Key Points
Question
Are trends in gestational age–specific neonatal mortality distinct for spontaneous and clinician-initiated deliveries?
Findings
In this cohort analysis of 22 million births, the proportion among spontaneous deliveries of births at 20 to 27, 28 to 31, 32 to 33, 34 to 36, and 37 to 38 weeks decreased and neonatal mortality rates decreased at 20 to 27 weeks (adjusted annual decline, 1%) and 28 to 31 weeks (adjusted annual decline, 6%). Among clinician-initiated deliveries, the proportion of births at 39 to 40 weeks increased; however, mortality rates decreased at 39 to 40 weeks by 1%.
Meaning
The decline in neonatal mortality rates among spontaneous deliveries occurred at 20 to 27 and 28 to 31 weeks; mortality rates among clinician-initiated deliveries declined at 39 to 40 weeks.
Abstract
Importance
Preterm and postterm deliveries have declined since 2005 in the United States, but the association between these changes and neonatal mortality remains unknown.
Objective
To estimate changes in the gestational age distribution among spontaneous and clinician-initiated deliveries between 2006 and 2013 and associated changes in neonatal mortality.
Design, Setting, and Participants
A retrospective cohort analysis was conducted of 22 million singleton live births without major malformations in the United States from 2006 to 2013. Data analysis was performed from August to October 2017.
Main Outcomes and Measures
Changes in gestational age distribution among spontaneous and clinician-initiated deliveries at extremely preterm (20-27 weeks), very preterm (28-31 weeks), moderately preterm (32-33 weeks), late preterm (34-36 weeks), early term (37-38 weeks), term (39-40), late term (41 weeks), and postterm (42-44 weeks) gestations and changes in neonatal mortality rates at less than 28 days between 2006 and 2013. These changes were estimated from log-linear Poisson regression models with robust variance, adjusted for confounders.
Results
Among 22 million births, 12 493 531 (56.7%) were spontaneous and 9 557 815 (43.3%) were clinician-initiated deliveries. Among spontaneous deliveries, the proportion of births at 20 to 27, 28 to 31, 32 to 33, 34 to 36, and 37 to 38 weeks declined. Among clinician-initiated deliveries, the proportion of births at 34 to 36 and 37 to 38 weeks declined and the proportion at 39 to 40 weeks increased. Among spontaneous deliveries, overall neonatal mortality rates declined from 1.8 to 1.3 per 1000 live births, mainly at 20 to 27 weeks (adjusted annual decline, 1%; 95% CI, −2% to −1%) and 28 to 31 weeks (adjusted annual decline, 6%; 95% CI, −8% to −5%). Among clinician-initiated deliveries, overall mortality rates remained unchanged (2.1 to 2.2 per 1000 live births). However, mortality rates declined (0.6 to 0.5 per 1000 live births) at 39 to 40 weeks by 1% (95% CI, −3% to −0.4%) annually, adjusted for confounders.
Conclusions and Relevance
In the United States, there was a decline in spontaneous deliveries associated with an overall decline in neonatal mortality. Although clinician-initiated deliveries increased at 39 to 40 weeks, neonatal mortality at that gestation declined.
This cohort analysis evaluates data from 22 million US singleton births to compare changes in the neonatal mortality rate between spontaneous and clinician-initiated deliveries from 2006 to 2013.
Introduction
Globally, a third of all preterm births each year are implicated in 1.2 million neonatal deaths.1,2,3 The economic burden of preterm birth is estimated at $26 billion in the United States alone.4 Efforts to determine the probability of and prevent preterm deliveries have met with mixed success.5 Preterm birth is associated with increased perinatal mortality and morbidity6,7,8 and neurodevelopmental deficits.9,10,11 A recent study showed that, among singleton births in the United States, births at late preterm (34-36 weeks), early term (38-39 weeks), and postterm (42-44 weeks) gestations declined between 2007 and 2015.12 However, perinatal mortality rates at these gestations increased by 1% to 4% annually. In contrast, births at term gestations (39-40 weeks) increased, but mortality rates at this gestation decreased by 1.5%. Whether these changes in gestational age differ for spontaneous and clinician-initiated deliveries remains uncertain.
Since 2005, a decline in both spontaneous and clinician-initiated deliveries of similar magnitudes in the United States has been reported.13,14,15,16,17 The administration of progesterone therapy to women with a history of spontaneous preterm birth18,19 and the reduction in elective deliveries at less than 39 weeks20 may have led to the decline in the number of deliveries at preterm gestations. We hypothesized that these reductions in the rates of preterm and early-term births in combination with advances in neonatal care are likely associated with the decline in neonatal mortality rates. Clinician-initiated deliveries are driven by the burden of impending maternal-fetal compromise,21 such as ischemic placental disease.22,23,24 Infants born of clinician-initiated deliveries at preterm gestations are at twice the risk of neonatal mortality or morbidity compared with spontaneous preterm deliveries.17,25,26 The recommendation of the American Congress of Obstetricians and Gynecologists to postpone nonindicated deliveries to 39 weeks20 may have resulted in a shift of births to 39 weeks or more, with an increase in spontaneous deliveries at late term and postterm gestations. Given the seemingly different pathophysiologic processes of spontaneous and clinician-initiated deliveries, it remains important to examine these distinct groups separately.
We designed this study to evaluate changes in the gestational age distribution among spontaneous and clinician-initiated deliveries between 2006 and 2013 and assess gestational age–specific trends in neonatal mortality rates among spontaneous and clinician-initiated deliveries.
