Abstract
Importance
Several trials demonstrated the impact of novel agent-based maintenance in newly diagnosed multiple myeloma (NDMM), but there is no current evidence demonstrating the superiority of one regimen over the other, owing to the lack of direct/indirect comparisons.
Objective
To analyze and compare the effectiveness of different maintenance regimens in NDMM via a network meta-analysis.
Data Sources
We performed 2 independent searches in PubMed and Cochrane databases, and then we identified all the records registered after 1999 and on or before November 20, 2017.
Study Selection
By blinded review, we identified prospective phase 3 randomized trials evaluating novel agent-based maintenance in patients with NDMM; the included studies compared at least 2 maintenance approaches; comparators included placebo and no maintenance. From 364 screened records, 11 studies were included.
Data Extraction and Synthesis
We followed (independent extraction) the guidelines provided by the PRISMA Report and the EQUATOR Network. The evidence was synthesized using a network meta-analysis (NMA). To allow comparison of all treatments, no maintenance was selected as common comparator and the effect of placebo was assumed to be the same as no treatment. The best option was identified by a Bayesian consistency model based on hazard ratio (HR), 95% credible interval (CrI), probability of being the best treatment (PbBT), and median ranking distribution (MedR).
Main Outcomes and Measures
Outcomes of interest were progression-free survival (PFS) and overall survival (OS).
Results
Eleven trials and 8 treatments including a total of 5073 participants were included. By PFS analysis, lenalidomide-based regimens (lenalidomide-prednisone, lenalidomide alone) were identified as the most effective options (HR, 0.39 [95% CrI, 0.28-0.53] and 0.47 [95% CrI, 0.39-0.55], respectively; MedR, 1 and 2; overall PbBT, 74%). Four treatments (thalidomide-interferon, thalidomide-bortezomib, bortezomib-prednisone, thalidomide alone) showed an HR in favor of maintenance. By OS analysis, lenalidomide alone was identified as the best option (HR, 0.76; 95% CrI, 0.51-1.16; MedR, 2; PbBT, 38%), followed by bortezomib-thalidomide and bortezomib-prednisone. Similar features were noticed in the restricted network including transplant trials, in the sensitivity analysis, and in most of the prognostic subgroups.
Conclusions and Relevance
Based on PFS and OS results of this NMA, lenalidomide maintenance appears to be the best treatment option, by synthesizing the available evidence of novel agent-based maintenance in the past 20 years.
Key Points
Question
What is the current best maintenance approach in patients with myeloma?
Findings
This network meta-analysis included 11 trials and 8 treatments including a total of 5073 participants and found that 6 maintenance treatments prolonged progression-free survival vs no maintenance: lenalidomide-based regimens were identified as the most effective options. On overall survival analysis, lenalidomide alone was identified as the best option; similar features were noticed in the restricted network including transplant trials, in the sensitivity analysis, and in most of the prognostic subgroups.
Meaning
By synthesizing the available evidence of novel agent-based maintenance in the last 20 years, lenalidomide maintenance can be currently considered the best treatment option.
This systematic review and network meta-analysis analyzes and compares different maintenance regimens for treating patients with newly diagnosed multiple myeloma and associated progression-free and overall survival.
Introduction
The continuous therapy (CT) approach has been evaluated extensively in patients with newly diagnosed multiple myeloma (NDMM), across age groups and treatment strategies upfront, in several trials with different designs. It generally consists of multiagent chemotherapy for a fixed time, followed by a less intensive but prolonged maintenance treatment. Some trials showed the benefit of thalidomide continuous therapy in terms of progression-free survival (PFS), with inconsistent results for overall survival (OS).1,2,3,4,5,6,7,8,9,10 The efficacy of continuous thalidomide is compromised by its poor tolerability, with peripheral neuropathy mainly limiting the long-term use. In a trial randomizing patients to bortezomib-based induction, autologous stem cell transplantation (ASCT) and bortezomib maintenance vs vincristine-doxorubicin-dexamethasone induction, ASCT and thalidomide maintenance, bortezomib-based therapy prolonged PFS, with a better safety profile.11,12 Bortezomib-thalidomide maintenance has also been evaluated both in the nontransplant and transplant settings.13,14,15,16,17 A meta-analysis showed improved PFS as well as OS with lenalidomide maintenance vs no maintenance/placebo in the posttransplant setting.18 Recent data from the Myeloma XI trial confirmed these findings.19
Despite the well-recognized importance of maintenance, there is no evidence demonstrating the overall superiority of 1 regimen over the others, owing to the lack of direct or indirect comparisons. Furthermore, there are several well-known factors that may affect outcome, such as baseline prognostic features (International Staging System [ISS] Stage20 and chromosomal abnormalities21). Direct comparisons in specific patient subsets are lacking as well.
We performed a systematic literature review to identify the randomized clinical trials (RCTs) in NDMM evaluating maintenance treatments using novel agents and then a network meta-analysis (NMA) to synthesize the efficacy (PFS, OS) of each regimen over the others. To do this, we collected and, whenever necessary, reanalyzed the data of each study included in the network. Our main goal was to compare the efficacy of each treatment vs no maintenance/placebo overall and in specific patient subgroups.
