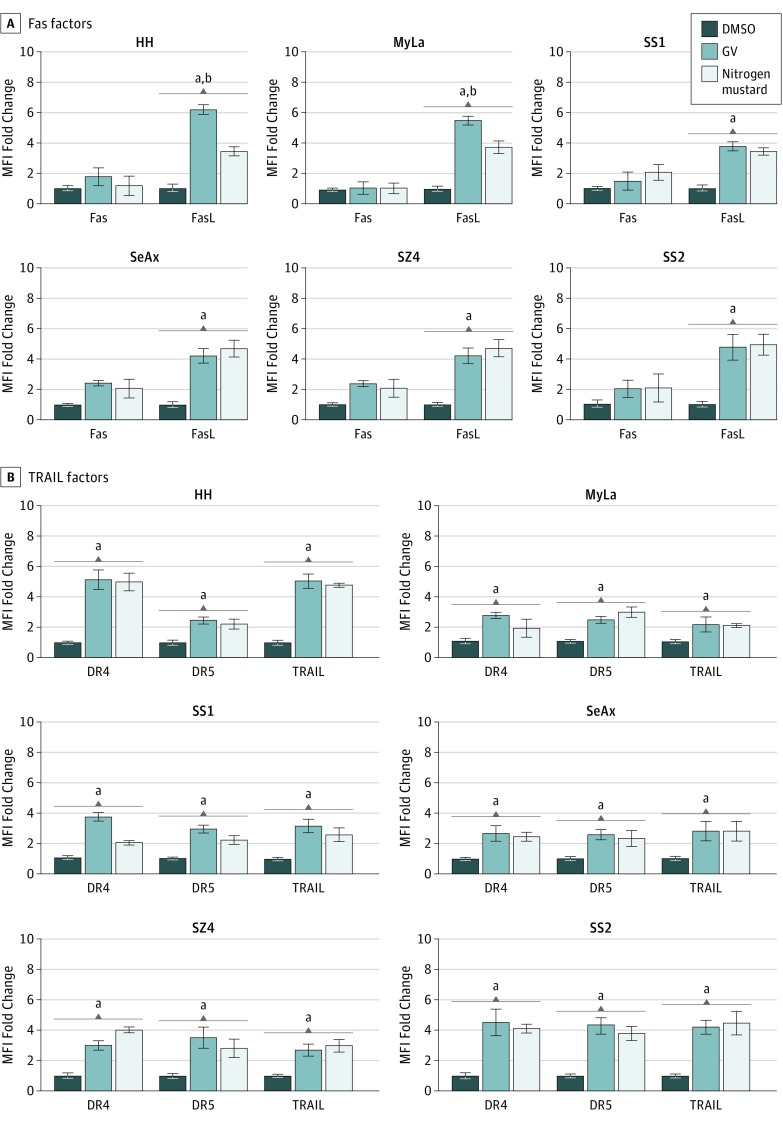
Because of the prominent augmentation of extrinsic pathway activity, we examined alterations of the Fas, TRAIL, and TNF apoptotic subpathways. As shown in Figure 3 and eFigure 2 and eFigure 3 in the Supplement, GV induced significant up-regulation of FasL and all 3 TRAIL components (TRAIL, DR4, and DR5) but not Fas or any TNF components. This finding suggests that GV-mediated extrinsic pathway apoptotic activity was dependent on the Fas and TRAIL subpathways. Furthermore, triggering of AICD requires a TCR signal transduction pathway that involves phosphorylation of PLC-γ1 followed by calcium flux and generation of ROS. As shown in Figure 3 and eFigure 3 in the Supplement, all these factors were enhanced by GV. In addition, in many instances, the enhancement was significantly greater than that of nitrogen mustard throughout this series of experiments.
Figure 3. Gentian Violet (GV)–Induced Upregulation of Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand (TRAIL) Pathway Factors and Fas Ligand (FasL).
Cells were treated with GV, nitrogen mustard (mechlorethamine), or dimethyl sulfoxide (DMSO) at 1 μmol/L for 24 hours. These different proteins or ions were detected by flow cytometry. DR indicates death receptor; MFI, mean fluorescence intensity; SS1, Sézary syndrome 1; SS2, Sézary syndrome 2.
aP < .01 compared with DMSO.
bP < .01 compared with nitrogen mustard.