Methods
Study Design and Data Sources
We undertook a retrospective cohort analysis of US singleton births without major malformations between 2006 and 2013. The data include live births linked to corresponding infant deaths (period-linked files) for over 99% of all births compiled by the National Center for Health Statistics. All deaths within the first year occurring in a given data year linked to their corresponding birth certificates composed the numerator file and whether the birth occurred in that year or the previous year. These data are collected by an attendant at delivery and transferred to the National Center for Health Statistics where they are cleaned, compiled, and deidentified. The study was restricted to births in states that used the 2003 revision of the birth certificate, first implemented in 2004. The study population included women who delivered nonmalformed, singleton live births between 20 and 44 weeks’ gestation. We analyzed births starting from 2006 because data through 2005 were based on uptake of the revised certificate in only a few states. Data analysis was performed from August to October 2017. The data used in this study are publicly available as fully deidentified, and ethics approval was deemed exempt under the human subjects research by the institutional review board of Columbia University.
The revised birth certificate was incorporated gradually by 19 states in 2006 (49% of births), 22 in 2007 (53% of births), 27 in 2008 (65% of births), 28 in 2009 (66% of births), 33 in 2010 (76% of births), 36 in 2011 (83% of births), and 38 in 2012 (86% of births). In 2013, 41 states and the District of Columbia (90% of births) used the revised birth certificate.
Gestational Age
Gestational age (completed weeks) was derived from the best obstetrical estimate of the infant’s gestation for births in 2007-2013 or a combined gestational age based on the best obstetrical estimate or a clinical estimate for births in 2006.27 The National Center for Health Statistics showed that the clinical estimate and the obstetrical estimate of gestational age were comparable.28 We analyzed the distribution of gestational age at birth according to previously proposed categories29: extremely preterm (20-27 weeks), very preterm (28-31 weeks), moderately preterm (32-33 weeks), late preterm (34-36 weeks), early term (37-38 weeks), term (39-40), late term (41 weeks), and postterm (42-44 weeks). We further categorized delivery type as spontaneous or clinician initiated. We defined clinician-initiated deliveries as women who underwent labor induction or a prelabor cesarean birth based on a validated algorithm.30 After assigning deliveries to the clinician-initiated group, the remaining deliveries were assigned to the spontaneous delivery group.
Neonatal Mortality
Neonatal mortality was defined to include live-born infants who died within the first 28 days. Mortality rates were expressed per 1000 live births.
Statistical Analysis
All analyses were performed separately for spontaneous or clinician-initiated deliveries. We examined 2 primary outcomes. First, we determined changes in birth rates at 20 to 27, 28 to 31, 32 to 33, 34 to 36, 37 to 38, 39 to 40, 41, and 42 to 44 weeks between 2006 and 2013; and second, we assessed temporal changes in neonatal mortality rates between 2006 and 2013 within gestational age groups.
Trends in neonatal mortality were examined in 2 ways. First, we looked at annual change in mortality rates between 2006 and 2013 and secondly as trends over the entire 8-year period. Annual changes were assessed by including the period variable denoting the year of delivery in the regression model as a single term. The assumption of linear changes in mortality rates was tested by modeling the period variable using restricted cubic spline transformation.31 The importance of a nonlinear pattern was assessed based on the likelihood ratio test by comparing 2 nested models32: 1 with the restricted cubic spline and the linear term with that of a model with a single linear term. Changes in the mortality rate over 8 years were assessed by including the period variable as 7 indicators, with 2006 as the reference, in the regression model. From this model, we extracted the regression coefficient for the 2013 period.
Temporal changes in the distribution of births and neonatal mortality rates among spontaneous or clinician-initiated deliveries were evaluated by fitting log-linear Poisson models with robust variance structure. From these models, we estimated the rate ratio as the measure of association and converted the rate ratios for the period variable to percentage change in the proportion of births over the 8-year period. We then examined changes in neonatal mortality within strata of gestational age. We first estimated the crude changes in mortality rates between 2006 and 2013 within gestational age groups and then adjusted this model for confounders.
Potential confounders considered were maternal age (<20, 20-24, 25-29, 30-34, 35-39, and ≥40 years), primiparity, maternal race/ethnicity (self-reported as non-Hispanic white, non-Hispanic black, Hispanic, and other), maternal educational level (highest level of education obtained, categorized as ≤8th grade; 9th-12th grade [high school]; associate degree, some college, or bachelor’s degree [some college]; and master’s or doctoral degree [beyond college]), and single marital status. Because race/ethnicity is associated with preterm birth,33 we adjusted for this variable in all analyses in addition to others listed.
Missing Data
Maternal educational level was missing in 255 304 (1.2%) and labor induction in 945 (<0.01%) pregnancies. These variables were analyzed 3 ways: (1) by assigning the missing data to the reference level of the covariate (ie, beyond college for educational level and labor that was not induced), (2) by assigning missing data as a separate level in the covariates and (3) complete case analysis. Because all 3 analyses produced identical results, we report the results of the complete case analysis.
Maternal smoking, a potential confounder, was missing in 2 480 859 pregnancies (11.3%). We imputed smoking using the monotone logistic regression method based on other sociodemographic covariates by creating 50 imputed data sets after 25 burn-in iterations.34 Once analysis of the 50 imputed data sets was completed, the results were combined. Findings were considered statistically significant at a 2-tailed P value <.05. Data analysis was conducted using SAS, version 9.4 (SAS Institute Inc).
Sensitivity Analysis
We examined maternal characteristics and changes in birth rates between 2007 and 2013 restricted to the 22 states that provided data consistently for all years. However, we were able to examine only changes in maternal characteristics between 2007 (the first year of data available to us) and 2013, as well as changes in birth rates over this period. Because we lacked data on neonatal deaths in the state-identified data, we were unable to examine trends in neonatal mortality.