Methods
Systematic Literature Review and Studies Selection
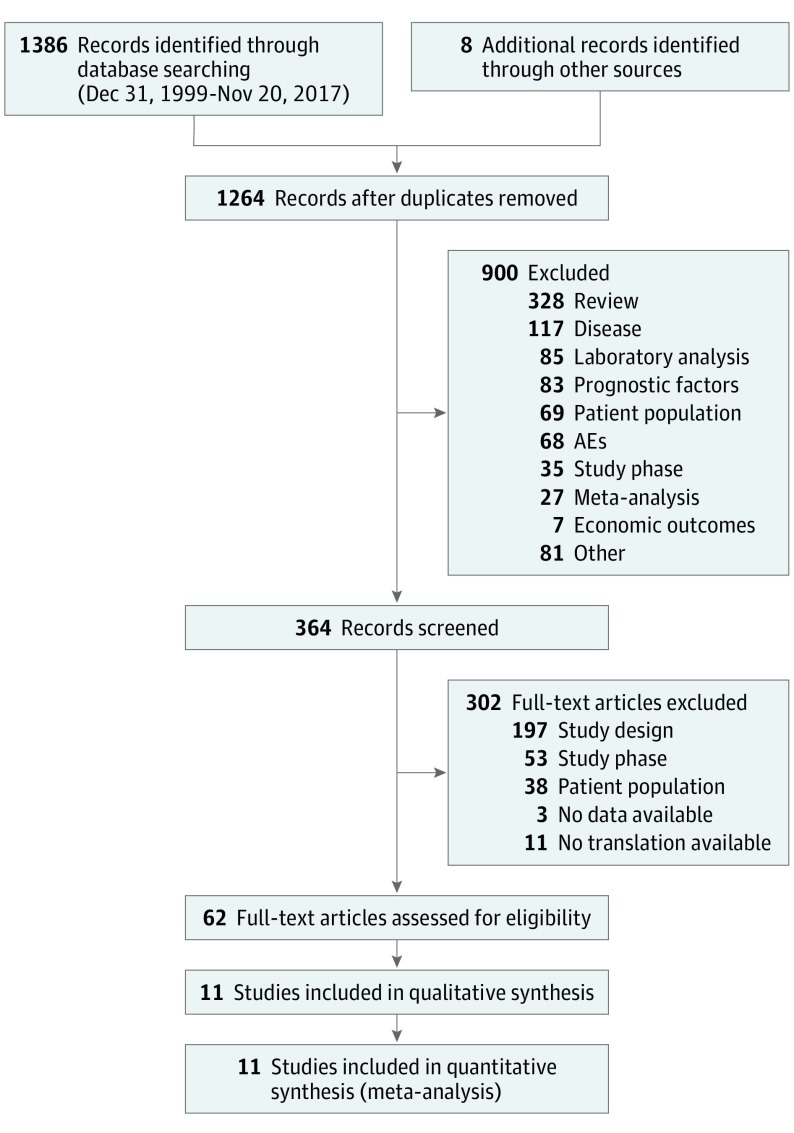
This meta-analysis adheres to the guidelines provided by the PRISMA report and the EQUATOR Network.22,23 We performed 2 independent searches (F.G. and U.P.) in the databases PubMed and Cochrane Central Register of Controlled Trials using the search terms “myeloma” and “maintenance.” We excluded records registered before December 31, 1999, because our aim was to focus on novel agents only. The entire search had a cut-off date of November 20, 2017, and was restricted to articles in English. To ensure that no RCTs were missing, we also considered additional sources (eAppendix in the Supplement), which were added manually to the PRISMA Flowchart (Figure 1).
Figure 1. PRISMA Flowchart.
Eligibility criteria were defined in terms of population, interventions, comparisons, outcomes, and study design (PICOS) criteria.22 The study population of interest comprised NDMM patients; the studies included are prospective phase 3 RCTs that compared at least 2 maintenance approaches; maintenance treatments should include 1 or more novel agents (thalidomide, lenalidomide, bortezomib) in at least 1 arm; comparators included placebo and no maintenance. To specifically evaluate the impact of maintenance, we excluded trials in which patients who received 2 different maintenance treatments underwent different and noncomparable premaintenance therapies (no stratification according to induction at the time of maintenance randomization) because in such trials the final outcomes could be related to the combined effect of both induction and maintenance. To analyze the impact of maintenance in the context of a current treatment approach, we excluded maintenance trials that enrolled only patients who did not receive novel agents during induction because this is no longer a standard approach. Nevertheless, these trials were subsequently included in a sensitivity analysis. Outcomes of interest included PFS and OS.
After removing duplicates by blinded review, titles and abstracts of the citations were screened for inclusion using Excel (version 2013, Microsoft Corporation) and then full-text articles were examined to assess suitability.
The Cochrane Collaboration’s tool for assessing risk of bias in RCTs was used.24 Risks of bias were assessed independently by 2 reviewers (F.G. and S.S.); when data were not reported in the main publications, we asked authors of the different articles to provide them (eAppendix in the Supplement).
Data Extraction
Data were independently extracted from all eligible RCTs that reported hazard ratios (HRs) and confidence intervals (CIs) for PFS and/or OS, or provided data to estimate HRs and CIs in case the study did not report these parameters. Where not available, the HR was estimated using the ratio between probabilities and the 95% CI was estimated using the P value (eAppendix in the Supplement).25
The HRs and P values for PFS and OS were collected for the main comparison (all patients included in the main analysis). We performed a restricted analysis of PFS and OS in patients previously treated with ASCT; in this analysis, for trials enrolling both patients who had received ASCT and not, only 95% CI and HR of the subgroup of ASCT patients were included.