NF-κB Expression and CTCL Cell Proliferation
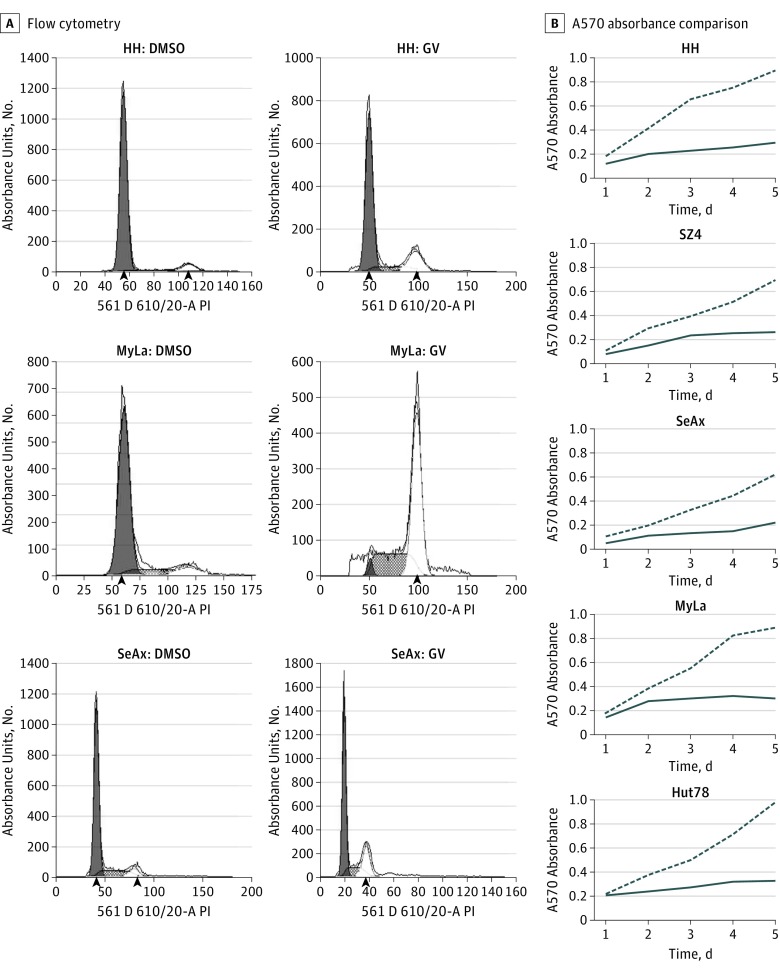
NF-κB is a known proliferative driver of CTCL, and overexpression of NF-κB components is a common feature of CTCL.19,20,21,22,23,24 As shown in eFigure 4 in the Supplement, immunoblot analysis demonstrated that canonical (NF-κB/RelA) and alternative (NF-κB2/RelB) NF-κB components were reduced by GV in CTCL lines and SS blood samples. Subsequent experiments showed that GV modestly increased inhibitory κB (IκB), a factor that inhibits NF-κB subunits. Furthermore, as shown in Figure 4, these changes were associated with decreased CTCL cell proliferation and cell cycle shifts with G2/S phase arrest.
Figure 4. Gentian Violet (CV) Inhibition of Cell Proliferation and Induction of Cell Cycle Arrest at G2/S Phases in Cutaneous T-Cell Lymphoma Lines.
Cells were treated with GV (1 μmol/L) or dimethyl sulfoxide (DMSO) (1 μmol/L). Cell cycle and proliferation were measured by propidium iodide staining and flow cytometry and MTT assay, respectively. A, Flow cytometry plots show cell cycle arrest after GV treatment (48 hours). Arrowheads indicate the the median fluorescence intensity of the G0/G1 and G2 populations. B, A570 absorbance comparison of GV- and DMSO-treated cells at days 1 to 5 shows decreased cell proliferation.
