Abstract
Objective
To assess the association between the use of sodium glucose cotransporter 2 (SGLT2) inhibitors and seven serious adverse events of current concern.
Design
Register based cohort study.
Setting
Sweden and Denmark from July 2013 to December 2016.
Participants
A propensity score matched cohort of 17 213 new users of SGLT2 inhibitors (dapagliflozin, 61%; empagliflozin, 38%; canagliflozin, 1%) and 17 213 new users of the active comparator, glucagon-like peptide 1 (GLP1) receptor agonists.
Main outcome measures
The primary outcomes were lower limb amputation, bone fracture, diabetic ketoacidosis, acute kidney injury, serious urinary tract infection, venous thromboembolism, and acute pancreatitis, as identified from hospital records. Hazard ratios and 95% confidence intervals were estimated by using Cox proportional hazards models.
Results
Use of SGLT2 inhibitors, as compared with GLP1 receptor agonists, was associated with an increased risk of lower limb amputation (incidence rate 2.7 v 1.1 events per 1000 person years, hazard ratio 2.32, 95% confidence interval 1.37 to 3.91) and diabetic ketoacidosis (1.3 v 0.6, 2.14, 1.01 to 4.52) but not with bone fracture (15.4 v 13.9, 1.11, 0.93 to 1.33), acute kidney injury (2.3 v 3.2, 0.69, 0.45 to 1.05), serious urinary tract infection (5.4 v 6.0, 0.89, 0.67 to 1.19), venous thromboembolism (4.2 v 4.1, 0.99, 0.71 to 1.38) or acute pancreatitis (1.3 v 1.2, 1.16, 0.64 to 2.12).
Conclusions
In this analysis of nationwide registers from two countries, use of SGLT2 inhibitors, as compared with GLP1 receptor agonists, was associated with an increased risk of lower limb amputation and diabetic ketoacidosis, but not with other serious adverse events of current concern.
Introduction
Sodium glucose cotransporter 2 (SGLT2) inhibitors present a valuable new therapeutic option for the treatment of type 2 diabetes, but concerns have been raised regarding their safety. In the CANVAS Program, patients randomised to the SGLT2 inhibitor canagliflozin experienced significantly higher rates of lower limb amputation (hazard ratio 1.97, 95% confidence interval 1.41 to 2.75) and bone fracture (1.26, 1.04 to 1.52) compared with patients receiving placebo.1 Case reports to the United States Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) have indicated that SGLT2 inhibitors could cause diabetic ketoacidosis,2 3 acute kidney injury,4 5 and serious urinary tract infection.2 SGLT2 inhibitors increase blood viscosity by inducing mild diuresis, which suggests that the risk of venous thromboembolism could be increased.6 7 The FDA is also investigating reports of acute pancreatitis associated with the use of SGLT2 inhibitors.8 9 10
Completed and ongoing clinical trials are conducted in selected populations and are too small to assess rare adverse events, whereas analyses of case reports have substantial limitations, including a lack of denominators and possible reporting bias. Only four controlled large scale observational studies have been conducted.11 12 13 14 Each study investigated one selected adverse event and three studies used study designs with method limitations,11 12 13 including immortal time bias and lag time bias.15 Therefore, the safety of SGLT2 inhibitors is still uncertain.
In this register based cohort study of patients from routine clinical practice, we used nationwide data from Sweden and Denmark to assess whether the use of SGLT2 inhibitors, as compared with an active comparator (glucagon-like peptide 1 (GLP1) receptor agonists), is associated with an increased risk of seven serious adverse events of current concern.
Methods
Study design
We conducted a cohort study from July 2013 to December 2016, with data from nationwide health and administrative registers in Sweden and Denmark. The registers included population registers (vital status, demographics), patient registers (comorbidities, outcomes), prescription registers (study drugs, comedications), Statistics Denmark and Statistics Sweden (socioeconomic variables), and the Swedish National Diabetes Register (glycated haemoglobin level, blood pressure, albuminuria, estimated glomerular filtration rate, body mass index, and smoking status), which are described in the supplementary materials.
We used an active comparator new-user study design and controlled for a wide range of potential confounders (patient characteristics that might be associated with both the outcome and the decision to initiate a drug) through a non-parsimonious propensity score model to minimise the risk of bias, including confounding by indication.16 We used GLP1 receptor agonists as the active comparator because of important shared features with SGLT2 inhibitors, including use in similar clinical situations (ie, as second line or third line diabetes drugs, with both drug classes likely considered for patients at high cardiovascular risk) and similar temporal trends of use in the two countries,1 17 18 19 20 21 but no known associations with the outcomes investigated in this study.
Study population
We included all patients in the two countries, aged 35 or over, who filled their first prescription for either an SGLT2 inhibitor or a GLP1 receptor agonist during the study period; the date of filling the first prescription constituted cohort entry. We excluded patients who had previously filled prescriptions for any of the study drugs; had no hospital contact or use of prescription drugs in the previous year; had dialysis or renal transplantation, endstage illness, drug misuse, or severe pancreatic disorders; or had a hospital stay for any reason in the 30 days before cohort entry (supplementary materials, table 1).
Patients were considered exposed to the study drug if prescriptions were refilled before the estimated end date of the most recent prescription. We included a grace period of 90 days to account for irregular drug intake and to capture events that occured shortly after treatment cessation (supplementary materials, table 2).
We estimated propensity scores by using logistic regression for the probability of starting an SGLT2 inhibitor conditional on the status of 66 covariates, defined and selected a priori, including sociodemographic characteristics, comorbidities, comedications, and healthcare utilisation, as current at cohort entry in Sweden and Denmark (supplementary materials, table 3). The nationwide registers provided complete information on disease history (for the previous 10 years) and prescription drug use in each country before cohort entry. Missing data on place of birth (<1%), civil status (<1%), and education (3%) were handled with use of missing categories.22
We matched SGLT2 inhibitor and GLP1 receptor agonist users (1:1 ratio, by country) according to propensity score, by using the nearest neighbour matching algorithm (caliper width 0.2 of the standard deviation of the logit score).23 24 Analyses were performed in a pooled dataset of the two countries.