Results
Of the 23 622 267 births between 2006 and 2013 based on the 2003 revision of birth certificates, we excluded 1 570 921 births for various criteria, leaving 22 051 346 nonmalformed singleton live births delivered at 20 to 44 weeks (eFigure in the Supplement). Newborns with diagnoses of anencephaly, spina bifida, cyanotic congenital heart disease, congenital diaphragmatic hernia, omphalocele, gastroschisis, limb reduction defect, cleft lip with or without cleft palate, or Down syndrome or other chromosomal disorders were excluded. There were 12 493 531 (56.7%) spontaneous deliveries and 9 557 815 (43.3%) clinician-initiated deliveries.
The distribution of maternal characteristics and changes in characteristics between 2006 and 2013 is shown in eTable 1 in the Supplement. Between 2006 and 2013, the proportion of pregnancies in women younger than 20 years declined by 30.2% (from 11.1% to 7.1%; 95% CI, −30.6 to −29.8), whereas the proportion of pregnancies among women 40 years or older increased by 20.5% (from 2.3% to 2.9%; 95% CI, 19.1 to 21.9). Rates of smoking changed by −35.3% (from 13.1% to 8.5%; 95% CI, −35.6% to −35.2%). Prevalence rates of hypertensive disease and diabetes increased over the study period, and labor induction rates declined.
Changes in Spontaneous and Clinician-Initiated Deliveries
The distributions of gestational age for spontaneous births between 2006 and 2013 are reported in Table 1 and Figure 1A, and the corresponding relative changes since 2006 are shown in Figure 1B. The proportion of births declined at 20 to 27 weeks from 0.7% to 0.6% (relative decline, 18%), 28 to 31 weeks from 0.8% to 0.6% (relative decline, 19%), 32 to 33 weeks from 1.0% to 0.8% (relative decline, 19%), 34 to 36 weeks from 7.4% to 6.0% (relative decline, 19%), and 37 to 38 weeks from 29.4% to 27.0% (relative decline, 8%). In contrast, the proportion of spontaneous births increased at 39 to 40 weeks from 54.4% to 58.6% (relative increase, 8%) and at 41 weeks from 5.9% to 6.0% (relative increase, 2%). Among clinician-initiated deliveries, the proportion of births declined at 34 to 36 weeks from 6.0% to 5.2% (relative decline, 12%) and at 37 to 38 weeks from 33.3% to 20.9% (relative decline, 37%) and increased at 39 to 40 weeks from 51.4% to 63.9% (relative increase, 24%) (Figure 2A).
Table 1. Distribution of Gestational Age Among Spontaneous and Clinician-Initiated Deliveries: US Singleton Live Births, 2006-2013.
| Gestational Age, wk | Births, No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | |
| Spontaneous Delivery | ||||||||
| 20-27 | 5413 (0.7) | 7719 (0.6) | 8841 (0.6) | 8832 (0.6) | 9987 (0.6) | 10 728 (0.6) | 10 839 (0.6) | 10 834 (0.6) |
| 28-31 | 6079 (0.8) | 8817 (0.7) | 9823 (0.7) | 9681 (0.6) | 10 733 (0.6) | 11 708 (0.6) | 12 163 (0.6) | 12 081 (0.6) |
| 32-33 | 8038 (1.0) | 11 612 (0.9) | 13 082 (0.9) | 12 892 (0.9) | 14 413 (0.9) | 15 329 (0.8) | 16 056 (0.8) | 15 966 (0.8) |
| 34-36 | 58 695 (7.4) | 85 896 (6.7) | 98 899 (6.6) | 96 575 (6.3) | 105 899 (6.3) | 114 734 (6.2) | 117 078 (6.1) | 116 307 (6.0) |
| 37-38 | 234 045 (29.4) | 366 813 (28.5) | 431 053 (28.7) | 431 037 (28.3) | 470 228 (28.1) | 508 752 (27.5) | 525 450 (27.4) | 527 207 (27.0) |
| 39-40 | 433 668 (54.4) | 718 367 (55.8) | 839 048 (55.9) | 864 927 (56.8) | 954 969 (57.1) | 1 068 341 (57.8) | 1 115 697 (58.2) | 1 143 430 (58.6) |
| 41 | 46 845 (5.9) | 79 869 (6.2) | 91 067 (6.1) | 90 737 (6.0) | 97 680 (5.8) | 109 853 (6.0) | 112 208 (5.9) | 116 772 (6.0) |
| 42-44 | 4410 (0.6) | 7985 (0.6) | 8295 (0.6) | 7739 (0.5) | 7693 (0.5) | 7769 (0.4) | 7818 (0.4) | 8010 (0.4) |
| Total | 797 193 (100) | 1 287 078 (100) | 1 500 108 (100) | 1 522 420 (100) | 1 671 602 (100) | 1 847 214 (100) | 1 917 309 (100) | 1 950 607 (100) |
| Clinician-Initiated Delivery | ||||||||
| 20-27 | 2889 (0.4) | 4440 (0.5) | 4994 (0.4) | 5232 (0.5) | 5768 (0.4) | 6361 (0.5) | 6913 (0.5) | 7045 (0.5) |
| 28-31 | 5097 (0.8) | 7768 (0.8) | 8639 (0.8) | 8914 (0.8) | 9938 (0.8) | 10 691 (0.8) | 11 014 (0.8) | 11 317 (0.8) |
| 32-33 | 5882 (0.9) | 8831 (0.9) | 9834 (0.9) | 10 039 (0.9) | 11 291 (0.9) | 12 297 (0.9) | 12 224 (0.9) | 12 933 (0.9) |
| 34-36 | 39 315 (6.0) | 58 235 (5.9) | 66 100 (5.8) | 65 603 (5.6) | 71 417 (5.5) | 74 108 (5.3) | 75 808 (5.3) | 77 035 (5.2) |
| 37-38 | 218 944 (33.3) | 318 867 (32.2) | 356 893 (31.5) | 337 054 (28.8) | 339 116 (26.1) | 328 070 (23.3) | 312 275 (21.8) | 306 942 (20.9) |
| 39-40 | 337 754 (51.4) | 516 835 (52.3) | 603 066 (53.3) | 658 450 (56.3) | 766 060 (59.1) | 869 823 (61.8) | 909 255 (63.4) | 938 557 (63.9) |
| 41 | 43 060 (6.6) | 67 521 (6.8) | 76 290 (6.7) | 78 926 (6.7) | 87 360 (6.7) | 99 651 (7.1) | 101 083 (7.0) | 108 360 (7.4) |
| 42-44 | 4188 (0.6) | 6455 (0.7) | 6700 (0.6) | 6266 (0.5) | 6375 (0.5) | 6592 (0.5) | 6308 (0.4) | 6747 (0.5) |
| Total | 657 129 (100) | 988 952 (100) | 1 132 516 (100) | 1 170 484 (100) | 1 297 325 (100) | 1 407 593 (100) | 1 434 880 (100) | 1 468 936 (100) |
Figure 1. Changes in the Gestational Age Distribution of Spontaneous Deliveries.