Association of GV-Induced Apoptosis and Methotrexate
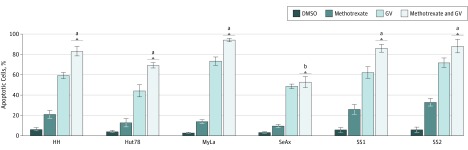
A prior study15 found that methotrexate enhances CTCL apoptosis in part by derepressing expression of the Fas DR by inhibiting methylation of its promoter. Because GV upregulated FasL, we theorized that the upregulation of its receptor Fas by methotrexate would be associated with even greater induction of apoptosis than would GV alone. As shown in Figure 5, combination GV and methotrexate treatment induced 70% to 90% apoptosis at 24 hours in CTCL lines and SS blood cells. This level of apoptosis was significantly greater than that observed with GV or methotrexate alone. The key role of Fas and FasL in this increased apoptosis was supported by the lack of enhanced apoptosis observed in the SeAx CTCL cell line. SeAx is known to be Fas null7 and therefore unable to respond to the Fas-enhancing activity of methotrexate.
Figure 5. Methotrexate-Induced Increase in Gentian Violet (GV)–Induced Apoptosis.
Cells were treated with GV (1 μmol/L), methotrexate (10 μmol/L), methotrexate and GV, or dimethyl sulfoxide (DMSO) (1 μmol/L) for 24 hours; apoptosis was detected by flow cytometry. There was no significant difference (P > .05) between methotrexate and GV vs GV treatment in SeAx cells. The Jurkat T-cell acute lymphoblastic luekemia line was included for comparison.
aP < .001 for methotrexate and GV treatment vs DMSO, methotrexate, and GV.
bP < .001 for methotrexate and GV treatment vs DMSO and methotrexate.
Discussion
In addition to its role as an antifungal and antibacterial agent, GV has anticancer properties.25,26,27,28,29,30 Prior work27,31,32,33 has found that GV inhibits nicotimamide adenine dinucleotide phosphate oxidases, NF-κB, and ANG-2 while inducing p53 and IκB in various cell types. Our current findings add to this knowledge base by detailing the association between GV and CTCL cells. We used in vitro and ex vivo approaches to show that GV induced major levels of apoptosis in CTCL lines and SS blood cells. As shown in Figure 1, Figure 2, and Figure 3 and eFigure 1 and eFigure 2 in the Supplement, the apoptosis involved mainly the extrinsic pathway in some cases and both the extrinsic and intrinsic pathways in others. Extrinsic apoptosis occurs largely via the Fas and TRAIL subpathways, whereas intrinsic apoptosis is associated with reduction in antiapoptotic factors, such as MCL-1. As shown in Figure 4 and eFigure 4 in the Supplement, while GV augments apoptosis, it diminishes CTCL proliferative drivers, such as NF-κB components, and enhances expression of IκB. This finding is associated with cell cycle arrest and reduced CTCL tumor cell proliferation, which simultaneously attack CTCL viability and growth.
As shown in Figure 5, the increase in GV-induced apoptosis in CTCL was greater when GV was combined with methotrexate. A previous study3 found that in addition to its role as an S-phase inhibitor that blocks purine synthesis, methotrexate has novel activity as a DNA methylation inhibitor by blocking synthesis of S-adenosylmethionine, the principal methyl donor for DNA methyltransferases. Therefore, methotrexate inhibits cell proliferation through the cell cycle and enhances apoptosis through epigenetic derepression of Fas, FasL, and other apoptotic factors by inhibiting promoter methylation. The specific role of Fas derepression is underscored by the inability of methotrexate to enhance SeAx apoptosis. This CTCL line is Fas null and therefore unable to upregulate Fas in response to methotrexate.7
The TCR is expressed on the cell surface in association with CD3 proteins to form the TCR/CD3 complex. During AICD, engagement of TCR/CD3 normally leads to activation of proximate tyrosine kinases, such as Zap70, and a subsequent downstream cascade that involves phosphorylation of PLC-γ1, calcium mobilization, generation of ROS, and FasL upregulation.5,11,34 As shown in Figure 3 and eFigure 3 in the Supplement, the ability of GV to enhance extrinsic apoptosis via the AICD mechanism is supported by the concomitant increases in phosphorylated PLC-γ1, calcium flux, ROS generation, and FasL observed in this study.
Limitations
The limitations of this study include analysis restricted to CTCL cell lines and blood samples from patients with SS. These are both advanced forms of CTCL and do not necessarily predict the response of early CTCL tumor cells to GV. In addition, the results reported here are preclinical findings based on in vitro and ex vivo experiments. Therefore, they cannot predict the actual efficacy or toxic effects of GV in the clinical setting.