Outcome measures
The primary outcome measures were lower limb amputation, bone fracture, diabetic ketoacidosis, acute kidney injury, serious urinary tract infection, venous thromboembolism, and acute pancreatitis, as captured from the patient registers. We also conducted analyses restricted to toe or metatarsal amputation and to major osteoporotic fracture as additional outcomes. Supplementary materials, table 4 shows outcome definitions according to ICD-10 (international classification of diseases, 10th edition) codes and procedure codes. Diagnoses recorded in Scandinavian health registers generally have high sensitivity and positive predictive value,25 26 although validation studies have only been conducted for some of the outcomes in the present study. Supplementary materials, table 5 shows validation studies in the national patient registers of diagnostic and procedure codes related to those used in our study.
Statistical analyses
Patients were followed from cohort entry to treatment cessation, crossover to the other study drug (ie, initiation of GLP1 receptor agonists among SGLT2 inhibitor users and vice versa), the outcome event, death, emigration, or the end of the study period (31 December 2016). We used Cox proportional hazards regression to calculate hazard ratios, analysing each outcome independently. The absolute risk difference was calculated as hazard ratio−1 multiplied by the rate in the comparator group.
We performed subgroup analyses for all outcomes by country, sex, age group, and history of major cardiovascular disease (supplementary materials, table 6). In additional analyses of diabetic ketoacidosis and acute kidney injury, we truncated follow-up at six months as reports to FAERS indicate that these events can occur shortly after treatment initiation.2 3 4 For diabetic ketoacidosis and lower limb amputation, we also performed a subgroup analysis according to insulin treatment status at baseline and according to history of peripheral artery disease or lower limb amputation, respectively (supplementary materials, table 7).
For all outcomes, we conducted four sensitivity analyses. Firstly, we used an intention-to-treat exposure definition. In this analysis, patients were considered exposed to the study drug throughout follow-up regardless of treatment cessation or crossover to the other study drug. Secondly, we used an additionally adjusted model. In this analysis, for the propensity score-matched cohort in Sweden, we adjusted the Cox models for additional covariates which provided information about disease severity and comorbidities related to diabetes, including glycated haemoglobin level, blood pressure, albuminuria, estimated glomerular filtration rate, body mass index, and smoking status (supplementary materials, table 8). Given the proportion of missing data for these variables (supplementary materials, table 8), we used multiple imputation (Markov chain Monte Carlo method) to handle missing data27; analyses were conducted by using 10 imputed datasets which were combined. Thirdly, we used a grace period of 30 days in the definition of current use of the study drug. Finally, we adjusted the models for country. We performed additional sensitivity analyses for bone fracture, using an outcome definition only including fractures that led to hospital stays, and excluding users of thiazolidinediones as these drugs have been associated with an increased risk of fracture.28 We performed additional sensitivity analysis for acute pancreatitis, excluding users of dipeptidyl peptidase 4 (DPP4) inhibitors because these drugs have been associated with acute pancreatitis.29 We performed the analysis with SAS version 9.4 software.
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for recruitment, design, or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Study population
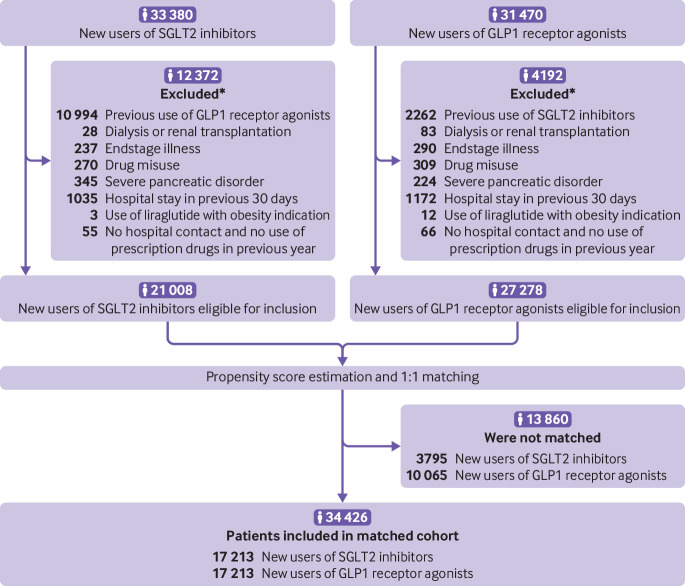
Figure 1 shows that we identified 21 008 new users of SGLT2 inhibitors and 27 278 new users of GLP1 receptor agonists who fulfilled the criteria for study eligibility. Table 1 shows that, compared with GLP1 receptor agonist users, SGLT2 inhibitor users were older, more likely to be men and to use DPP4 inhibitors at baseline, and less likely to have an obesity diagnosis and to use insulin at baseline. Figure 1 and table 1 show that after 1:1 propensity score matching, 34 426 patients (17 213 in each group) were included in the study cohort; the two groups were well balanced on all baseline characteristics. Of the SGLT2 inhibitor users, 61% used dapagliflozin, 38% empagliflozin, and 1% canagliflozin. Patient characteristics before and after propensity score matching for each country separately are shown in supplementary materials, tables 9 and 10. In the analyses of the seven primary outcome measures, median follow-up time in the cohort ranged between 270 and 274 days (supplementary materials, table 11).
Fig 1.
Flowchart of patient inclusion in the study cohort. SGLT2=sodium glucose cotransporter 2; GLP1=glucagon-like peptide 1. *A patient could be excluded for more than one reason.
Table 1.
