Abstract
This analysis investigates the prevalence of financial relationships between oncologist authors of clinical drug trials and the pharmaceutical industry and how often such financial interests are unreported.
In medical research, a financial conflict of interest (FCOI) may affect the conduct and reporting of clinical trials.1 The presence of FCOIs in clinical trials of oncology drugs that receive US Food and Drug Administration (FDA) approval is of particular concern, because these trials may change the trajectory of cancer care. These trials also generate high–impact factor publications, prestige for authors, revenue for the pharmaceutical companies, and newsworthy headlines. The primary objective of this investigation was to quantify the prevalence and magnitude of industry-oncologist financial relationships in trials of oncology drugs that received FDA approval. The secondary objective was to identify the proportion of undisclosed FCOIs among oncologist authors.
Methods
We searched the FDA Hematology/Oncology (Cancer) Approvals & Safety Notifications website from August 26, 2017, to August 31, 2017, for oncology drugs approved from January 1, 2016, to August 31, 2017.2 We extracted clinical trial registry numbers from the FDA approval or the corresponding pharmaceutical company press release and matched the registry number with a published trial. We then extracted industry payments data for each US-based oncologist author from the Open Payments Database. Only payments received during the conduct of the trial from the trial sponsor were considered. We considered the financial relationship to extend from the trial registration date to the publication of the trial. We cross-referenced Open Payments data with the disclosure statements from published clinical trials. Trial registry numbers were taken from the FDA approval or the pharmaceutical company press release, and that registry number was used to identify the published trial. If the registry did not have a link to the published trial, we searched PubMed using the registry number or keywords (a combination of the registry number, intervention drug, and disease or condition for the trial). The search was limited to trials because FDA approvals are based on clinical trials. This study did not involve human subjects and was not subject to institutional review board approval.
The 4 categories of payments were general (personal payments, eg, speaking fees and travel reimbursement), research (eg, fees for enrolling patients and for study coordination), associated research (eg, grants), and ownership (eg, stock). We excluded food payments from the general category and did not encounter any ownership payments. Any disputed payments were subtracted from the oncologist author’s total payments.
Results
We identified 1007 authors, of whom 344 oncologist authors from 43 published trials were included in the study. A total of 263 oncologist-authors (76.5%) received at least 1 industry payment. The excluded authors were either not from the United States, not a physician, or not an oncologist. The median value of general payments to the 344 oncologist authors was $2828 (interquartile range [IQR], $0-$19 628), and the median value of associated research payments was $164 644 (IQR, $0-$551 926). Compared with the other categories, research payments were uncommon. Cumulatively, the 344 oncologist authors received a total of $216 627 353. Overall, 110 oncologist authors (32%) did not fully disclose their payments from the trial sponsor. The Table shows total, disclosed, and undisclosed payments by individual category.
Table. Total Payments, Disclosed Payments, and Undisclosed Payments From the Pharmaceutical Industry to 344 Oncologist Authors of Clinical Trials.
| Payments | Payment Category | ||
|---|---|---|---|
| Generala | Research | Associated Research | |
| Total, $ | |||
| Median (IQR) | 2828 (0-19 628) | 0 (0-0) | 164 644 (0-551 926) |
| Mean (SD) | 18 336 (107 087) | 1494 (10 841) | 609 870 (1 843 467) |
| Sum | 6 318 031 | 513 885 | 209 795 437 |
| Disclosed, $ | |||
| Median (IQR) | 1170 (0-20 506) | 0 (0-0) | 81 591 (0-518 546) |
| Mean (SD) | 19 544 (128 467) | 1173 (5500) | 563 049 (1 900 634) |
| Sum | 4 573 269 | 273 207 | 131 753 549 |
| Undisclosed, $ | |||
| Median (IQR) | 3783 (58-18 793) | 0 (0-0) | 292 273 (44 909-667 547) |
| Mean (SD) | 15 861 (28 249) | 2188 (17 457) | 709 472 (1 719 681) |
| Sum | 1 744 762 | 240 678 | 78 041 888 |
Abbreviation: IQR, interquartile range.
Excluding food.