Conclusions
Despite extensive analysis, no uniform targetable mutation has been discovered to date that accounts for the pathogenesis of CTCL.13 Therefore, our research has focused on interventions that enhance CTCL apoptosis and impede tumor cell proliferation. Our current findings indicate that GV has these important characteristics. Used throughout the world for more than a century as an antimicrobial agent and tissue dye, it is well tolerated topically on the skin and oral mucosa.32 Our preclinical data suggest that GV might be an effective topical agent for early-stage CTCL. Although purple at a neutral pH, GV is rendered colorless or nearly so when the pH is adjusted in either direction. The well-established antimicrobial actions of GV would be advantageous for the dermatophyte and bacterial infections that sometimes complicate the treatment of patients with CTCL.35,36,37 In particular, staphylococcal infections can be problematic in patients with CTCL and have been implicated in exacerbating the severity of CTCL.35,36There is also a social justice aspect to GV. In an era of high-priced anticancer drugs that are inaccessible to many patients throughout the world, GV (like methotrexate) is an inexpensive compound that is widely available. Physicians around the globe are familiar with its use. Clinical studies will be needed to determine the efficacy of topical GV therapy for early-stage CTCL; however, our preclinical data provide justification for further investigations.
eFigure 1. Gentian Violet induced apoptosis involves extrinsic and intrinsic pathways
eFigure 2. Levels of TNF pathway factors are not altered by gentian violet
eFigure 3. Gentian Violet leads to up-regulation of phosphorylated phospholipase C-gamma 1 (pPLC-gamma 1), calcium influx and reactive oxygen species (ROS)
eFigure 4. Gentian Violet leads to decreased NF-kB subunit expression and
increased IkB
References
- 1.Meech SJ, Edelson R, Walsh P, Norris DA, Duke RC. Reversible resistance to apoptosis in cutaneous T cell lymphoma. Ann N Y Acad Sci. 2001;941:46-58. doi: 10.1111/j.1749-6632.2001.tb03710.x [DOI] [PubMed] [Google Scholar]
- 2.Braun FK, Fecker LF, Schwarz C, et al. . Blockade of death receptor-mediated pathways early in the signaling cascade coincides with distinct apoptosis resistance in cutaneous T-cell lymphoma cells. J Invest Dermatol. 2007;127(10):2425-2437. doi: 10.1038/sj.jid.5700868 [DOI] [PubMed] [Google Scholar]
- 3.Contassot E, Kerl K, Roques S, et al. . Resistance to FasL and tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in Sézary syndrome T-cells associated with impaired death receptor and FLICE-inhibitory protein expression. Blood. 2008;111(9):4780-4787. doi: 10.1182/blood-2007-08-109074 [DOI] [PubMed] [Google Scholar]
- 4.Contassot E, French LE. Targeting apoptosis defects in cutaneous T-cell lymphoma. J Invest Dermatol. 2009;129(5):1059-1061. doi: 10.1038/jid.2009.14 [DOI] [PubMed] [Google Scholar]
- 5.Klemke CD, Brenner D, Weiss EM, et al. . Lack of T-cell receptor-induced signaling is crucial for CD95 ligand up-regulation and protects cutaneous T-cell lymphoma cells from activation-induced cell death. Cancer Res. 2009;69(10):4175-4183. doi: 10.1158/0008-5472.CAN-08-4631 [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Nihal M, Siddiqui J, Vonderheid EC, Wood GS. Low FAS/CD95 expression by CTCL correlates with reduced sensitivity to apoptosis that can be restored by FAS upregulation. J Invest Dermatol. 2009;129(5):1165-1173. doi: 10.1038/jid.2008.309 [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Siddiqui J, Nihal M, Vonderheid EC, Wood GS. Structural alterations of the FAS gene in cutaneous T-cell lymphoma (CTCL). Arch Biochem Biophys. 2011;508(2):185-191. doi: 10.1016/j.abb.2010.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Salva KA, Stutz N, Longley BJ, Spiegelman VS, Wood GS. Quantitative gene analysis of methylation and expression (Q-GAME) in fresh or fixed cells and tissues. Exp Dermatol. 2014;23(5):304-309. doi: 10.1111/exd.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stutz N, Johnson RD, Wood GS. The Fas apoptotic pathway in cutaneous T-cell lymphomas: frequent expression of phenotypes associated with resistance to apoptosis. J Am Acad Dermatol. 2012;67(6):1327.e1-1327.e10. doi: 10.1016/j.jaad.2012.05.035 [DOI] [PubMed] [Google Scholar]