Changes among spontaneous deliveries (A) and neonatal mortality among spontaneous deliveries following adjustment for confounders (B): US singleton live births, 2006-2013.
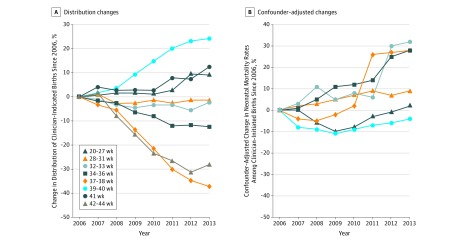
Figure 2. Changes in the Gestational Age Distribution of Clinician-Initiated Deliveries.
Changes among clinician-initiated deliveries (A) and neonatal mortality among clinician-initiated deliveries following adjustment for confounders (B): US singleton live births, 2006-2013.
Changes in Neonatal Mortality Rates Among Spontaneous and Clinician-Initiated Deliveries
Among spontaneous deliveries, neonatal mortality rates declined from 1.8 to 1.3 per 1000 live births (Table 2), resulting in an adjusted annual decline of 3% (95% CI, −4% to −2%). Adjusted relative annual changes in neonatal mortality rate (2006 as reference), among spontaneous deliveries is shown in Figure 2B. Among spontaneous deliveries, neonatal mortality rates at 20 to 27 weeks declined from 387.8 to 360.1 per 1000 live births, an adjusted annual decline by 1% (95% CI, −2% to −1%), from 32.4 to 22.3 per 1000 live births at 28 to 31 weeks, an adjusted annual decline of 6% (95% CI, −8% to −5%), and from 0.7 to 0.4 per 1000 live births at 41 weeks, an adjusted annual decline of 6% (95% CI, −10% to −1%) (Table 3). Tests to evaluate whether these annual changes were linear were generally satisfied (eTable 2 in the Supplement). Adjusted change in mortality rates over the 8-year period are given in eTable 3 in the Supplement.
Table 2. Neonatal Mortality Rates Based on Gestational Age Among Spontaneous and Clinician-Initiated Deliveries: US Singleton Live Births, 2006-2013.
| Gestational Age, wk | Neonatal Deaths, No. (Rate per 1000 Live Births) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | |
| Spontaneous Delivery | ||||||||
| 20-27 | 2099 (387.8) | 2943 (381.3) | 3464 (391.8) | 3406 (385.6) | 3770 (377.5) | 3938 (367.1) | 3926 (362.2) | 3901 (360.1) |
| 28-31 | 197 (32.4) | 267 (30.3) | 327 (33.3) | 309 (31.9) | 306 (28.5) | 286 (24.4) | 253 (20.8) | 269 (22.3) |
| 32-33 | 94 (11.7) | 137 (11.8) | 138 (10.5) | 137 (10.6) | 186 (12.9) | 162 (10.6) | 157 (9.8) | 171 (10.7) |
| 34-36 | 161 (2.7) | 260 (3.0) | 293 (3.0) | 315 (3.3) | 358 (3.4) | 373 (3.3) | 346 (3.0) | 352 (3.0) |
| 37-38 | 190 (0.8) | 290 (0.8) | 320 (0.7) | 307 (0.7) | 382 (0.8) | 368 (0.7) | 406 (0.8) | 387 (0.7) |
| 39-40 | 195 (0.4) | 308 (0.4) | 357 (0.4) | 369 (0.4) | 375 (0.4) | 440 (0.4) | 448 (0.4) | 460 (0.4) |
| 41 | 33 (0.7) | 42 (0.5) | 51 (0.6) | 49 (0.5) | 40 (0.4) | 58 (0.4) | 40 (0.5) | 50 (0.4) |
| 42-44 | 2 (0.5) | 10 (1.3) | 12 (1.4) | 9 (1.2) | 16 (2.1) | 15 (1.9) | 20 (2.6) | 9 (1.1) |
| Total | 1425 (1.8) | 2076 (1.6) | 2369 (1.6) | 2357 (1.6) | 2554 (1.5) | 2586 (1.4) | 2470 (1.3) | 2521 (1.3) |
| Clinician-Initiated Delivery | ||||||||
| 20-27 | 692 (239.5) | 1061 (239.0) | 1116 (223.5) | 1122 (214.4) | 1272 (220.5) | 1556 (244.6) | 1621 (234.5) | 1698 (241.0) |
| 28-31 | 186 (36.5) | 288 (37.1) | 311 (36.0) | 340 (38.1) | 346 (34.8) | 432 (40.4) | 422 (38.3) | 438 (38.7) |
| 32-33 | 91 (15.5) | 141 (16.0) | 184 (18.7) | 155 (15.4) | 187 (16.6) | 194 (15.8) | 240 (19.6) | 257 (19.9) |
| 34-36 | 200 (5.1) | 299 (5.1) | 351 (5.3) | 369 (5.6) | 354 (5.5) | 422 (5.7) | 473 (6.2) | 486 (6.3) |
| 37-38 | 239 (1.1) | 332 (1.0) | 370 (1.0) | 362 (1.1) | 379 (1.1) | 458 (1.4) | 432 (1.4) | 430 (1.4) |
| 39-40 | 191 (0.6) | 267 (0.5) | 329 (0.5) | 331 (0.5) | 389 (0.5) | 447 (0.5) | 460 (0.5) | 499 (0.5) |
| 41 | 21 (0.5) | 41 (0.6) | 47 (0.6) | 48 (0.6) | 41 (0.5) | 57 (0.6) | 56 (0.6) | 63 (0.6) |
| 42-44 | 4 (1.0) | 9 (1.4) | 11 (1.6) | 8 (1.3) | 13 (2.0) | 6 (0.9) | 3 (0.5) | 13 (1.9) |
| Total | 1367 (2.1) | 2013 (2.0) | 2287 (2.0) | 2283 (2.0) | 2490 (1.9) | 2905 (2.1) | 3005 (2.1) | 3196 (2.2) |
Table 3. Temporal Changes in Neonatal Mortality Rates Based on Gestational Age Among Spontaneous and Clinician-Initiated Deliveries: US Singleton Live Births, 2006-2013a.
| Gestational Age, wk | Mean Annual Change, % (95% CI)b | |||
|---|---|---|---|---|
| Spontaneous Deliveries | Clinician-Initiated Deliveries | |||
| Unadjusted | Adjustedc | Unadjusted | Adjustedc | |
| 20-27 | −1 (−2 to −1) | −1 (−2 to −1) | 1 (0.1 to 1) | 1 (0.2 to 2) |
| 28-31 | −7 (−8 to −5) | −6 (−8 to −5) | 1 (−1 to 3) | 1 (0.2 to 3) |
| 32-33 | −2 (−4 to 1) | −2 (−4 to 1) | 3 (1 to 6) | 4 (1 to 6) |
| 34-36 | 1 (−1 to 2) | 1 (−1 to 3) | 3 (2 to 5) | 4 (2 to 6) |
| 37-38 | −1 (−2 to 1) | −1 (−2 to 1) | 5 (4 to 7) | 5 (4 to 7) |
| 39-40 | −1 (−3 to 0.3) | −1 (−3 to 1) | −1 (−2 to 1) | −1 (−3 to −0.3) |
| 41 | −6 (−11 to −2) | −6 (−10 to −1) | 0 (−5 to 5) | −1 (−5 to 4)d |
| 42-44 | 9 (1 to 19) | e | 0 (−10 to 11) | e |
| Total | −3 (−4 to −3) | −3 (−4 to −2) | 1 (1 to 2) | 1 (1 to 3) |
Change in neonatal mortality rates between 2006 and 2013 were estimated from log-linear Poisson regression models with robust variance estimation.
Annual change reflects the yearly change in neonatal mortality, with the mean determined over the 8-year period.
Changes were adjusted for maternal age, primiparity, maternal race/ethnicity, educational level, single marital status, and maternal smoking (following imputation of missing data).
Changes were adjusted for maternal age, primiparity, maternal race, and maternal smoking (following imputation of missing data).
Models did not converge.
Neonatal mortality rates among clinician-initiated deliveries increased from 2.1 to 2.2 per 1000 live births (Table 2), resulting in an adjusted annual increase of 1% (95% CI, 1% to 3%). Adjusted relative annual changes in neonatal mortality (2006 as reference) among clinician-initiated deliveries is shown in Figure 2B. Mortality rates increased at 32 to 33 weeks from 15.5 to 19.9 per 1000 live births (adjusted annual increase, 4%; 95% CI, 1% to 6%), from 5.1 to 6.3 per 1000 live births at 34 to 36 weeks (adjusted annual increase, 4%; 95% CI, 2% to 6%), and from 1.1 to 1.4 per 1000 live births at 37 to 38 weeks (adjusted annual increase, 5%; 95% CI, 4% to 7%) (Table 3). However, mortality rates at 39 to 40 weeks declined from 0.6 to 0.5 per 1000 live births (adjusted annual decline, 1%; 95% CI −3% to −0.4%). Adjusted change in mortality rates over the 8-year period are also reported in Table 3. Tests to evaluate whether these annual changes were linear were generally satisfied (eTable 2 in the Supplement).
Sensitivity Analysis
Analyses restricted to the 22 states that reported data between 2007 and 2013 showed similar maternal characteristics (eTable 4 in the Supplement), as well as gestational age-specific changes (eTable 5 in the Supplement) among spontaneous and clinician-initiated deliveries compared with the primary analysis.
Discussion
This study of nonmalformed singleton live births in the United States revealed that, between 2006 and 2013, the proportion of births among spontaneous deliveries declined at 20 to 31 and 41 weeks; neonatal mortality rates also declined at these same gestational ages. Among clinician-initiated deliveries, the proportion of births declined at 32 to 40 weeks; neonatal mortality rates at 32 to 38 weeks increased, whereas the rates declined at 39 to 40 weeks. A sensitivity analysis restricted to the states that reported data in 2007 showed that the distribution of maternal characteristics remained fairly similar between the full cohort of births and the restricted cohort between 2007 and 2013. This finding suggests that the changing maternal profile across years is less likely to have affected the trends in neonatal mortality rates.
Previous studies in the United States have shown that the temporal increase in clinician-initiated preterm deliveries were largely responsible for the overall increase in all preterm births.14,17,35 Moreover, these increases were associated with a favorable reduction in neonatal mortality rates.17 The reduction in neonatal mortality is also likely associated with improvements in neonatal care. Obstetrical interventions, such as use of 17-hydroxyprogesterone caproate,18,19 vaginal progesterone,36 and cerclage,37,38 in high-risk pregnancies may partly explain the declines in spontaneous preterm deliveries.20
The distribution in gestational ages among clinician-initiated deliveries may be related to an increase in expectant management between 37 and 38 weeks, although prophylactic use of low-dose aspirin in women with ischemic placental disease39,40 may have also contributed to these increases to some extent.41 The largest decline in clinician-initiated deliveries was seen at 37 to 38 weeks, which may reflect hospital policies or quality initiatives aimed at reducing elective deliveries at less than 39 weeks.20,42,43 Furthermore, it is also likely that obstetricians may have been more selective in limiting their use of induction or prelabor cesarean delivery at 37 to 38 weeks to pregnancies with more severe indications for clinician-initiated delivery. It appears that, at 39 to 40 weeks, there has been an increase in clinician-initiated deliveries (during which mortality rates declined). These births may differ in risk profile from clinician-initiated deliveries later in gestation. For extremely and moderately preterm births, the decision for an obstetrical intervention is largely driven by maternal-fetal indications, whereas the births closer to term are likely a mix of indicated and elective deliveries.
With the exception of spontaneous deliveries at 20 to 31 and 41 weeks, mortality rates remained unaffected by declining birth rates in all other gestational age groups. It is likely that prenatal interventions—by prolonging gestational age—coupled with improved neonatal care may have resulted in lower mortality rates. However, mortality rates among clinician-initiated deliveries, increased slightly at 32 to 33 and 34 to 36 weeks, more dramatically at 37 to 38 weeks, and substantially at 39 to 40 weeks. The increase in neonatal mortality at 37 to 38 weeks may have been an unintended consequence of the recommendation to postpone elective deliveries prior to 39 weeks.20 It seems plausible that early-term deliveries that are successfully delayed to 39 weeks or later may have fewer, less severe indications for obstetrical intervention, therefore representing more truly elective interventions. If this supposition were true, the resulting 37- to 38-week birth group would comprise increasingly higher-risk infants over time as the trend toward delaying to 39 weeks or more became more widespread. Furthermore, stillbirths at less than 39 weeks may increase when facilities implement hard-stop rules for clinician-initiated delivery,44 a finding that is concordant with trends in neonatal mortality seen at less than 39 weeks.
Strengths and Limitations
The classification of births based on the precipitating process leading to delivery (spontaneous and clinician initiated) provide insights. Gestational age was based on the best obstetrical estimate, a method that portends high sensitivity and specificity, as well as predictive values of 98% for defining preterm delivery.45 The assignment of births to spontaneous or clinician-initiated groups was based on a recently developed algorithm that has undergone external validation.30 Although population changes in gestational age at delivery have been the topic of previous investigations,46,47 we are unaware of studies that have evaluated the extent to which such shifts in spontaneous and clinician-initiated deliveries may have affected trends in neonatal mortality in recent years in the United States.
This study also has some limitations. First, the potential for misclassification of some variables on the vital records remains, as well as the obstetric estimate of gestation.48 In addition, there is the potential for misclassification of major birth defects.49 Second, the associations for mortality among clinician-initiated deliveries being likely affected by bias due to confounding by indication remains; such a bias may have attenuated the effect sizes.23 The algorithm to distinguish spontaneous from clinician-initiated deliveries was based on data ascertained from a few hospitals,30 so it remains unclear whether this method is broadly generalizable across the United States. Third, a small proportion of women may have had more than 1 pregnancy during the study period, but because the data do not identify women with more than 1 pregnancy, we were unable to adjust for this clustering. Although inclusion of multiple pregnancies will not affect the effect estimates, the 95% CI estimates will be biased. Fourth, artifacts owing to the birth registration at the borderline of viability,50,51 as well as shift across time where stillbirths are now more likely coded as periviable gestations, may have affected the trends.
Conclusions
Among singleton births in the United States, spontaneous deliveries at 20 to 31 and 41 weeks' gestation declined between 2006 and 2013, and neonatal mortality rates declined at these gestations. Despite a decline in clinician-initiated deliveries at 32 to 38 weeks, mortality rates at these gestations increased. However, mortality rates at 39 to 40 weeks—the gestational time during which the proportion of births increased—among clinician-initiated deliveries declined. An examination of how these trends differ based on the changing landscape of births by race52 and socioeconomic disparities that underlie preterm births53 remains important.
eFigure. Births Included
eTable 1. Distribution of Maternal Characteristics Across all Years, as Well as in 2006 and 2013: United States Singleton Live Births
eTable 2. Assessment of the Linearity Assumption in the Evaluation of Average Annual Change in Neonatal Mortality Rates Between 2006 and 2013
eTable 3. Temporal Changes in Neonatal Mortality Rates Over the 8-Year Period Based on Gestational Age Among Spontaneous and Clinician-Initiated Deliveries: United States Singleton Live Births, 2006-2013
eTable 4. Distribution of Maternal Characteristics Across All Years, as Well as in 2007 and 2013: United States Singleton Live Births
eTable 5. Distribution of Gestational Age Among Spontaneous and Clinician-Initiated Deliveries: United States Singleton Live Births, 2007-2013
References
- 1.Chang HH, Larson J, Blencowe H, et al. ; Born Too Soon Preterm Prevention Analysis Group . Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013;381(9862):223-234. doi: 10.1016/S0140-6736(12)61856-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blencowe H, Cousens S, Chou D, et al. ; Born Too Soon Preterm Birth Action Group . Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(suppl 1):S2. doi: 10.1186/1742-4755-10-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C; GAPPS Review Group . Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10(suppl 1):S1. doi: 10.1186/1471-2393-10-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman RE, Butler AS, eds. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 5.Norman JE, Shennan AH. Prevention of preterm birth—why can’t we do any better? Lancet. 2013;381(9862):184-185. doi: 10.1016/S0140-6736(12)61956-4 [DOI] [PubMed] [Google Scholar]
- 6.Hibbard JU, Wilkins I, Sun L, et al. ; Consortium on Safe Labor . Respiratory morbidity in late preterm births. JAMA. 2010;304(4):419-425. doi: 10.1001/jama.2010.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy UM, Ko CW, Raju TN, Willinger M. Delivery indications at late-preterm gestations and infant mortality rates in the United States. Pediatrics. 2009;124(1):234-240. doi: 10.1542/peds.2008-3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manuck TA, Rice MM, Bailit JL, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network . Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215(1):103.e1-103.e14, e114. doi: 10.1016/j.ajog.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359(3):262-273. doi: 10.1056/NEJMoa0706475 [DOI] [PubMed] [Google Scholar]
- 10.Petrini JR, Dias T, McCormick MC, Massolo ML, Green NS, Escobar GJ. Increased risk of adverse neurological development for late preterm infants. J Pediatr. 2009;154(2):169-176. doi: 10.1016/j.jpeds.2008.08.020 [DOI] [PubMed] [Google Scholar]
- 11.Romeo DM, Di Stefano A, Conversano M, et al. Neurodevelopmental outcome at 12 and 18 months in late preterm infants. Eur J Paediatr Neurol. 2010;14(6):503-507. doi: 10.1016/j.ejpn.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 12.Ananth CV, Goldenberg RL, Friedman AM, Vintzileos A. Association of temporal changes in gestational age with perinatal mortality in the United States, 2007-2015. JAMA Pediatr. 2018;172(7):627-634. doi: 10.1001/jamapediatrics.2018.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gyamfi-Bannerman C, Ananth CV. Trends in spontaneous and indicated preterm delivery among singleton gestations in the United States, 2005-2012. Obstet Gynecol. 2014;124(6):1069-1074. doi: 10.1097/AOG.0000000000000546 [DOI] [PubMed] [Google Scholar]
- 14.Richards JL, Kramer MS, Deb-Rinker P, et al. Temporal trends in late preterm and early term birth rates in 6 high-income countries in North America and Europe and association with clinician-initiated obstetric interventions. JAMA. 2016;316(4):410-419. doi: 10.1001/jama.2016.9635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, et al. Effect of late-preterm birth and maternal medical conditions on newborn morbidity risk. Pediatrics. 2008;121(2):e223-e232. doi: 10.1542/peds.2006-3629 [DOI] [PubMed] [Google Scholar]
- 16.Dietz PM, Rizzo JH, England LJ, et al. Early term delivery and health care utilization in the first year of life. J Pediatr. 2012;161(2):234-239.e1. doi: 10.1016/j.jpeds.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 17.Lisonkova S, Hutcheon JA, Joseph KS. Temporal trends in neonatal outcomes following iatrogenic preterm delivery. BMC Pregnancy Childbirth. 2011;11:39. doi: 10.1186/1471-2393-11-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meis PJ, Klebanoff M, Thom E, et al. ; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network . Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385. doi: 10.1056/NEJMoa035140 [DOI] [PubMed] [Google Scholar]
- 19.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419-424. doi: 10.1067/mob.2003.41 [DOI] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists ACOG committee opinion no. 561: nonmedically indicated early-term deliveries. Obstet Gynecol. 2013;121(4):911-915. doi: 10.1097/01.AOG.0000428649.57622.a7 [DOI] [PubMed] [Google Scholar]
- 21.Joseph KS, Demissie K, Kramer MS. Obstetric intervention, stillbirth, and preterm birth. Semin Perinatol. 2002;26(4):250-259. doi: 10.1053/sper.2002.34769 [DOI] [PubMed] [Google Scholar]
- 22.Ananth CV, Smulian JC, Vintzileos AM. Ischemic placental disease: maternal versus fetal clinical presentations by gestational age. J Matern Fetal Neonatal Med. 2010;23(8):887-893. doi: 10.3109/14767050903334885 [DOI] [PubMed] [Google Scholar]
- 23.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195(6):1557-1563. doi: 10.1016/j.ajog.2006.05.021 [DOI] [PubMed] [Google Scholar]
- 24.Ananth CV, Vintzileos AM. Medically indicated preterm birth: recognizing the importance of the problem. Clin Perinatol. 2008;35(1):53-67, viii. doi: 10.1016/j.clp.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 25.Barros FC, Vélez MdP. Temporal trends of preterm birth subtypes and neonatal outcomes. Obstet Gynecol. 2006;107(5):1035-1041. doi: 10.1097/01.AOG.0000215984.36989.5e [DOI] [PubMed] [Google Scholar]
- 26.Chen A, Feresu SA, Barsoom MJ. Heterogeneity of preterm birth subtypes in relation to neonatal death. Obstet Gynecol. 2009;114(3):516-522. doi: 10.1097/AOG.0b013e3181b473fc [DOI] [PubMed] [Google Scholar]
- 27.National Center for Health Statistics Guide for completing the facility worksheets for the certificate of live birth https://www.cdc.gov/nchs/data/dvs/guidetocompletefacilitywks.pdf. Updated May 2016. Accessed September 12, 2018.