Subgroup analyses according to prognostic features were performed. For these analyses, we included only studies with available data on both ISS stage and cytogenetic risk. The HRs and P values for PFS were collected for the subsequent subsets: ISS stages I/II, ISS stage III; high-risk chromosomal abnormalities detected by FISH (del(17p) and/or t(14;14) and/or t(14;16)), standard-risk (absence of del(17p), t(14;14), t(14;16)). For these subgroup analyses we focused on PFS only owing to the small number of patients in each subgroup, which could be a limitation, even more relevant in the OS analysis, given the lower number of events. Whenever data were either unavailable in the full-text articles, or noncomparable owing to different risk categorization, these were provided by the cooperative groups.
Network Meta-analysis
The NMA was conducted using the natural log transformations of HRs, and their 95% CIs to estimate standard errors (SEs).
To include all trials within 1 framework, we had to choose a common comparator, and we chose no maintenance/placebo. We assumed that placebo treatment was equivalent to no maintenance and that there were no differences in efficacy due to dosages or schemes (for thalidomide, lenalidomide, lenalidomide-prednisone, and bortezomib-thalidomide). We used the R-Project statistical software (version 3.1.1, R Foundation) and the gemtc and R2WinBUGS statistical packages to perform the analysis with WinBugs (version 14, The BUGS Project). Because no loop or design inconsistencies were present, the NMA was conducted following the Bayesian consistency framework.26
The simulation was performed using the Markov Chain Monte Carlo (MCMC) technique with 3 different chains, and each of them produced 500 000 interactions with 300 000 burn-in samples and 15 thinning rates. The output of Bayesian NMA is a posterior distribution of relative effect size, and we obtained the HR as a mean value, and 95% credible interval (95% CrI) as the 2.5th and 97.5th percentiles. Subsequent to performing all the simulations, we calculated the percentage of simulations in which every single treatment ranked first to determine its probability of being the best treatment (PbBT). For each treatment, we estimated the median value of the ranking distribution for all the simulations (MedR). This allowed us to consider not only when a treatment ranked first, but also any other ranking it could obtain. The best option was identified on the basis of MedR, HR with 95% CrI, and PbBT.
Results
Study Selection
Sixty-two publications were included, corresponding to 11 trials (Figure 1) (eAppendix in the Supplement).
All trials started enrollment after 2001, 4 trials enrolled only patients ineligible for ASCT, 4 trials included both patients eligible and not eligible for ASCT, and 3 trials enrolled only patients eligible for ASCT. Eight maintenance options were evaluated: no maintenance/placebo, interferon, thalidomide alone, thalidomide-bortezomib, thalidomide-interferon, lenalidomide alone, lenalidomide-prednisone, and bortezomib-prednisone. eTable 1S in the Supplement summarizes the main characteristics of the included trials and each maintenance treatment schedule.13,16,17,18,19,27,28,29,30,31,32,33,34,35,36,37 Overall, the included trials presented minimal risk of bias (eAppendix in the Supplement).
Primary Analysis: PFS and OS With Different Maintenance Strategies
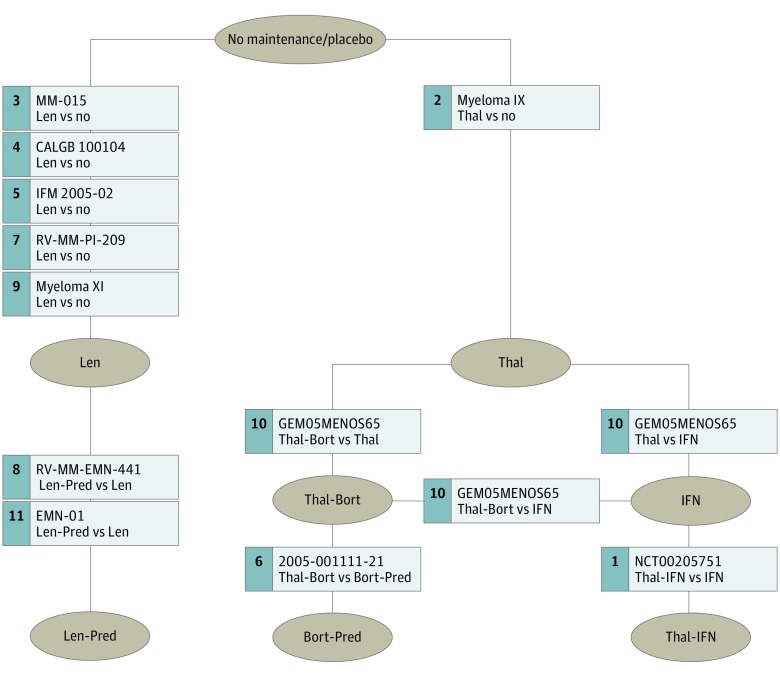
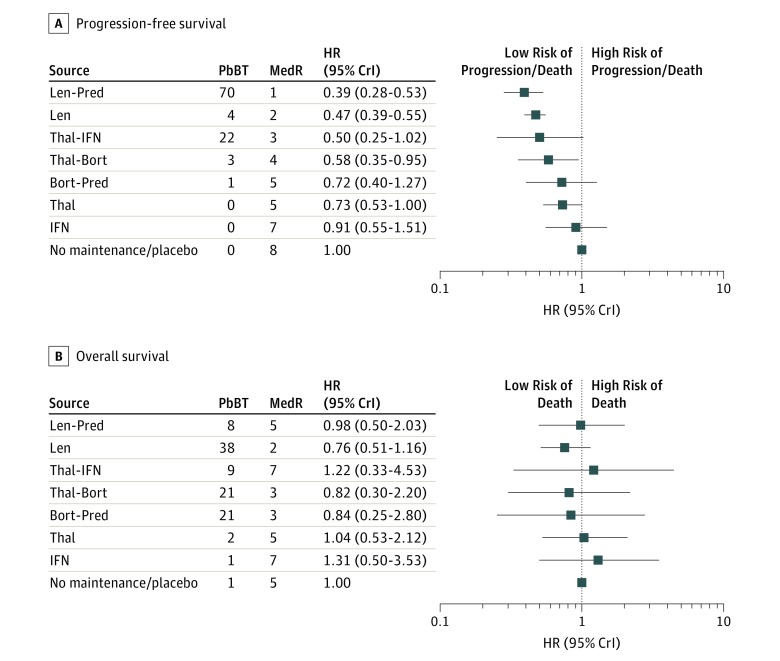
The primary analysis network was composed of all the selected trials (5073 patients) (Figure 2). On PFS analysis, lenalidomide-based regimens had the most favorable HR (lenalidomide-prednisone HR, 0.39 [95% CrI, 0.28-0.53]; lenalidomide alone HR, 0.47 [95% CrI, 0.39-0.55]); they ranked, on median, first and second in all the simulations; overall, they resulted in the most effective options in 74% of the simulations. Four treatments (thalidomide-interferon, thalidomide-bortezomib, bortezomib-prednisone, thalidomide alone) showed an HR in favor of maintenance (HR range, 0.50-0.73); only 1 (interferon) did not show any benefit. On OS analysis, lenalidomide alone was identified as the best option, based again on HR, MedR, and PbBT, followed by thalidomide-bortezomib and bortezomib-prednisone. No benefit was suggested with the other regimens (Figure 3) (eFigure 1S in the Supplement).