- 10.Ni X, Zhang C, Talpur R, Duvic M. Resistance to activation-induced cell death and bystander cytotoxicity via the Fas/Fas ligand pathway are implicated in the pathogenesis of cutaneous T cell lymphomas. J Invest Dermatol. 2005;124(4):741-750. doi: 10.1111/j.0022-202X.2005.23657.x [DOI] [PubMed] [Google Scholar]
- 11.Fargnoli MC, Edelson RL, Berger CL, et al. . Diminished TCR signaling in cutaneous T cell lymphoma is associated with decreased activities of Zap70, Syk and membrane-associated Csk. Leukemia. 1997;11(8):1338-1346. doi: 10.1038/sj.leu.2400745 [DOI] [PubMed] [Google Scholar]
- 12.Chitgopeker P, Sahni D. T-cell receptor gene rearrangement detection in suspected cases of cutaneous T-cell lymphoma. J Invest Dermatol. 2014;134(4):1-5. doi: 10.1038/jid.2014.73 [DOI] [PubMed] [Google Scholar]
- 13.Damsky WE, Choi J. Genetics of cutaneous t cell lymphoma: from bench to bedside. Curr Treat Options Oncol. 2016;17(7):33. doi: 10.1007/s11864-016-0410-8 [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Salva KA, Wood GS. c-CBL E3 ubiquitin ligase is overexpressed in cutaneous T-cell lymphoma: its inhibition promotes activation-induced cell death. J Invest Dermatol. 2015;135(3):861-868. doi: 10.1038/jid.2014.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Wood GS. Reduction of Fas/CD95 promoter methylation, upregulation of Fas protein, and enhancement of sensitivity to apoptosis in cutaneous T-cell lymphoma. Arch Dermatol. 2011;147(4):443-449. doi: 10.1001/archdermatol.2010.376 [DOI] [PubMed] [Google Scholar]
- 16.Docampo R, Moreno SNJ. The metabolism and mode of action of gentian violet. Drug Metab Rev. 1990;22(2-3):161-178. doi: 10.3109/03602539009041083 [DOI] [PubMed] [Google Scholar]
- 17.Berrios RL, Arbiser JL. Effectiveness of gentian violet and similar products commonly used to treat pyodermas. Dermatol Clin. 2011;29(1):69-73. doi: 10.1016/j.det.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Wood GS. c-CBL ubiquitin E3 ligase is over-expressed in Sézary syndrome and its inhibition restores T-cell receptor signaling, upregulates FAS-Ligand expression and promotes activation-induced cell death. J Invest Dermatol. 2014;134:S32-S32. [Google Scholar]
- 19.Sors A, Jean-Louis F, Pellet C, et al. . Down-regulating constitutive activation of the NF-κB canonical pathway overcomes the resistance of cutaneous T-cell lymphoma to apoptosis. Blood. 2006;107(6):2354-2363. doi: 10.1182/blood-2005-06-2536 [DOI] [PubMed] [Google Scholar]
- 20.Sors A, Jean-Louis F, Bégué E, et al. . Inhibition of IκB kinase subunit 2 in cutaneous T-cell lymphoma down-regulates nuclear factor-κB constitutive activation, induces cell death, and potentiates the apoptotic response to antineoplastic chemotherapeutic agents. Clin Cancer Res. 2008;14(3):901-911. doi: 10.1158/1078-0432.CCR-07-1419 [DOI] [PubMed] [Google Scholar]
- 21.Cleere R, Long A, Kelleher D, O’Neill LAJ. Autocrine regulation of the transcription factor NF-κB by TNF-α in the human T cell lymphoma line Hut 78. Biochem Soc Trans. 1995;23(1):113S. doi: 10.1042/bst023113s [DOI] [PubMed] [Google Scholar]
- 22.O’Connell MA, Cleere R, Long A, O’Neill LAJ, Kelleher D. Cellular proliferation and activation of NF-κB are induced by autocrine production of tumor necrosis factor α in the human T lymphoma line HuT 78. J Biol Chem. 1995;270(13):7399-7404. doi: 10.1074/jbc.270.13.7399 [DOI] [PubMed] [Google Scholar]
- 23.Giri DK, Aggarwal BB. Constitutive activation of NF-κB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells: autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273(22):14008-14014. doi: 10.1074/jbc.273.22.14008 [DOI] [PubMed] [Google Scholar]