Baseline characteristics of users of sodium glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP1) receptor agonists, before and after propensity score matching. Values are numbers (percentages) unless stated otherwise
| Characteristic | Before | After | |||
|---|---|---|---|---|---|
| SGLT2 inhibitors | GLP1 receptor agonists | SGLT2 inhibitors | GLP1 receptor agonists | ||
| No of patients | 21 008 | 27 278 | 17 213 | 17 213 | |
| Men | 13 113 (62) | 15 712 (58) | 10 482 (61) | 10 449 (61) | |
| Mean (SD) age (years) | 62 (10) | 60 (11) | 61 (10) | 61 (10) | |
| SGLT2 inhibitor*: | |||||
| Dapagliflozin | 12 821 (61) | NA | 10 454 (61) | NA | |
| Empagliflozin | 7864 (37) | NA | 6506 (38) | NA | |
| Canagliflozin | 324 (2) | NA | 254 (1) | NA | |
| Country: | |||||
| Sweden | 11 891 (57) | 17 783 (65) | 10 512 (61) | 10 512 (61) | |
| Denmark | 9117 (43) | 9495 (35) | 6701 (39) | 6701 (39) | |
| Place of birth: | |||||
| Scandinavia | 17 242 (82) | 23 614 (87) | 14 607 (85) | 14 556 (85) | |
| Rest of Europe | 1671 (8) | 1677 (6) | 1184 (7) | 1205 (7) | |
| Outside Europe | 2052 (10) | 1950 (7) | 1399 (8) | 1426 (8) | |
| Missing | 43 (<1) | 37 (<1) | 23 (<1) | 26 (<1) | |
| Civil status: | |||||
| Married or living with partner† | 12 172 (58) | 15 130 (55) | 9819 (57) | 9823 (57) | |
| Single | 8778 (42) | 12 088 (44) | 7348 (43) | 7343 (43) | |
| Missing | 58 (<1) | 60 (<1) | 46 (<1) | 47 (<1) | |
| Education: | |||||
| Primary school, secondary school, or vocational training | 16 518 (79) | 21 264 (78) | 13 509 (78) | 13 500 (78) | |
| Short tertiary education | 1447 (7) | 2157 (8) | 1253 (7) | 1278 (7) | |
| Medium or long tertiary education | 2495 (12) | 3326 (12) | 2064 (12) | 2048 (12) | |
| Missing | 548 (3) | 531 (2) | 387 (2) | 387 (2) | |
| Year of cohort entry‡: | |||||
| 2013 | 1005 (5) | 2845 (10) | 833 (5) | 1746 (10) | |
| 2014 | 3648 (17) | 6434 (24) | 3034 (18) | 4009 (23) | |
| 2015 | 6268 (30) | 8544 (31) | 5161 (30) | 5387 (31) | |
| 2016 | 10 087 (48) | 9455 (35) | 8185 (48) | 6071 (35) | |
| Comorbidities: | |||||
| Acute coronary syndrome | 1608 (8) | 2070 (8) | 1300 (8) | 1316 (8) | |
| Other ischaemic heart disease | 3468 (17) | 4478 (16) | 2786 (16) | 2794 (16) | |
| Heart failure or cardiomyopathy | 1195 (6) | 1881 (7) | 1026 (6) | 1058 (6) | |
| Valve disorders | 402 (2) | 563 (2) | 326 (2) | 332 (2) | |
| Stroke | 836 (4) | 1114 (4) | 679 (4) | 655 (4) | |
| Other cerebrovascular disease | 918 (4) | 1274 (5) | 757 (4) | 733 (4) | |
| Atrial fibrillation | 1462 (7) | 2043 (7) | 1222 (7) | 1207 (7) | |
| Other arrhythmia | 834 (4) | 1109 (4) | 673 (4) | 687 (4) | |
| Coronary revascularisation in the past year | 282 (1) | 334 (1) | 230 (1) | 219 (1) | |
| Other cardiac surgery or invasive procedure in the past year | 107 (1) | 151 (1) | 86 (<1) | 85 (<1) | |
| Chronic obstructive pulmonary disease | 662 (3) | 1136 (4) | 586 (3) | 559 (3) | |
| Other lung disease | 1298 (6) | 2257 (8) | 1175 (7) | 1160 (7) | |
| Venous thromboembolism | 495 (2) | 809 (3) | 440 (3) | 445 (3) | |
| Cancer | 1433 (7) | 1884 (7) | 1152 (7) | 1175 (7) | |
| Liver disease | 420 (2) | 609 (2) | 349 (2) | 376 (2) | |
| Rheumatic disease | 598 (3) | 870 (3) | 499 (3) | 498 (3) | |
| Psychiatric disorder | 1914 (9) | 3279 (12) | 1705 (10) | 1729 (10) | |
| Fracture in the past year | 326 (2) | 449 (2) | 267 (2) | 268 (2) | |
| Acute pancreatitis | 198 (1) | 234 (1) | 156 (1) | 142 (1) | |
| Serious urinary tract infection | 405 (2) | 716 (3) | 361 (2) | 341 (2) | |
| Lower limb amputation | 34 (<1) | 34 (<1) | 27 (<1) | 23 (<1) | |
| Arterial disease | 1244 (6) | 1762 (6) | 1035 (6) | 1049 (6) | |
| Renal disease | 755 (4) | 1726 (6) | 708 (4) | 735 (4) | |
| Obesity§ | 2742 (13) | 6197 (23) | 2634 (15) | 2726 (16) | |
| Diabetic eye complications | 2605 (12) | 4403 (16) | 2274 (13) | 2315 (13) | |
| Diabetic ketoacidosis | 34 (<1) | 68 (<1) | 29 (<1) | 30 (<1) | |
| Diabetes, other complications | 3777 (18) | 6366 (23) | 3252 (19) | 3251 (19) | |
| Hospital stays in the past year for: | |||||
| Cardiovascular causes | 809 (4) | 1160 (4) | 682 (4) | 672 (4) | |
| Type 2 diabetes | 163 (1) | 369 (1) | 145 (1) | 134 (1) | |
| Other causes | 2372 (11) | 3596 (13) | 2001 (12) | 2020 (12) | |
| Outpatient visits in the past year for: | |||||
| Cardiovascular causes | 1701 (8) | 2429 (9) | 1433 (8) | 1395 (8) | |
| Type 2 diabetes | 3364 (16) | 6235 (23) | 3054 (18) | 3104 (18) | |
| Other causes | 10 858 (52) | 15 310 (56) | 9091 (53) | 9077 (53) | |
| Diabetes drugs used in the past six months: | |||||
| Metformin | 17 203 (82) | 20 790 (76) | 13 793 (80) | 13 830 (80) | |
| Sulphonylureas | 4957 (24) | 5513 (20) | 3937 (23) | 3955 (23) | |