Figure 2. Primary Analysis Network.
Thal indicates thalidomide; IFN, interferon; Len, lenalidomide; Bort, bortezomib; Pred, prednisone.
Figure 3. Forest Plot of Network Meta-analysis Results (Primary Analysis).
Thal indicates thalidomide; IFN, interferon; Len, lenalidomide; Bort, bortezomib; Pred, prednisone; PFS, progression-free survival; OS, overall survival; PbBT, probability of being the best treatment; MedR, median value of the ranking distribution for all the simulations. A, PFS results. B, OS results.
Restricted Analysis: PFS and OS in the ASCT Setting
Seven trials (Myeloma IX, CALGB-100104, IFM-2005-02, RV-MM-PI-209, RV-MM-EMN-441, Myeloma XI, GEM05MENOS65) were included (2917 patients) (eFigure 2S, eTable 4S in the Supplement).
Results were similar to those of the primary analysis network. Regarding PFS, lenalidomide-based maintenance regimens were identified as the best options; thalidomide-bortezomib and Thal showed a HR in favor of maintenance (HR range, 0.58-0.73), and no benefit was noticed with interferon. On OS analysis, lenalidomide-based regimens were the best option; no benefit with any other regimen was noticed (eFigure 2S in the Supplement; panels B and C).
Subgroup Analyses: Prognostic Features
Eight trials (NCT00205751, Myeloma IX, IFM-2005-02, 2005-001111-21, RV-MM-PI-209, RV-MM-EMN-441, GEM05MENOS65, EMN-01) were included in the subgroup analysis (eFigure 3S and eTable 5S in the Supplement). Two trials were excluded (CALGB 100104, MM-015) because no data on cytogenetic abnormalities were available; 1 trial (Myeloma XI) was not included because the publication of the main article is still underway.
Results of the subgroup analysis in patients with good prognosis (ISS stage I or II disease [n = 2144] and standard-risk chromosomal abnormalities [n = 1657]) suggested lenalidomide-based maintenance as the best option in this subset, on the basis of HR, MedR, and PbBT. Subgroup analysis on patients with poor prognosis, although limited by a lower sample size, suggested a major benefit of bortezomib-based maintenance in patients with ISS stage III disease (n = 626), but failed to detect the advantage of any regimen over no maintenance/placebo in patients with high-risk chromosomal abnormalities (n = 392) (eFigure 4S in the Supplement).
Sensitivity Analyses
A sensitivity analysis was conducted on a slightly broader network of 14 trials (6516 patients) (eFigure 5S in the Supplement). This analysis added to the primary analysis 3 trials (IFM9902, MY10 and ALLG MM6) and 3 maintenance options (thalidomide-pamidronate, thalidomide-prednisone, and prednisone)8,9,10 in a population of patients not previously exposed to novel agents. Results of the analysis for PFS suggested lenalidomide-based therapies as the best option. In terms of OS, the trend was again similar to that of the main analysis, except for the OS advantage with thalidomide-pamidronate.
Discussion
Maintenance treatment is defined as any therapy administered after the completion of the induction period in patients either responsive or nonprogressive, with the goal of prolonging survival.38,39 The optimal maintenance therapy should be a convenient treatment, with a good compliance and tolerability. In the past 20 years, several trials have evaluated maintenance with either 1 or more of these drugs. Most trials showed the benefit of long-term novel agent-based treatment in both young, ASCT-eligible patients, and elderly patients. Nevertheless, direct comparisons of such regimens are lacking, and it is hard to draw conclusions.