- 24.Izban KF, Ergin M, Qin JZ, et al. . Constitutive expression of NF-κB is a characteristic feature of mycosis fungoides: implications for apoptosis resistance and pathogenesis. Hum Pathol. 2000;31(12):1482-1490. doi: 10.1053/hupa.2000.20370 [DOI] [PubMed] [Google Scholar]
- 25.Rao S, Morris R, Rice ZP, Arbiser JL. Regression of diffuse B-cell lymphoma of the leg with intralesional gentian violet. Exp Dermatol. 2018;27(1):93-95. doi: 10.1111/exd.13418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukawera E, Chartier S, Williams V, Pagano PJ, Lapointe R, Grandvaux N. Redox-modulating agents target NOX2-dependent IKKε oncogenic kinase expression and proliferation in human breast cancer cell lines. Redox Biol. 2015;6:9-18. doi: 10.1016/j.redox.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garufi A, D’Orazi V, Arbiser JL, D’Orazi G. Gentian violet induces wtp53 transactivation in cancer cells. Int J Oncol. 2014;44(4):1084-1090. doi: 10.3892/ijo.2014.2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arbiser JL, Bips M, Seidler A, Bonner MY, Kovach C. Combination therapy of imiquimod and gentian violet for cutaneous melanoma metastases. J Am Acad Dermatol. 2012;67(2):e81-e83. doi: 10.1016/j.jaad.2011.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietrobono S, Morandi A, Gagliardi S, et al. . Down-regulation of SOX2 underlies the inhibitory effects of the triphenylmethane gentian violet on melanoma cell self-renewal and survival. J Invest Dermatol. 2016;136(10):2059-2069. doi: 10.1016/j.jid.2016.06.610 [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi M, Murata T. Potential suppressive effects of gentian violet on human breast cancer MDA-MB-231 cells in vitro: comparison with gemcitabine. Oncol Lett. 2016;12(2):1605-1609. doi: 10.3892/ol.2016.4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry BN, Govindarajan B, Bhandarkar SS, et al. . Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J Invest Dermatol. 2006;126(10):2316-2322. doi: 10.1038/sj.jid.5700413 [DOI] [PubMed] [Google Scholar]
- 32.Maley AM, Arbiser JL. Gentian violet: a 19th century drug re-emerges in the 21st century. Exp Dermatol. 2013;22(12):775-780. doi: 10.1111/exd.12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi M, Vikulina T, Arbiser JL, Weitzmann MN. Suppression of NF-κB activation by gentian violet promotes osteoblastogenesis and suppresses osteoclastogenesis. Curr Mol Med. 2014;14(6):783-792. doi: 10.2174/1566524014666140724104842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willerslev-Olsen A, Krejsgaard T, Lindahl LM, et al. . Staphylococcal enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood. 2016;127(10):1287-1296. doi: 10.1182/blood-2015-08-662353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willerslev-Olsen A, Krejsgaard T, Lindahl LM, et al. . Bacterial toxins fuel disease progression in cutaneous T-cell lymphoma. Toxins (Basel). 2013;5(8):1402-1421. doi: 10.3390/toxins5081402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackow CM, Cather JC, Hearne V, Asano AT, Musser JM, Duvic M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor V beta gene expansion. Blood. 1997;89(1):32-40. [PubMed] [Google Scholar]
- 37.Duvic M, Feasel AM, Schwartz CA, Cather JC. Enterococcal eschars in cutaneous T-cell lymphoma tumors: a distinct clinical entity. Clin Lymphoma. 2000;1(2):141-145. doi: 10.3816/CLM.2000.n.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Gentian Violet induced apoptosis involves extrinsic and intrinsic pathways
eFigure 2. Levels of TNF pathway factors are not altered by gentian violet
eFigure 3. Gentian Violet leads to up-regulation of phosphorylated phospholipase C-gamma 1 (pPLC-gamma 1), calcium influx and reactive oxygen species (ROS)
eFigure 4. Gentian Violet leads to decreased NF-kB subunit expression and
increased IkB