- 28.Martin JA, Osterman MJ, Kirmeyer SE, Gregory EC. Measuring gestational age in vital statistics data: transitioning to the obstetric estimate. Natl Vital Stat Rep. 2015;64(5):1-20. [PubMed] [Google Scholar]
- 29.Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309(23):2445-2446. doi: 10.1001/jama.2013.6235 [DOI] [PubMed] [Google Scholar]
- 30.Klebanoff MA, Yossef-Salameh L, Latimer C, et al. Development and validation of an algorithm to determine spontaneous versus provider-initiated preterm birth in US vital records. Paediatr Perinat Epidemiol. 2016;30(2):134-140. doi: 10.1111/ppe.12267 [DOI] [PubMed] [Google Scholar]
- 31.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551-561. doi: 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 32.Spiegelman D. %glmcurv9. Harvard T. H. Chan School of Public Health. https://www.hsph.harvard.edu/donna-spiegelman/software/glmcurv9/. Accessed January 22, 2018.
- 33.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75-84. doi: 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin DB. Multiple Imputation for Nonresponse Surveys. New York, NY: Wiley & Sons; 1987. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 35.Ananth CV, Joseph KS, Oyelese Y, Demissie K, Vintzileos AM. Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstet Gynecol. 2005;105(5, pt 1):1084-1091. doi: 10.1097/01.AOG.0000158124.96300.c7 [DOI] [PubMed] [Google Scholar]
- 36.Hassan SS, Romero R, Vidyadhari D, et al. ; PREGNANT Trial . Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18-31. doi: 10.1002/uog.9017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤ 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308-317. doi: 10.1002/uog.15953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-375.e8. doi: 10.1016/j.ajog.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ananth CV. Ischemic placental disease: a unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin Perinatol. 2014;38(3):131-132. doi: 10.1053/j.semperi.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 40.Ananth CV, Friedman AM. Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Semin Perinatol. 2014;38(3):151-158. doi: 10.1053/j.semperi.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 41.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110-120.e6. [DOI] [PubMed] [Google Scholar]
- 42.Oshiro BT, Kowalewski L, Sappenfield W, et al. A multistate quality improvement program to decrease elective deliveries before 39 weeks of gestation. Obstet Gynecol. 2013;121(5):1025-1031. doi: 10.1097/AOG.0b013e31828ca096 [DOI] [PubMed] [Google Scholar]
- 43.Schoen CN, Tabbah S, Iams JD, Caughey AB, Berghella V. Why the United States preterm birth rate is declining. Am J Obstet Gynecol. 2015;213(2):175-180. doi: 10.1016/j.ajog.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 44.Ehrenthal DB, Hoffman MK, Jiang X, Ostrum G. Neonatal outcomes after implementation of guidelines limiting elective delivery before 39 weeks of gestation. Obstet Gynecol. 2011;118(5):1047-1055. doi: 10.1097/AOG.0b013e3182319c58 [DOI] [PubMed] [Google Scholar]
- 45.Dietz PM, Bombard JM, Hutchings YL, et al. Validation of obstetric estimate of gestational age on US birth certificates. Am J Obstet Gynecol. 2014;210(4):335.e1-335.e5. doi: 10.1016/j.ajog.2013.10.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeitlin J, Szamotulska K, Drewniak N, et al. ; Euro-Peristat Preterm Study Group . Preterm birth time trends in Europe: a study of 19 countries. BJOG. 2013;120(11):1356-1365. doi: 10.1111/1471-0528.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lisonkova S, Sabr Y, Butler B, Joseph KS. International comparisons of preterm birth: higher rates of late preterm birth are associated with lower rates of stillbirth and neonatal death. BJOG. 2012;119(13):1630-1639. doi: 10.1111/j.1471-0528.2012.03403.x [DOI] [PubMed] [Google Scholar]
- 48.Martin JA, Wilson EC, Osterman MJ, Saadi EW, Sutton SR, Hamilton BE. Assessing the quality of medical and health data from the 2003 birth certificate revision: results from two states. Natl Vital Stat Rep. 2013;62(2):1-19. [PubMed] [Google Scholar]
- 49.Salemi JL, Tanner JP, Sampat DP, et al. Evaluation of the sensitivity and accuracy of birth defects indicators on the 2003 revision of the US birth certificate: has data quality improved? Paediatr Perinat Epidemiol. 2017;31(1):67-75. doi: 10.1111/ppe.12326 [DOI] [PubMed] [Google Scholar]
- 50.Joseph KS, Liu S, Rouleau J, et al. ; Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System . Influence of definition based versus pragmatic birth registration on international comparisons of perinatal and infant mortality: population based retrospective study. BMJ. 2012;344:e746. doi: 10.1136/bmj.e746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goyal NK, DeFranco E, Kamath-Rayne BD, Beck AF, Hall ES. County-level variation in infant mortality reporting at early previable gestational ages. Paediatr Perinat Epidemiol. 2017;31(5):385-391. doi: 10.1111/ppe.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howell EA, Janevic T, Hebert PL, Egorova NN, Balbierz A, Zeitlin J. Differences in morbidity and mortality rates in black, white, and Hispanic very preterm infants among New York City hospitals. JAMA Pediatr. 2018;172(3):269-277. doi: 10.1001/jamapediatrics.2017.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kramer MS. Socioeconomic disparities in preterm birth. Paediatr Perinat Epidemiol. 2015;29(3):169-171. doi: 10.1111/ppe.12186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Births Included
eTable 1. Distribution of Maternal Characteristics Across all Years, as Well as in 2006 and 2013: United States Singleton Live Births
eTable 2. Assessment of the Linearity Assumption in the Evaluation of Average Annual Change in Neonatal Mortality Rates Between 2006 and 2013
eTable 3. Temporal Changes in Neonatal Mortality Rates Over the 8-Year Period Based on Gestational Age Among Spontaneous and Clinician-Initiated Deliveries: United States Singleton Live Births, 2006-2013
eTable 4. Distribution of Maternal Characteristics Across All Years, as Well as in 2007 and 2013: United States Singleton Live Births
eTable 5. Distribution of Gestational Age Among Spontaneous and Clinician-Initiated Deliveries: United States Singleton Live Births, 2007-2013