The primary objective of this meta-analysis was to synthetize all the available evidence on maintenance with thalidomide, bortezomib, and lenalidomide in NDMM (ASCT and non-ASCT settings) and to contribute to identifying the best maintenance approach. We used HRs as effect measure for survival and included all the treatments evaluated during the last 20 years into a single network, including the most recently published studies. To enable the comparison, we had to arbitrarily choose a common comparator, and we opted for no maintenance/placebo.
Results of analysis suggested that lenalidomide was the best maintenance option. Lenalidomide and lenalidomide-prednisone were associated with the best PFS advantage. Lenalidomide alone was associated with the best OS advantage. This is not surprising: first, 5 randomized trials showed a significant PFS advantage with lenalidomide alone vs no maintenance/placebo19,30,31,32,40,41; second, a recent meta-analysis demonstrated a significant OS advantage of lenalidomide alone vs no maintenance/placebo in the post-ASCT setting18; and third, 2 trials showed a moderate PFS benefit adding prednisone to lenalidomide vs lenalidomide alone, but no OS advantage.35,36,37
Use of thalidomide-interferon, thalidomide alone, thalidomide-bortezomib, and bortezomib-prednisone showed an HR for PFS in favor of maintenance but no OS benefit. Unlike lenalidomide alone, all these regimens were evaluated in a single trial each; their estimated lower effectiveness was therefore based on single-trial results, which could be a limitation.
Long-term use of lenalidomide undoubtedly has advantages, owing to the lack of neuropathy, which is the main factor limiting the long-term use of both thalidomide and bortezomib. The main toxic effects associated with lenalidomide maintenance include neutropenia, thrombocytopenia, cutaneous eruption and, in the long term, diarrhea.
Use of interferon alone showed no PFS or OS benefit in comparison with no maintenance/placebo. These results are in contrast to some previous studies42,43,44 conducted in the prenovel agent era, and could be partly related to a stronger effect of interferon in patients not previously exposed to novel drugs.
Results of our main analysis were confirmed in the post-ASCT setting, supporting the recent approval of the drug by the US Food and Drug Administration and the European Medicines Agency in this setting.
Yet, it remains to be defined as to whether all patients require maintenance therapy or not or, most importantly, if the choice of the agent/s should be dictated by disease characteristics. The benefit of lenalidomide in high-risk patients is still a matter of debate, based on conflicting results of published studies, and on the potentially higher effectiveness of bortezomib in this setting. Sub-analyses of several trials30,32,34,35 suggested the suboptimal efficacy of lenalidomide alone in high-risk patients, but recent results of the Myeloma XI trial did show a benefit also in high-risk disease19; yet, patients who received lenalidomide maintenance in the Myeloma XI trial were previously treated with IMiDs and were sensitive to the drug. Various studies showed the efficacy of bortezomib in high-risk patients11,12,14,15,45; nevertheless, this was associated more often with a bortezomib-based induction and consolidation/maintenance rather than with maintenance itself. Updated results of the HOVON-65/GMMG-HD4 trial suggested that the negative effect of del17 was abrogated by bortezomib-based induction and maintenance, whereas there was only a trend for improved OS in patients with t(4;14) and 1q gain.12 A recent retrospective comparison of bortezomib vs lenalidomide therapy in elderly patients suggested an advantage of bortezomib upfront in patients with either del17, t(4;14) or t(14;16).46 Again, head-to-head comparisons in specific subgroups are lacking. To our knowledge, this is the first NMA to evaluate which drug is associated with best results for maintenance therapy in patients with different prognostic features. Patients with good prognosis (ISS stages I and II and standard-risk chromosomal abnormalities) highly benefit from maintenance, and lenalidomide-based therapy was associated with the best outcomes in this subset. Subgroup analyses in high-risk patients are limited by the lower number of patients. Despite these low numbers, bortezomib-based maintenance seems to be more beneficial for patients with ISS stage III disease. No specific advantage of any maintenance over the other was noticed in patients with high-risk chromosomal abnormalities. It remains uncertain as to whether this is related to the small sample size, the different cut-off used to define positivity, or the extremely poor prognosis of these patients. Considering the poor prognosis related to these chromosomal abnormalities, the conflicting RCT results on the efficacy of lenalidomide alone, the absence of clear advantage of 1 agent over the other, and the retrospective nature of this analysis, we cannot conclude that lenalidomide alone is sufficient for high-risk patients and a better choice would probably be to combine lenalidomide with proteasome inhibitors, instead of using only 1 agent.47
Assumptions and Limitations