| DPP4 inhibitors | 8315 (40) | 7681 (28) | 5948 (35) | 6007 (35) | |
| Insulin, fast acting | 2796 (13) | 6824 (25) | 2774 (16) | 2836 (16) | |
| Insulin, intermediate and long acting | 5105 (24) | 11 818 (43) | 5063 (29) | 5050 (29) | |
| Other antidiabetics (glitazones, glinides, acarbose) | 825 (4) | 945 (3) | 646 (4) | 653 (4) | |
| No diabetes drug | 1038 (5) | 1522 (6) | 942 (5) | 905 (5) | |
| Time (years) since first diabetes drug: | |||||
| <1 | 1648 (8) | 2405 (9) | 1480 (9) | 1427 (8) | |
| 1-2 | 2463 (12) | 3069 (11) | 2117 (12) | 2074 (12) | |
| 3-4 | 2943 (14) | 3599 (13) | 2473 (14) | 2448 (14) | |
| 5-6 | 3173 (15) | 3765 (14) | 2529 (15) | 2582 (15) | |
| ≥7 | 10 781 (51) | 14 440 (53) | 8614 (50) | 8682 (50) | |
| Other drugs used in the past year: | |||||
| ARB or ACE-I | 14 402 (69) | 19 059 (70) | 11 928 (69) | 11 944 (69) | |
| Calcium channel blocker | 6729 (32) | 9262 (34) | 5619 (33) | 5611 (33) | |
| Loop diuretic | 2463 (12) | 4722 (17) | 2264 (13) | 2308 (13) | |
| Other diuretic | 3558 (17) | 5231 (19) | 3058 (18) | 3043 (18) | |
| Beta blocker | 7414 (35) | 10 196 (37) | 6231 (36) | 6224 (36) | |
| Digoxin | 454 (2) | 619 (2) | 381 (2) | 377 (2) | |
| Nitrate | 1505 (7) | 1939 (7) | 1212 (7) | 1211 (7) | |
| Platelet inhibitors | 7447 (35) | 9353 (34) | 5937 (34) | 5964 (35) | |
| Anticoagulant | 1470 (7) | 2132 (8) | 1237 (7) | 1240 (7) | |
| Lipid lowering drug | 15 046 (72) | 19 374 (71) | 12 273 (71) | 12 261 (71) | |
| Antidepressant | 3139 (15) | 5206 (19) | 2841 (17) | 2855 (17) | |
| Antipsychotic | 701 (3) | 989 (4) | 609 (4) | 584 (3) | |
| Anxiolytic, hypnotic, or sedative | 3154 (15) | 4618 (17) | 2711 (16) | 2728 (16) | |
| Beta 2 agonist inhalant | 1725 (8) | 2887 (11) | 1538 (9) | 1522 (9) | |
| Anticholinergic inhalant | 538 (3) | 854 (3) | 473 (3) | 469 (3) | |
| Glucocorticoid inhalant | 1819 (9) | 2964 (11) | 1618 (9) | 1602 (9) | |
| Oral glucocorticoid | 1365 (6) | 2039 (7) | 1170 (7) | 1123 (7) | |
| Non-steroidal anti-inflammatory drug | 4828 (23) | 6544 (24) | 4029 (23) | 4056 (24) | |
| Opioid | 3474 (17) | 5384 (20) | 2982 (17) | 2989 (17) | |
| Number of drugs used in the past year: | |||||
| 0-4 | 2620 (12) | 2515 (9) | 1944 (11) | 1932 (11) | |
| 5-9 | 8972 (43) | 10 046 (37) | 7046 (41) | 7096 (41) | |
| 10-14 | 5995 (29) | 8360 (31) | 5102 (30) | 5090 (30) | |
| ≥15 | 3421 (16) | 6357 (23) | 3121 (18) | 3095 (18) | |
DDP4=dipeptidyl peptidase 4; ARB=angiotensin receptor blocker; ACE-I=angiotensin converting enzyme inhibitor; NA=not applicable.
One patient received both dapagliflozin and empagliflozin at cohort entry.
Including married or living with partner in Denmark and married in Sweden.
Not included in propensity score.
Diagnosis of obesity in secondary care.
Primary outcomes
Table 2 shows the results of the primary outcome analyses. Use of SGLT2 inhibitors, as compared with GLP1 receptor agonists, was associated with an increased risk of lower limb amputation (hazard ratio 2.32, 95% confidence interval 1.37 to 3.91) and diabetic ketoacidosis (2.14, 1.01 to 4.52) but not with bone fracture (1.11, 0.93 to 1.33), acute kidney injury (0.69, 0.45 to 1.05), serious urinary tract infection (0.89, 0.67 to 1.19), venous thromboembolism (0.99, 0.71to 1.38), or acute pancreatitis (1.16, 0.64 to 2.12).
Table 2.
Primary outcome analyses of association between use of sodium glucose cotransporter 2 (SGLT2) inhibitors compared with glucagon-like peptide 1 (GLP1) receptor agonists and risk of serious adverse events
| Adverse event | SGLT2 inhibitors (n=17 213) | GLP1 receptor agonists (n=17 213) | Hazard ratio (95% CI) | Absolute risk difference (95% CI) | |||
|---|---|---|---|---|---|---|---|
| No of events | No of events per 1000 patient years | No of events | No of events per 1000 patient years | ||||
| Lower limb amputation | 40 | 2.7 | 22 | 1.1 | 2.32 (1.37 to 3.91) | 1.5 (0.4 to 3.3) | |
| Bone fracture | 228 | 15.4 | 263 | 13.9 | 1.11 (0.93 to 1.33) | 1.5 (−1.0 to 4.6) | |
| Diabetic ketoacidosis | 19 | 1.3 | 11 | 0.6 | 2.14 (1.01 to 4.52) | 0.7 (0.0 to 2.0) | |
| Acute kidney injury | 34 | 2.3 | 62 | 3.2 | 0.69 (0.45 to 1.05) | −1.0 (−1.8 to 0.2) | |
| Serious urinary tract infection | 80 | 5.4 | 114 | 6.0 | 0.89 (0.67 to 1.19) | −0.7 (−2.0 to 1.1) | |
| Venous thromboembolism | 63 | 4.2 | 79 | 4.1 | 0.99 (0.71 to 1.38) | 0.0 (−1.2 to 1.6) | |
| Acute pancreatitis | 20 | 1.3 | 23 | 1.2 | 1.16 (0.64 to 2.12) | 0.2 (−0.4 to 1.3) | |
Additional outcomes
The hazard ratio for use of SGLT2 inhibitors versus GLP1 receptor agonists was 1.55 (95% confidence interval 0.87 to 2.77) for toe or metatarsal amputation and 1.05 (0.80 to 1.39) for major osteoporotic bone fracture (supplementary materials, table 12).
Subgroup and additional analyses
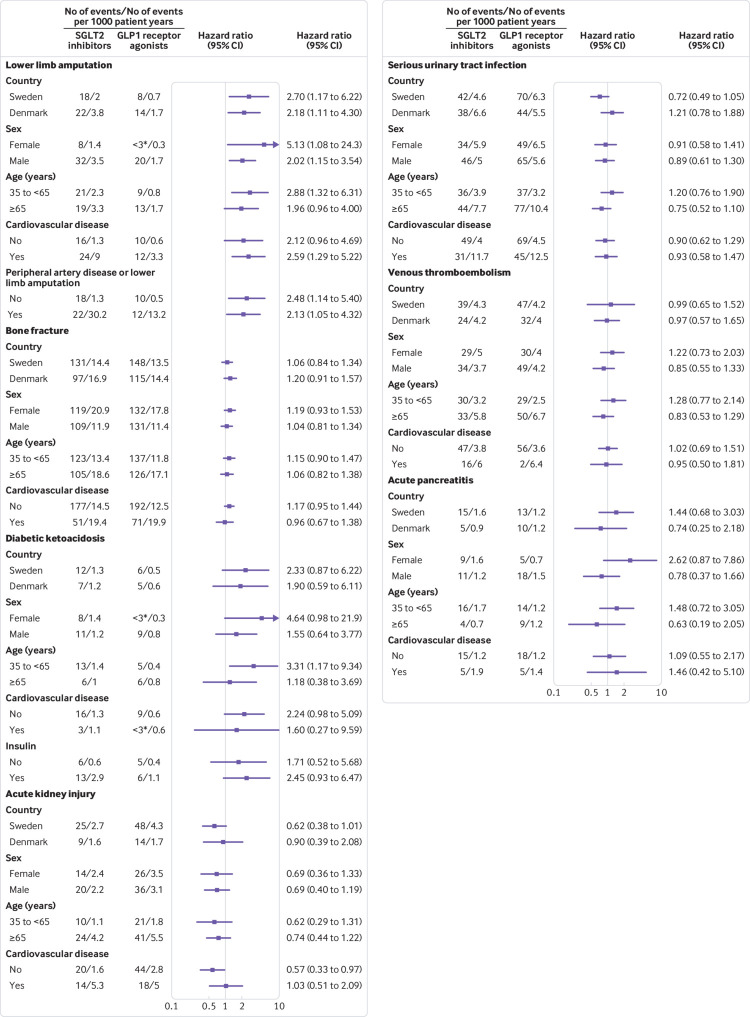
Figure 2 shows the results of the subgroup analyses. For each outcome, hazard ratios in analyses by country, sex, age group, and history of cardiovascular disease were similar to those observed in the primary analyses, although several of these subgroup analyses were based on a limited number of events. Hazard ratios were also similar in the analysis of diabetic ketoacidosis according to insulin treatment status and in the analysis of lower limb amputation according to history of peripheral artery disease or lower limb amputation. Hazard ratios in the additional analyses of diabetic ketoacidosis and acute kidney injury in which follow-up was truncated at six months after treatment initiation were similar to those in the primary analyses (supplementary materials, table 14).
Fig 2.
Subgroup analyses of serious adverse events among sodium glucose cotransporter 2 (SGLT2) inhibitor users compared with glucagon-like peptide 1 (GLP1) receptor agonist users. P values for homogeneity (all >0.05) are shown in supplementary materials, table 13. *Number of events not specified for <3 according to regulations on reporting Danish register data.
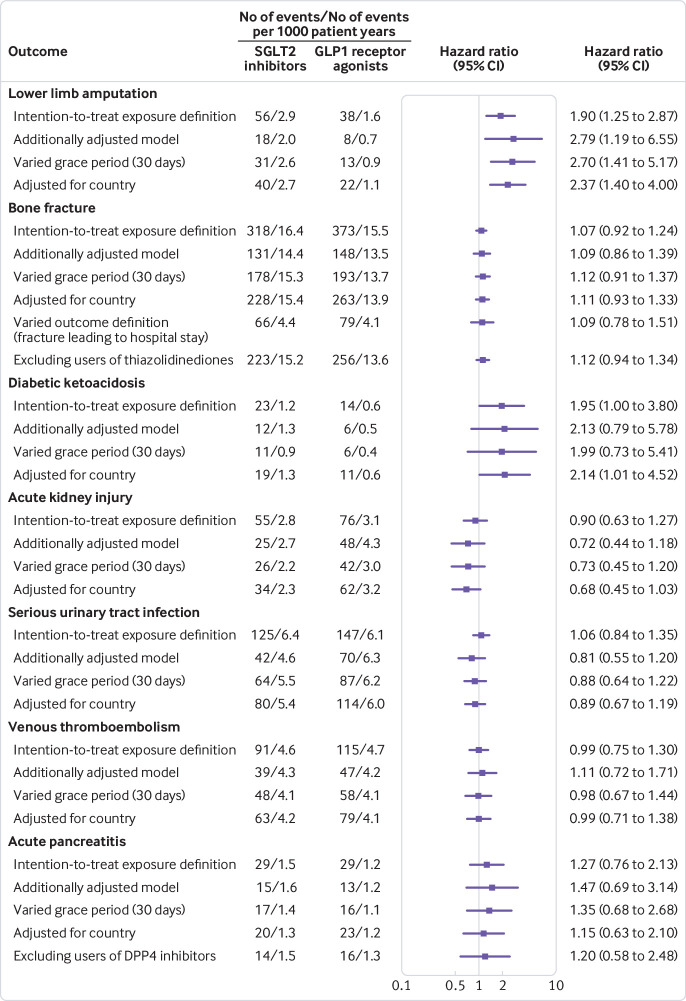
Sensitivity analyses
Figure 3 shows the results of the sensitivity analyses. Hazard ratios were similar to those in the primary analyses when using an exposure definition on intention-to-treat basis, a grace period of 30 days in the definition of current use of the study drug, and when adjusting the models for country. In the Swedish cohort (baseline characteristics in supplementary materials, table 15), additional adjustment of the models for glycated haemoglobin, blood pressure, albuminuria, estimated glomerular filtration rate, body mass index, and smoking status did not materially affect the findings. In the analysis of bone fracture, hazard ratios were not affected by the exclusion of recent users of thiazolidinediones or by the use of an outcome definition that only included fractures leading to hospital stays. In the analysis of acute pancreatitis, hazard ratios were not affected by the exclusion of recent users of DPP4 inhibitors.
Fig 3.
Sensitivity analyses of serious adverse events among sodium glucose cotransporter 2 (SGLT2) inhibitor users compared with glucagon-like peptide 1 (GLP1) receptor agonist users. DPP4=dipeptidyl peptidase 4.
Discussion
Using nationwide registers in two countries, we examined the risk of serious adverse events associated with the use of SGLT2 inhibitors. As compared with GLP1 receptor agonists, SGLT2 inhibitor use was associated with twofold increases in the risk of both lower limb amputation and diabetic ketoacidosis, but no noticeable increase in risk was observed for the other investigated adverse events. The findings should be interpreted in the context of limitations of observational studies and the uncertainty of the effect estimates. Based on the upper limit of the confidence interval, our findings are inconsistent with a relative risk increase of more than 33% for bone fracture, 5% for acute kidney injury, 19% for serious urinary tract infection, 38% for venous thromboembolism, and 112% for acute pancreatitis.
The twofold increase in risk of lower limb amputation associated with the use of SGLT2 inhibitors observed in our study is in line with the findings from the CANVAS Program, which randomised patients with high cardiovascular risk to canagliflozin or placebo.1 Importantly, our results were consistent in patients with and without cardiovascular disease and with and without peripheral arterial disease or previous amputation; the event rates, however, were substantially higher in the subgroups with such history. A pooled analysis of clinical trials of dapagliflozin was underpowered to assess lower limb amputations,30 and no imbalance in this outcome in patients receiving empagliflozin versus placebo was observed in the EMPA-REG OUTCOME trial.31 Whether the increase in lower limb amputations is a class effect for SGLT2 inhibitors, or specific to individual drugs, needs further study.
In our study, the use of SGLT2 inhibitors was not associated with the risk of bone fracture. Increased risk of fractures in patients using canagliflozin was observed in the CANVAS Program but not in the other trials of canagliflozin.1 32 A meta-analysis of clinical trials revealed no imbalance in fracture rates between those treated with empagliflozin versus placebo.33 Falls owing to volume depletion has been suggested as an underlying mechanism for fractures during use of SGLT2 inhibitors, but it has also been suggested that the drugs affect bone metabolism, as indicated by decreased hip bone mineral density after treatment with canagliflozin.34 However, in analyses restricted to osteoporotic fractures, our results remained similar.
The twofold increased risk of diabetic ketoacidosis observed in our study is in accordance with an analysis of insurance claims in the US, which showed an increase in risk that was of similar magnitude, comparing SGLT2 inhibitors with DPP4 inhibitors.14 Analyses of FEARS data showed higher spontaneous reporting rates of diabetic ketoacidosis in SGLT2 inhibitor users than in DPP4 inhibitor users,3 and rates of this adverse event were numerically higher among those receiving active treatment in the CANVAS Program (0.6 v 0.3 events per 1000 person years),1 and in a meta-analysis of clinical trials of SGLT2 inhibitors (odds ratio 1.96, 95% confidence interval 0.77 to 4.98).35 In addition, higher rates of diabetic ketoacidosis have been observed among those receiving SGLT2 inhibitors in trials that include patients with type 1 diabetes.36 37 Viewed together, a relatively coherent picture of an increased risk of diabetic ketoacidosis associated with the use of SGLT2 inhibitors has emerged, although the absolute risk increase seems small.
Reports to FAERS and trials showing a transient decrease in glomerular filtration rate after initiation of SGLT2 inhibitors have given rise to the concern that SGLT2 inhibitors might lead to acute kidney injury.4 5 30 38 In our study, we found no such association; these findings are in line with those from meta-analyses of trials of SGLT2 inhibitors,30 33 35 the EMPA-REG OUTCOME trial,38 and the CANVAS Program where rates of acute kidney injury were similar or numerically lower among patients randomized to SGLT2 inhibitors.1
We found no association between the use of SGLT2 inhibitors and the risk of serious urinary tract infection. Whereas reports to FAERS and an imbalance in the proportion of patients experiencing urosepsis in the EMPA-REG OUTCOME trial (0.4% empagliflozin v 0.1% placebo) indicated that SGLT2 inhibitors can lead to serious urinary tract infections,2 18 such associations were not observed in the CANVAS Program and in a meta-analysis of empagliflozin trials.1 33
We found no increase in the risk of venous thromboembolism associated with the use of SGLT2 inhibitors. It has been speculated that SGLT2 inhibitors might increase the risk of venous thromboembolism by increasing blood viscosity.6 7 A meta-analysis of clinical trials, including data from regulatory submissions, showed a hazard ratio of 1.54 (95% confidence interval 0.63 to 3.79) for venous thromboembolism in patients receiving active treatment versus placebo,6 but there was no imbalance in event rates in the subsequently presented CANVAS Program or in meta-analyses of SGLT2 inhibitor trials.1 30 33 35
Reports to FAERS have prompted the FDA to investigate the risk of acute pancreatitis associated with use of SGLT2 inhibitors.8 9 10 Clinical trials have been too small to assess this potential adverse event. We found no association between the use of SGLT2 inhibitors and acute pancreatitis.
Strengths and weaknesses in relation to other studies
Using nationwide registers to include data from a large number of patients seen in routine clinical practice and implementing a controlled study design that sought to limit the risk of different sources of bias, our study substantially expands on current knowledge regarding the safety of SGLT2 inhibitors and quantifies the risk of serious adverse events potentially linked to the drug class. To date, four controlled large scale observational studies, each investigating one selected outcome (lower limb amputation and diabetic ketoacidosis),11 12 13 14 have been conducted on SGLT2 inhibitors and potential adverse events. Three of these studies used a design that permitted patients with a history of comparator drug use to enter the cohort.11 12 13 Therefore, the studies could suffer from compromised confounding control,16 as indicated by the imbalance in diabetic drugs at baseline between users of SGLT2 inhibitors versus comparators, even after propensity score matching. In addition, the implementation of a hierarchical selection of SGLT2 inhibitor treatment episodes in those studies, gives rise to immortal time bias in patients initiating an SGLT2 inhibitor after having used a comparator drug during the study period.11 12 13 15 Further, lag time bias could result from the use of any other glucose lowering drug as comparator,15 because some of these drugs are typically used at earlier or later stages of diabetes than SGLT2 inhibitors.19
We attempted to limit the risk of confounding by using a new-user design in which patients had no history of either study drug at cohort entry, a non-parsimonious propensity score and rigorous matching, as well as GLP1 receptor agonists as the comparator. In sensitivity analyses in Sweden (61% of the overall cohort), we also adjusted for glycated haemoglobin level, blood pressure, albuminuria, estimated glomerular filtration rate, body mass index, and smoking status; the consistency of results between these analyses and our main analyses indicate that confounding owing to these variables was minimal, although some of these analyses had few events for each variable used for multivariate adjustment.
Limitations
Our study has limitations. Firstly, we performed analyses for SGLT2 inhibitors as a drug class; dapagliflozin (61%) and empagliflozin (38%) were the most common SGLT2 inhibitors in our study population and use of canagliflozin was rare. The mechanisms underlying the potential adverse events associated with SGLT2 inhibitors are unknown. Class-wide effects, including volume depletion (lower limb amputation,39 bone fracture,32 diabetic ketoacidosis,40 acute kidney injury,41 and venous thromboembolism),6 increased levels of phosphate and secondary hyperparathyroidism (bone fracture),42 non-insulin dependent glucose lowering and increased glucagon levels (diabetic ketoacidosis),40 uricosuria (acute kidney injury),41 and glucosuria (serious urinary tract infection) have been suggested.43 However, pharmacologic properties and off-target effects specific to individual drugs have been described and reduced hip bone mineral density has been observed after treatment with canagliflozin.34 44 45 Uncertainty regarding the potential effects specific to individual SGLT2 inhibitors remains and assessment of adverse events for individual SGLT2 inhibitors remains a topic for additional examination.
Secondly, the number of events in many of the subgroup analyses were low, resulting in wide confidence intervals. More well powered analyses will require further accumulation of clinical data on the use of SGLT2 inhibitors.
Thirdly, we defined drug exposure based on filled prescriptions; low adherence might bias the results towards the null.
Fourthly, although diagnoses recorded in Scandinavian health registers generally have high sensitivity and positive predictive value,25 26 validation studies of the outcomes in our study have only been performed for venous thromboembolism, acute pancreatitis, and certain fractures, infections, and amputations.25 26 46 47 Outcome misclassification could have introduced bias in our analyses; such misclassification is unlikely to be differential in the context of this study design and would thus only introduce bias, if any, towards the null. A study from the US showed that diagnostic codes of acute kidney injury in administrative databases have high specificity although registration of events could be incomplete48; the event rates observed in our study should be interpreted with caution.
Finally, residual and unmeasured confounding affecting the findings in our study cannot be ruled out.
Conclusion
In this analysis of nationwide registers from two countries, SGLT2 inhibitors, as compared with GLP1 receptor agonists, were associated with an increased risk of lower limb amputations and diabetic ketoacidosis, but not with other serious adverse events of current concern.
What is already known on this topic
Sodium glucose cotransporter 2 (SGLT2) inhibitors are increasingly popular drugs for the treatment of type 2 diabetes
Data from clinical trials, case reports, and observational studies have indicated that use of this drug class could be associated with serious adverse events, including lower limb amputation, bone fracture, diabetic ketoacidosis, acute kidney injury, serious urinary tract infection, venous thromboembolism, and acute pancreatitis
What this study adds
The use of SGLT2 inhibitors is associated with twofold increases in the risk of lower limb amputation and diabetic ketoacidosis
Web Extra.
Extra material supplied by the author
Supplementary materials: Sources of data, tables 1-16, and figure 1
Contributors: PU, HS, and BP initiated the study and had access to all the data for this project. HS performed the statistical analysis. PU and BP wrote the first draft of the paper. All authors contributed to the acquisition, analysis, or interpretation of data and to the critical revision of the manuscript for important intellectual content. PU, HS, and BP had full access to the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. BP supervised the study and is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The study was supported by the Swedish Heart-Lung Foundation, the Swedish Cancer Society, the Nordic Cancer Union, the Novo Nordisk Foundation, and the Swedish Society for Medical Research. BP was supported by an investigator grant from the Strategic Research Area Epidemiology program at Karolinska Institutet. HS was supported by a career development investigator grant from Lundbeck Foundation. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and have the following declarations: CJ reports personal fees from Pfizer and Bayer outside the submitted work; BE reports personal fees from Amgen, AstraZeneca, Boerhringer Ingelheim, Eli Lilly, Merck Sharp and Dohme, Mundipharma, Navamedic, Novo Nordisk, and RLS Global outside the submitted work, and grants from Sanofi outside the submitted work; and SG reports personal fees and research grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp and Dohme, Novo Nordisk, and Sanofi outside of the submitted work. The other authors did not have any potential conflicts to report.
Ethical approval: The study was approved by the Regional Ethics Committee in Stockholm, Sweden, and the Danish Data Protection Agency. In Denmark, ethics approval is not required for register based research. Informed consent was not required.
Data sharing: No additional data are available.
The lead author (PU) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1. Neal B, Perkovic V, Mahaffey KW, et al. CANVAS Program Collaborative Group Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017;377:644-57. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM475487.pdf.
- 3. Blau JE, Tella SH, Taylor SI, Rother KI. Ketoacidosis associated with SGLT2 inhibitor treatment: Analysis of FAERS data. Diabetes Metab Res Rev 2017;33:e2924. 10.1002/dmrr.2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). https://www.fda.gov/downloads/Drugs/DrugSafety/UCM506772.pdf
- 5. Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: Analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis 2017;27:1108-13. 10.1016/j.numecd.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 6. Wu JHY, Foote C, Blomster J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2016;4:411-9. 10.1016/S2213-8587(16)00052-8 [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. FDA Adverse Events Reporting System (FAERS) - Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS) between April – June 2015. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm484292.htm
- 8.U.S. Food and Drug Administration. FDA Adverse Events Reporting System (FAERS) - Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS) April - June 2016. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/surveillance/adversedrugeffects/ucm523358.htm
- 9. Chowdhary M, Kabbani AA, Chhabra A. Canagliflozin-induced pancreatitis: a rare side effect of a new drug. Ther Clin Risk Manag 2015;11:991-4. 10.2147/TCRM.S86641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verma R. Canagliflozin-Associated Acute Pancreatitis. Am J Ther 2016;23:e972-3. 10.1097/MJT.0000000000000135 [DOI] [PubMed] [Google Scholar]
- 11. Udell JA, Yuan Z, Rush T, Sicignano NM, Galitz M, Rosenthal N. Cardiovascular Outcomes and Risks After Initiation of a Sodium Glucose Cotransporter 2 Inhibitor: Results From the EASEL Population-Based Cohort Study (Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World). Circulation 2018;137:1450-9. 10.1161/CIRCULATIONAHA.117.031227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yuan Z, DeFalco FJ, Ryan PB, et al. Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium-glucose co-transporter-2 inhibitors in the USA: A retrospective cohort study. Diabetes Obes Metab 2018;20:582-9. 10.1111/dom.13115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Desai M, Ryan PB, et al. Incidence of diabetic ketoacidosis among patients with type 2 diabetes mellitus treated with SGLT2 inhibitors and other antihyperglycemic agents. Diabetes Res Clin Pract 2017;128:83-90. 10.1016/j.diabres.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 14. Fralick M, Schneeweiss S, Patorno E. Risk of Diabetic Ketoacidosis after Initiation of an SGLT2 Inhibitor. N Engl J Med 2017;376:2300-2. 10.1056/NEJMc1701990 [DOI] [PubMed] [Google Scholar]
- 15. Suissa S. Lower Risk of Death With SGLT2 Inhibitors in Observational Studies: Real or Bias? Diabetes Care 2018;41:6-10. 10.2337/dc17-1223 [DOI] [PubMed] [Google Scholar]
- 16. Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2015;2:221-8. 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marso SP, Daniels GH, Brown-Frandsen K, et al. LEADER Steering Committee. LEADER Trial Investigators Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016;375:311-22. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015;373:2117-28. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018 . Diabetes Care 2018;41(Suppl 1):S73-85. 10.2337/dc18-S008 [DOI] [PubMed] [Google Scholar]
- 20.MEDSTAT.DK. The Danish Health Data Authority. www.medstat.dk
- 21.Statistics database: medications. Swedish National Board of Health and Welfare. [Internet]. Available from: http://www.socialstyrelsen.se/statistik/statistikdatabas/lakemedel.
- 22. D’Agostino RB, Rubin DB. Estimating and Using Propensity Scores with Partially Missing Data. J Am Stat Assoc 2000;95:749-59 10.1080/01621459.2000.10474263. [DOI] [Google Scholar]
- 23. Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J 2009;51:171-84. 10.1002/bimj.200810488 [DOI] [PubMed] [Google Scholar]
- 24. Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf 2012;21(Suppl 2):69-80. 10.1002/pds.3263 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449-90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harel O, Zhou X-H. Multiple imputation: review of theory, implementation and software. Stat Med 2007;26:3057-77. 10.1002/sim.2787 [DOI] [PubMed] [Google Scholar]
- 28. Zhu Z-N, Jiang Y-F, Ding T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone 2014;68:115-23. 10.1016/j.bone.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 29. Tkáč I, Raz I. Combined Analysis of Three Large Interventional Trials With Gliptins Indicates Increased Incidence of Acute Pancreatitis in Patients With Type 2 Diabetes. Diabetes Care 2017;40:284-6. 10.2337/dc15-1707 [DOI] [PubMed] [Google Scholar]
- 30. Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: A pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab 2018;20:620-8. 10.1111/dom.13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and Assessment of Lower-Limb Amputations in the EMPA-REG OUTCOME Trial. Diabetes Care 2018;41:e4-5. 10.2337/dc17-1551 [DOI] [PubMed] [Google Scholar]
- 32. Watts NB, Bilezikian JP, Usiskin K, et al. Effects of Canagliflozin on Fracture Risk in Patients With Type 2 Diabetes Mellitus. J Clin Endocrinol Metab 2016;101:157-66. 10.1210/jc.2015-3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kohler S, Zeller C, Iliev H, Kaspers S. Safety and Tolerability of Empagliflozin in Patients with Type 2 Diabetes: Pooled Analysis of Phase I-III Clinical Trials. Adv Ther 2017;34:1707-26. 10.1007/s12325-017-0573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of Bone Mineral Density and Bone Biomarkers in Patients With Type 2 Diabetes Treated With Canagliflozin. J Clin Endocrinol Metab 2016;101:44-51. 10.1210/jc.2015-1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang X-L, Zhu Q-Q, Chen YH, et al. Cardiovascular Safety, Long-Term Noncardiovascular Safety, and Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors in Patients With Type 2 Diabetes Mellitus: A Systemic Review and Meta-Analysis With Trial Sequential Analysis. J Am Heart Assoc 2018;7:e007165. 10.1161/JAHA.117.007165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garg SK, Henry RR, Banks P, et al. Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N Engl J Med 2017;377:2337-48. 10.1056/NEJMoa1708337 [DOI] [PubMed] [Google Scholar]
- 37. Umpierrez GE. Diabetes: SGLT2 inhibitors and diabetic ketoacidosis - a growing concern. Nat Rev Endocrinol 2017;13:441-2. 10.1038/nrendo.2017.77 [DOI] [PubMed] [Google Scholar]
- 38. Wanner C, Inzucchi SE, Lachin JM, et al. EMPA-REG OUTCOME Investigators Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 2016;375:323-34. 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 39. Fadini GP, Avogaro A. SGLT2 inhibitors and amputations in the US FDA Adverse Event Reporting System. Lancet Diabetes Endocrinol 2017;5:680-1. 10.1016/S2213-8587(17)30257-7 [DOI] [PubMed] [Google Scholar]
- 40. Handelsman Y, Henry RR, Bloomgarden ZT, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement on the Association of Sglt-2 Inhibitors and Diabetic Ketoacidosis. Endocr Pract 2016;22:753-62. 10.4158/EP161292.PS [DOI] [PubMed] [Google Scholar]
- 41. Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol 2016;12:711-2. 10.1038/nrneph.2016.159 [DOI] [PubMed] [Google Scholar]
- 42. Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 2015;3:8-10. 10.1016/S2213-8587(14)70227-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract 2014;103:373-81. 10.1016/j.diabres.2013.12.052 [DOI] [PubMed] [Google Scholar]
- 44. Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J Pharmacol Sci 2016;130:159-69. 10.1016/j.jphs.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 45. Ohgaki R, Wei L, Yamada K, et al. Interaction of the Sodium/Glucose Cotransporter (SGLT) 2 inhibitor Canagliflozin with SGLT1 and SGLT2. J Pharmacol Exp Ther 2016;358:94-102. 10.1124/jpet.116.232025 [DOI] [PubMed] [Google Scholar]
- 46. Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open 2016;6:e012832. 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bruun C, Siersma V, Guassora AD, Holstein P, de Fine Olivarius N. Amputations and foot ulcers in patients newly diagnosed with type 2 diabetes mellitus and observed for 19 years. The role of age, gender and co-morbidity. Diabet Med 2013;30:964-72. 10.1111/dme.12196 [DOI] [PubMed] [Google Scholar]
- 48. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014;9:682-9. 10.2215/CJN.07650713 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials: Sources of data, tables 1-16, and figure 1