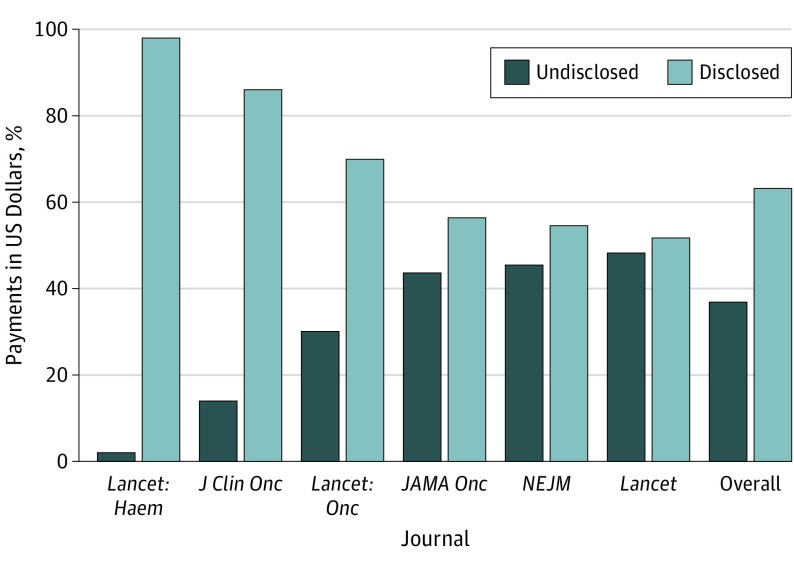
Oncologist authors published most often in The Lancet Oncology (n = 115), The New England Journal of Medicine (n = 100), and The Lancet (n = 70). The Lancet (26 of 70 authors; 37.1%) and The New England Journal of Medicine (46 of 100 authors; 46.0%) had the highest proportions of oncologist authors with undisclosed payments. Furthermore, as the Figure shows, oncologist authors in these 2 journals also had the highest percentages of undisclosed dollars received (The Lancet, 49%; $22 519 822 of $46 534 132; The New England Journal of Medicine, 45%; $34 426 582 of $75 701 366).
Figure. Disclosed and Undisclosed Payments Received by Oncologist Authors of Clinical Drug Trials, by Journal.
J Clin Onc indicates Journal of Clinical Oncology; JAMA Onc, JAMA Oncology; Lancet: Haem, The Lancet Haemotology; Lancet: Onc, The Lancet Oncology; and NEJM, The New England Journal of Medicine.
Discussion
Our study found that financial relationships between oncologist authors and the pharmaceutical industry may be common, expensive, and frequently undisclosed. One-third of oncologist authors failed to completely disclose payments from the sponsor of the published clinical trial. One-quarter of oncologist authors did not receive any payments, which indicates that abstaining from industry payments during a clinical trial is possible.
Historically, opinions regarding FCOIs in medical research have differed. Most parties would agree that the relationship between the pharmaceutical industry and physicians has benefited patients with cancer through the development of drugs that improve survival and quality of life. However, there is widespread concern that the pharmaceutical industry’s role in medical practice unduly influences professional behavior and judgement.3,4 In clinical trials of FDA-approved oncology drugs, bias, either real or potential, is more concerning because these oncology drugs are often associated with marginal improvement in survival5 but exorbitant costs.6
Our study is limited by the scope of our analysis, which spans only 20 months, and our findings may not be generalizable to all oncologist authors of other clinical trials. Furthermore, the authors included in our sample published clinical trials that investigated novel drugs or drugs for novel indications, which may indicate that the oncologist authors included in our study have closer ties to industry sponsors than do other authors.
These findings suggest that Open Payments data may be used to ensure complete disclosure of industry payments to oncologist authors.
References
- 1.Lo B, Field MJ, eds; Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, Education, and Practice. Conflict of interest in medical research, education, and practice. Washington, DC: National Academies Press (US); 2010. [PubMed] [Google Scholar]
- 2.US Food and Drug Administration Hematology/Oncology (Cancer) Approvals & Safety Notifications. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm. Published January 16, 2018. Accessed January 25, 2018.
- 3.Rosenbaum L. Beyond moral outrage: weighing the trade-offs of COI regulation. N Engl J Med. 2015;372(21):2064-2068. doi: 10.1056/NEJMms1502498 [DOI] [PubMed] [Google Scholar]
- 4.De Jesus-Morales K, Prasad V. Closed financial loops: when they happen in government, they’re called corruption; in medicine, they’re just a footnote. Hastings Cent Rep. 2017;47(3):9-14. doi: 10.1002/hast.700 [DOI] [PubMed] [Google Scholar]
- 5.Fojo T, Mailankody S, Lo A. Unintended consequences of expensive cancer therapeutics—the pursuit of marginal indications and a me-too mentality that stifles innovation and creativity: the John Conley Lecture. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1225-1236. doi: 10.1001/jamaoto.2014.1570 [DOI] [PubMed] [Google Scholar]
- 6.Mailankody S, Prasad V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol. 2015;1(4):539-540. doi: 10.1001/jamaoncol.2015.0373 [DOI] [PubMed] [Google Scholar]