To perform this NMA and include all trials in the same network, we made 2 assumptions. The first is that the effect of placebo was the same as no maintenance. Trials using placebo as control vs observation have the advantage of reducing bias in reporting quality of life and in grading adverse events (such as anxiety, fatigue, pain), whereas knowing or not knowing which participants are receiving therapy may partially affect subjective evaluation. Nevertheless, the main aims of our analysis were PFS and OS; events like progression and death are not influenced by the use of placebo or simple observation, therefore we believe that the assumption that placebo is equivalent to no maintenance can be acceptable for this type of analysis. Of note, only 3 trials included in our NMA used placebo, and they all evaluated lenalidomide maintenance; the estimation of the effect of lenalidomide maintenance therapy is based on 5 trials and this further minimizes the risk of bias. The second assumption is that different thalidomide doses (50-200 mg), lenalidomide schedules (28 days continuously or 21/28 days), and prednisone doses (50-25 mg) had an equivalent effect. Although this latter assumption might be a limitation, there are data suggesting similar survival in patients randomized to different thalidomide doses48; likewise, slightly different schedules of lenalidomide maintenance therapy induced similar median PFS31,32,33,34; in the EMN441 trial, 50 mg of prednisone was not well tolerated in the long term, and median time to dose reductions (25 mg) was 6 months; thereafter prednisone could be administered for a longer time, thus making similar the dose administered in the 2 trials.35
We excluded from this NMA trials that evaluated continuous therapy upfront but administered 2 different maintenance treatments after different and noncomparable premaintenance therapies because in such trials the final outcome was probably related to the combined effect of both induction and maintenance, rather than the maintenance therapy itself. The impact of some of these continuous approaches has been evaluated in 2 NMAs, which reported a PFS and OS advantage of lenalidomide-dexamethasone (Rd) over the other therapies, including MPT and VMP (fixed duration) in elderly patients.49,50 More recently, Bort-Rd for 8 cycles, followed by continuous Rd showed better PFS and OS vs continuous Rd alone in patients without an immediate intent to undergo ASCT.51 In the transplant setting, randomized clinical trials showed the efficacy of long-term thalidomide7,52 (but mainly compared with a treatment not including any novel agent) and the superiority of Bort-based pretransplant induction followed by bortezomib maintenance therapy over vincristine-doxorubicin-dexamethasone pretransplant induction followed by thalidomide maintenance therapy.11,12
To analyze the impact of maintenance therapy in the context of current treatment approaches, we excluded from our primary analysis 3 trials8,9,10 that evaluated thalidomide maintenance therapy post-ASCT in patients who did not receive novel agents-based induction. Because this could be a limitation, these trials were subsequently included in a sensitivity analysis, which had similar PFS results to the main analysis; an unexpected OS advantage was noticed with thalidomide-pamidronate. This could at least in part be related to the estimation required to calculate the HR, and/or to a stronger effect of thalidomide in patients not previously treated with novel drugs. An advantage in favor of thalidomide-based maintenance therapy was not evident in the other trials.
We did not report on adverse events, drug discontinuations, and quality of life because our main objectives were PFS and OS. In addition, because we used no treatment as common comparator, data on adverse events, discontinuations, and quality of life were not available and the comparison across trials was possible. These are indeed key factors: the oral administration, good tolerability, and no worsening in quality of life53 support the use of lenalidomide for most patients in the context of the currently available drugs. An increase in second primary malignant disease with prolonged lenalidomide therapy has been reported,31,32,33,54 but the survival benefit overcame the risk in all the trials. The optimal dose (10 vs 15 mg) and schedule (21/28 days or continuously) are still open issues. Main grade 3 to 4 nonhematologic toxic effects were similar in patients treated with different doses and schedules in the trials evaluating lenalidomide maintenance therapy post-ASCT, but both grade 3 and 4 neutropenia and thrombocytopenia were lower in patients treated with 10 mg 21/28 days compared with 10-mg escalated to 15 mg and administered continuously.31,32,33,34 The optimal duration is still to be determined. Some trials evaluated maintenance therapy until progression, others for up to 2 to 3 years, but no randomized clinical trial addressed so far the benefit of treatment until progression vs 2 to 3 years. Because of toxicity, thalidomide could be often administered only for a few months and bortezomib administration was planned for a maximum of 2 to 3 years. Although lenalidomide therapy was planned in most of the studies as treatment until progression, the mean duration of treatment was approximately 30 months.18 Duration of therapy is therefore strictly related to tolerability, and most of the trials showed that the effective duration of maintenance therapy is limited to 2 to 3 years. Another unanswered question is the benefit of upfront continuous lenalidomide vs lenalidomide-containing triplets at first relapse because there are no data available on the effectiveness of the currently approved lenalidomide-containing triplets in patients refractory to lenalidomide.
Conclusions
Despite the assumptions and limitations of this NMA, our results support the use of lenalidomide maintenance therapy in most patients. Still, better treatment options are required in patients with aggressive disease, who may benefit from combinations of proteasome inhibitors and immunomodulatory agents. There are ongoing trials evaluating maintenance therapy with second-generation proteasome inhibitors alone or plus immunomodulatory agents and with monoclonal antibodies. These well-tolerated drugs, which could have a potential efficacy in standard but also in high-risk disease, could become available in the maintenance therapy setting in the near future, and increase the treatment options for patients with MM.
eAppendix
References
- 1.Palumbo A, Bringhen S, Caravita T, et al. ; Italian Multiple Myeloma Network, GIMEMA . Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367(9513):825-831. doi: 10.1016/S0140-6736(06)68338-4 [DOI] [PubMed] [Google Scholar]
- 2.Waage A, Gimsing P, Fayers P, et al. ; Nordic Myeloma Study Group . Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116(9):1405-1412. doi: 10.1182/blood-2009-08-237974 [DOI] [PubMed] [Google Scholar]
- 3.Facon T, Mary JY, Hulin C, et al. ; Intergroupe Francophone du Myélome . Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209-1218. doi: 10.1016/S0140-6736(07)61537-2 [DOI] [PubMed] [Google Scholar]
- 4.Beksac M, Haznedar R, Firatli-Tuglular T, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86(1):16-22. doi: 10.1111/j.1600-0609.2010.01524.x [DOI] [PubMed] [Google Scholar]
- 5.Wijermans P, Schaafsma M, Termorshuizen F, et al. ; Dutch-Belgium Cooperative Group HOVON . Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28(19):3160-3166. doi: 10.1200/JCO.2009.26.1610 [DOI] [PubMed] [Google Scholar]
- 6.Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-3670. doi: 10.1200/JCO.2008.21.0948 [DOI] [PubMed] [Google Scholar]
- 7.Barlogie B, Pineda-Roman M, van Rhee F, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112(8):3115-3121. doi: 10.1182/blood-2008-03-145235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27(11):1788-1793. doi: 10.1200/JCO.2008.18.8573 [DOI] [PubMed] [Google Scholar]
- 9.Stewart AK, Trudel S, Bahlis NJ, et al. A randomized phase 3 trial of thalidomide and prednisone as maintenance therapy after ASCT in patients with MM with a quality-of-life assessment: the National Cancer Institute of Canada Clinicals Trials Group Myeloma 10 Trial. Blood. 2013;121(9):1517-1523. doi: 10.1182/blood-2012-09-451872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attal M, Harousseau J-L, Leyvraz S, et al. ; Inter-Groupe Francophone du Myélome (IFM) . Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289-3294. doi: 10.1182/blood-2006-05-022962 [DOI] [PubMed] [Google Scholar]
- 11.Sonneveld P, Schmidt-Wolf IGH, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946-2955. doi: 10.1200/JCO.2011.39.6820 [DOI] [PubMed] [Google Scholar]
- 12.Goldschmidt H, Lokhorst HM, Mai EK, et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2018;32(2):383-390. doi: 10.1038/leu.2017.211 [DOI] [PubMed] [Google Scholar]
- 13.Rosiñol L, Oriol A, Teruel AI, et al. Bortezomib and thalidomide maintenance after stem cell transplantation for multiple myeloma: a PETHEMA/GEM trial. Leukemia. 2017;31(9):1922-1927. doi: 10.1038/leu.2017.35 [DOI] [PubMed] [Google Scholar]
- 14.Palumbo A, Bringhen S, Larocca A, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol. 2014;32(7):634-640. doi: 10.1200/JCO.2013.52.0023 [DOI] [PubMed] [Google Scholar]
- 15.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28(34):5101-5109. doi: 10.1200/JCO.2010.29.8216 [DOI] [PubMed] [Google Scholar]
- 16.Mateos M-V, Oriol A, Martínez-López J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11(10):934-941. doi: 10.1016/S1470-2045(10)70187-X [DOI] [PubMed] [Google Scholar]
- 17.Mateos M-V, Oriol A, Martínez-López J, et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood. 2014;124(12):1887-1893. doi: 10.1182/blood-2014-05-573733 [DOI] [PubMed] [Google Scholar]
- 18.McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J Clin Oncol. 2017;35(29):3279-3289. doi: 10.1200/JCO.2017.72.6679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson G, Davies FE, Pawlyn C, et al. Lenalidomide maintenance significantly improves outcomes compared to observation irrespective of cytogenetic risk: results of the Myeloma XI trial. Blood. 2017;130(Suppl_1):Abstract #436 [ASH 2017 59th Meeting]. [Google Scholar]
- 20.Greipp PR, San Miguel J, Durie BGM, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412-3420. doi: 10.1200/JCO.2005.04.242 [DOI] [PubMed] [Google Scholar]
- 21.Munshi NC, Anderson KC, Bergsagel PL, et al. ; International Myeloma Workshop Consensus Panel 2 . Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117(18):4696-4700. doi: 10.1182/blood-2010-10-300970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 23.Systematic reviews/Meta-analyses/HTA | Study Designs | The EQUATOR Network. UK EQUATOR Centre, Centre for Statistics in Medicine, NDORMS, University of Oxford. https://www.equator-network.org/?post_type=eq_guidelines&eq_guidelines_study_design=systematic-reviews-and-meta-analyses&eq_guidelines_clinical_specialty=0&eq_guidelines_report_section=0&s=. Accessed February 23, 2018.
- 24.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman DG, Bland JM. How to obtain the P value from a confidence interval. BMJ. 2011;343:d2304. doi: 10.1136/bmj.d2304 [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98-110. doi: 10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig H, Adam Z, Tóthová E, et al. Thalidomide maintenance treatment increases progression-free but not overall survival in elderly patients with myeloma. Haematologica. 2010;95(9):1548-1554. doi: 10.3324/haematol.2009.020586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan GJ, Gregory WM, Davies FE, et al. ; National Cancer Research Institute Haematological Oncology Clinical Studies Group . The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012;119(1):7-15. doi: 10.1182/blood-2011-06-357038 [DOI] [PubMed] [Google Scholar]
- 29.Morgan GJ, Davies FE, Gregory WM, et al. Long-term follow-up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res. 2013;19(21):6030-6038. doi: 10.1158/1078-0432.CCR-12-3211 [DOI] [PubMed] [Google Scholar]
- 30.Palumbo A, Hajek R, Delforge M, et al. ; MM-015 Investigators . Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366(19):1759-1769. doi: 10.1056/NEJMoa1112704 [DOI] [PubMed] [Google Scholar]
- 31.Holstein SA, Jung S-H, Richardson PG, et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial. Lancet Haematol. 2017;4(9):e431-e442. doi: 10.1016/S2352-3026(17)30140-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770-1781. doi: 10.1056/NEJMoa1114083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Attal M, Lauwers-Cances V, Marit G, et al. ; IFM Investigators . Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791. doi: 10.1056/NEJMoa1114138 [DOI] [PubMed] [Google Scholar]
- 34.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905. doi: 10.1056/NEJMoa1402888 [DOI] [PubMed] [Google Scholar]
- 35.Gay F, Oliva S, Petrucci MT, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16(16):1617-1629. doi: 10.1016/S1470-2045(15)00389-7 [DOI] [PubMed] [Google Scholar]
- 36.Bringhen S, Offidani M, Musto P, et al. Long term outcome of lenalidomide-dexamethasone (Rd) vs melphalan-lenalidomide-prednisone (MPR) vs cyclophosphamide-prednisone-lenalidomide (CPR) as induction followed by lenalidomide-prednisone (RP) vs lenalidomide (R) as maintenance in a community-based newly diagnosed myeloma population. Blood. 2017;130(Suppl_1):Abstract #901 [ASH 2017 59th Meeting]. [Google Scholar]
- 37.Magarotto V, Bringhen S, Offidani M, et al. Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood. 2016;127(9):1102-1108. doi: 10.1182/blood-2015-08-662627 [DOI] [PubMed] [Google Scholar]
- 38.Ludwig H, Durie BGM, McCarthy P, et al. ; International Myeloma Working Group . IMWG consensus on maintenance therapy in multiple myeloma. Blood. 2012;119(13):3003-3015. doi: 10.1182/blood-2011-11-374249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reece DE. Posttransplantation maintenance therapy and optimal frontline therapy in myeloma. Hematology Am Soc Hematol Educ Program. 2011;2011(1):197-204. doi: 10.1182/asheducation-2011.1.197 [DOI] [PubMed] [Google Scholar]
- 40.Attal M, Lauwers-Cances V, Hulin C, et al. ; IFM 2009 Study . Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376(14):1311-1320. doi: 10.1056/NEJMoa1611750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide is a highly effective maintenance therapy in myeloma patients of all ages; results of the phase III Myeloma XI Study. Blood. 2016;128(22):Abstract #1143. [Google Scholar]
- 42.Mandelli F, Avvisati G, Amadori S, et al. Maintenance treatment with recombinant interferon alfa-2b in patients with multiple myeloma responding to conventional induction chemotherapy. N Engl J Med. 1990;322(20):1430-1434. doi: 10.1056/NEJM199005173222005 [DOI] [PubMed] [Google Scholar]
- 43.Cunningham D, Powles R, Malpas J, et al. A randomized trial of maintenance interferon following high-dose chemotherapy in multiple myeloma: long-term follow-up results. Br J Haematol. 1998;102(2):495-502. doi: 10.1046/j.1365-2141.1998.00795.x [DOI] [PubMed] [Google Scholar]
- 44.Fritz E, Ludwig H. Interferon-alpha treatment in multiple myeloma: meta-analysis of 30 randomised trials among 3948 patients. Ann Oncol Off J Eur Soc Med Oncol. 2000;11(11):1427-1436. [DOI] [PubMed] [Google Scholar]
- 45.Cavo M, Tacchetti P, Patriarca F, et al. ; GIMEMA Italian Myeloma Network . Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075-2085. doi: 10.1016/S0140-6736(10)61424-9 [DOI] [PubMed] [Google Scholar]
- 46.Larocca A, Offidani M, Musto P, et al. Impact of bortezomib- or lenalidomide-based induction treatment on high risk cytogenetic transplant-ineligible patients with newly diagnosed multiple myeloma enrolled in the Gimema-MM-03-05 and EMN01 trials. Blood. 2017;130(Suppl_1):Abstract #744 [ASH 2017 59th Meeting]. [Google Scholar]
- 47.Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28(3):690-693. doi: 10.1038/leu.2013.335 [DOI] [PubMed] [Google Scholar]
- 48.Yakoub-Agha I, Hulin C, Doyen C, et al. A multicenter prospective randomized study testing non-inferiority of thalidomide 100 mg/day as compared with 400 mg/day in patients with refractory/relapsed multiple myeloma: first results of the final analysis of the IFM 01-02 study. Blood. 2005;106(11):Abstract #364. [Google Scholar]
- 49.Weisel K, Doyen C, Dimopoulos M, et al. A systematic literature review and network meta-analysis of treatments for patients with untreated multiple myeloma not eligible for stem cell transplantation. Leuk Lymphoma. 2017;58(1):153-161. doi: 10.1080/10428194.2016.1177772 [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Chen J, He YA, et al. Comparing efficacy and survivals of initial treatments for elderly patients with newly diagnosed multiple myeloma: a network meta-analysis of randomized controlled trials. Onco Targets Ther. 2016;10:121-128. doi: 10.2147/OTT.S123680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527. doi: 10.1016/S0140-6736(16)31594-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lokhorst HM, Schmidt-Wolf I, Sonneveld P, et al. ; Dutch-Belgian HOVON; German GMMG . Thalidomide in induction treatment increases the very good partial response rate before and after high-dose therapy in previously untreated multiple myeloma. Haematologica. 2008;93(1):124-127. doi: 10.3324/haematol.11644 [DOI] [PubMed] [Google Scholar]
- 53.Abonour R, Durie BGM, Jagannath S, et al. Health-related quality of life of patients with newly diagnosed multiple myeloma receiving any or lenalidomide maintenance after autologous stem cell transplant in the Connect MM disease registry. Blood. 2016;128(22):Abstract #537. [Google Scholar]
- 54.Palumbo A, Bringhen S, Kumar SK, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014;15(3):333-342. doi: 10.1016/S1470-2045(13)70609-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix