Key Points
Question
What is the clinical relevance of somatic driver alterations in estrogen receptor–positive, HER2-negative early breast cancer in postmenopausal women?
Findings
In a secondary analysis of the Breast International Group 1-98 randomized clinical trial, a sampling plan selected 764 samples for targeted DNA sequencing and after adjustment for clinicopathologic factors, amplifications on 11q13 and 8p11 and an increasing number of driver alterations were associated with a significantly increased risk of distant recurrence. PIK3CA mutations were associated with significantly greater benefit with letrozole compared with tamoxifen therapy.
Meaning
Classification of somatic driver alterations based on DNA analysis provides valuable prognostic information that may aid treatment decision making in the adjuvant setting.
Abstract
Importance
A range of somatic driver alterations has been described in estrogen receptor–positive, HER2-negative (ER+/HER2−) early breast cancer (BC); however, the clinical relevance is unknown.
Objective
To investigate associations of driver alterations with prognosis and the role of PIK3CA mutations in prediction of benefit associated with endocrine therapy in postmenopausal patients with ER+/HER2− early BC treated with tamoxifen or letrozole.
Design, Setting, and Participants
The Breast International Group (BIG) 1-98 trial randomized 8010 postmenopausal patients with hormone receptor–positive, operable, invasive BC to monotherapy with letrozole, tamoxifen, or a sequential strategy for 5 years. Driver alterations were characterized using next-generation sequencing in primary tumors from a subset of 764 patients from 7329 eligible patients with ER+/HER2− BC, with 841 distant recurrences after a median of 8.1 years of follow-up. To correct for the oversampling of distant recurrences, weighted analysis methods were used. This analysis was conducted from April 4, 2016, to November 30, 2016.
Main Outcomes and Measures
The prevalence of driver alterations, associations with clinicopathologic factors, distant recurrence-free interval, and treatment interactions were analyzed. Multivariable analyses were performed to adjust for clinicopathologic factors.
Results
Of 764 samples, 538 (70.4%), including 140 distant recurrence events, were successfully sequenced. Nineteen driver alterations were observed with 5% or greater frequency, with a mean of 4 alterations (range, 0-15) per tumor. PIK3CA mutations were the most common (49%) and were significantly associated with reduction in the risk for distant recurrence (hazard ratio [HR], 0.57; 95% CI, 0.38-0.85; P = .006). TP53 mutations (HR, 1.92; 95% CI, 1.21-3.04; P = .006), amplifications on 11q13 (HR, 2.14; 95% CI, 1.36-3.37; P = .001) and 8p11 (HR, 3.02; 95% CI, 1.88-4.84; P < .001), and increasing number of driver alterations (HR per additional alteration, 1.18; 95% CI, 1.11-1.25; P < .001) were associated with significantly greater risk. Amplifications on 11q13 and 8p11 remained significant predictors in multivariable analysis, but not PIK3CA and TP53 mutations. Patients with tumors harboring kinase or helical domain PIK3CA mutations derived significantly greater benefit from letrozole over tamoxifen than patients whose tumors did not (P interaction = .002).
Conclusions and Relevance
In ER+/HER2− postmenopausal, early-stage BC, amplifications on 11q13 and 8p11 were significantly associated with increased risk for distant recurrence and PIK3CA mutations were predictive of greater magnitude of benefit from letrozole. With these findings, DNA-based classification may aid adjuvant treatment decision making in this setting.
Trial Registration
ClinicalTrials.gov Identifier: NCT00004205
This secondary analysis of a randomized clinical trial examines the association between driver alterations and the risk of mutations and prognosis in women with postmenopausal hormone receptor–positive, HER2-negative breast cancer.
Introduction
Large-scale, next-generation sequencing studies have provided valuable insights into the genomic landscape of primary breast cancers (BCs).1,2,3,4,5 These studies have highlighted the diverse spectrum of driver alterations and mutational processes in estrogen receptor–positive, HER2-negative (ER+/HER2−) disease, the most commonly diagnosed BC subtype. Despite these findings, the clinical implications of driver alterations including the most frequent—PIK3CA (Entrez 5290) mutations—remains to be fully elucidated.
Women with a diagnosis of early-stage ER+/HER2− BC typically receive adjuvant systemic therapy consisting of endocrine therapy, often preceded by chemotherapy. In addition to markers of anatomic tumor burden,6 a number of gene expression–based markers exist to aid in estimation of prognosis and decision making regarding adjuvant chemotherapy.7,8,9 These markers are based on continuous gene expression variables and the characterization of phenotype resulting from underlying genetic and epigenetic processes. A common theme of gene expression signatures is the observation that higher rates of proliferation portend poorer prognosis.10
For adjuvant endocrine therapy, the Breast International Group (BIG) 1-98 study was a seminal, phase 3 clinical trial that evaluated the superiority of 5 years of letrozole over tamoxifen in postmenopausal women with early-stage, hormone receptor–positive BC. At a median of 8.1 years’ follow-up, 5 years of letrozole treatment demonstrated a significant reduction in the risk of death compared with tamoxifen alone, establishing aromatase inhibition as the standard of care for postmenopausal women with hormone receptor–positive BC.11,12,13 Other endocrine therapy options now also exist with the type, sequencing, duration, and possible future incorporation of cyclin-dependent kinase 4/6 inhibitors as considerations in therapeutic decision making.14 Clinically useful biomarkers to aid in individualizing these options are lacking.
PIK3CA mutations are particularly enriched in luminal BCs,1 associated with improved outcomes and sensitivity to endocrine therapy in ER+ BC,15 and have been shown to be associated with the initiation of ER+ tumors.16 Therefore, owing to its association with estrogen signaling, we hypothesized that letrozole, being the more potent antiestrogen, would have higher clinical benefit than tamoxifen in patients with tumors harboring PIK3CA mutations.
In this study, we aimed to characterize the clinical relevance of oncogenic drivers in postmenopausal patients with early-stage ER+/HER2− BC enrolled in the BIG 1-98 study. Our primary objective was to investigate the prognostic associations of driver alterations with distant recurrence-free interval. As a secondary objective, we evaluated whether PIK3CA mutations could be predictive of a greater magnitude of benefit with letrozole over tamoxifen and hence could have implications for selection of adjuvant endocrine treatment.
Methods
Patients
BIG 1-98 was a multicenter, randomized, double-blind, phase 3 trial in which 8010 postmenopausal patients with hormone receptor–positive, operable, invasive BC were randomly assigned to monotherapy with letrozole (2.5 mg orally daily) or tamoxifen (20 mg orally daily) for 5 years, a sequential strategy of letrozole for 2 years followed by tamoxifen for 3 years, or the reverse sequence. After a median follow-up of 8.1 years, 1022 distant recurrences were observed. Details of the study have been reported previously.11,12,13,17 All patients provided written informed consent. Ethics committees and relevant health authorities approved the protocol. Tumor samples were collected retrospectively in accordance with institutional guidelines and national laws. The International Breast Cancer Study Group Biological Project Working Group approved this investigation.
Tumor Sequencing and Variant Calling
Library preparation, hybridization-based capture, and next-generation sequencing were performed using Foundation Medicine’s T5-targeted panel of 287 known cancer genes in a Clinical Laboratory Improvement Amendments–certified and College of American Pathologists–accredited laboratory using a previously published method (eTable 1 in the Supplement).18 Samples with a minimum median exon depth of coverage of 150 × were eligible for analysis.
After filtering for known germline variants in publicly available databases, only alterations predicted with the accredited variant annotation pipeline were used in these analyses.18 These alterations were annotated as known, likely, ambiguous, or unknown. To reduce false-positives, only alterations annotated as known or likely to be pathogenic were included for subsequent analyses as driver alterations. All sequencing and annotation were done without knowledge of patients’ treatment assignments or outcomes.
Patient Selection
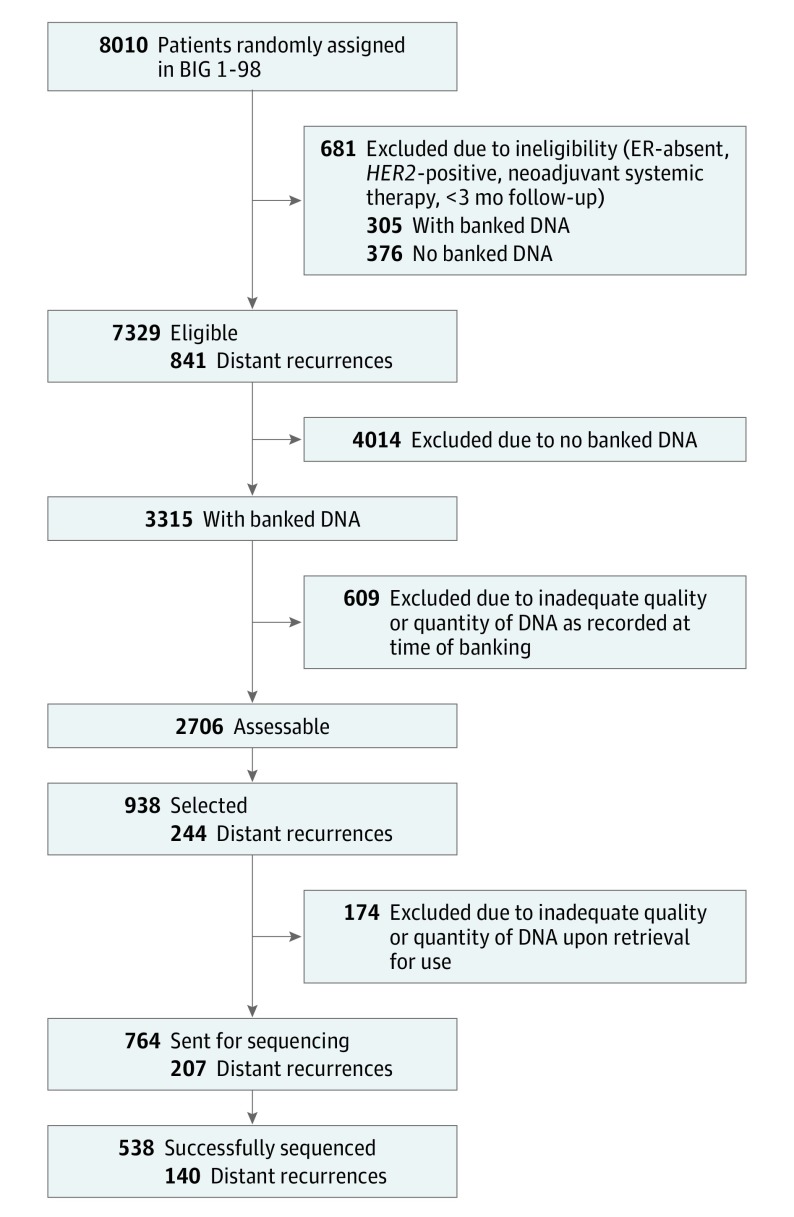
Patient selection is summarized in Figure 1. Patients were ineligible if the tumor was ER-absent or HER2-positive on central pathology assessment, neoadjuvant endocrine or chemotherapy was administered, or follow-up was less than 3 months. Of the 8010 randomized patients in the BIG 1-98 trial, there were 7329 eligible patients with 841 (11.5%) distant recurrences. From the 2706 patients with banked, assessable DNA extracted from formalin-fixed, paraffin-embedded archival tumor samples, 938 patients were selected, using a sampling plan identifying all patients having a distant recurrence and a stratified random sampling of those without recurrence (stratified by randomization option [2 groups vs 4 groups], treatment assignment, nodal status [negative vs positive], and luminal A- or B-like status19). After further exclusion for inadequate DNA quality or quantity, 764 samples were sent for sequencing. To correct for oversampling of distant recurrences, weighted analysis methods were used. Sampling weights were calculated for 72 classes, defined by the presence or absence of distant recurrence and the 36 strata, as the inverse of the sampling fractions. Analysis was conducted from April 4, 2016, to November 30, 2016.
Figure 1. Flow Diagram of Patient Selection for the Analysis Population.
ER indicates estrogen receptor.
Statistical Analysis
At the design stage, we estimated that, with 350 (10%) distant recurrence events in the eligible cohort and a sampling fraction of 0.10 for a total sample size of 700 patient samples, there would be 80% power to detect hazard ratios (HRs) for association of driver alterations with distant recurrence-free interval of 1.5 for alteration prevalence of 20%, and HR of 2.0 for alteration prevalence of 5%, with nominal α = .05 tests.20
The primary end point of interest was distant recurrence-free interval, defined as time from randomization to recurrence at a distant site. Patients without distant recurrence were censored at the date of last follow-up or death. Driver alterations were categorized as present or absent (for short variants, as mutated vs wild type; for amplifications, as amplified vs nonamplified). Because oncogenic driver mutations in PIK3CA cluster in distinct functional protein domains,21 we annotated PIK3CA mutations by affected protein domain: kinase or helical domain mutations. PIK3CA mutations not affecting kinase or helical protein domains were uncommon, less well described, and annotated as other. Exploratory analyses by previously described PIK3CA mutation hotspots (N345, C420, P539, E542, E545, E546, H1047) were performed.22
The generalized Horvitz-Thompson weighted method (inverse probability weighting) was applied to all analyses using the sample weights previously described.23 For clinicopathologic associations, weighted χ2 tests were used for categorical variables (age, tumor size, nodal status, and grade) and weighted t tests were used for Ki-67 (percent). Pairwise associations were investigated without sampling weights, characterized by odds ratios and using Fisher exact test to generate P values; multiple testing correction using the Benjamini-Hochberg method was applied using a false discovery rate of less than 0.2. For the remainder of the analyses, we deemed an unadjusted, 2-sided value of P < .05 to be significant.
Weighted Cox proportional hazards regression models adjusted for treatment arm were used to determine prognostic associations. For each driver alteration, models were adjusted for treatment arm and included the following variables: age, tumor size, nodal status, and grade. Treatment-by-PIK3CA genotype interactions were used to assess the outcome of monotherapy in tumors with or without a kinase or helical domain PIK3CA mutation (both wild-type and other PIK3CA mutations). Hazard ratios and 95% CIs were generated using robust SEs, with a Wald test for significance. Weighted Kaplan-Meier curves were generated for visualization purposes. Subpopulation treatment effect pattern plot (STEPP)24 methodology was used in exploratory analyses to illustrate the association between Ki-67 level, PIK3CA mutation status, and disease outcome.
All statistical analyses were prespecified. We used R software, version 3.3.1 (R Foundation) and Stata, version 14 (StataCorp) for the statistical analyses.
Results
Driver Alterations in ER+/HER2− Postmenopausal Early-Stage BC
Of 764 samples, 538 (70.4%) successfully underwent DNA sequencing and met quality metrics. The median exon depth of targeted genes ranged from 151 × to 1397 × , with an average of 476 × . Patient characteristics are summarized in eTable 2 in the Supplement alongside the eligible patients from the BIG 1-98 trial (n = 7329).
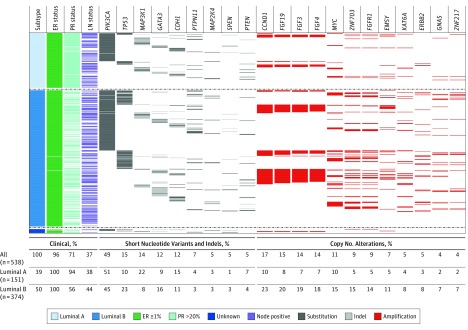
Nineteen driver alterations had a prevalence of 5% or greater, including 9 genes with recurrent mutations and 10 genes with recurrent focal amplifications (Figure 2; eTable 3 in the Supplement). PIK3CA mutations were the most common (49%), followed by CCND1 (Entrez 595) amplifications (17%), and TP53 (Entrez 7157) short-variants (15%). PIK3CA-mutated tumors were commonly coaltered (76%) and exhibited heterogeneous coalteration partners (eFigures 1, 2, and 3 in the Supplement). Coalteration frequencies by PIK3CA genotype were similar, with the exception of amplifications on chromosome 11q13, which were significantly more common in wild-type tumors (eFigure 4 in the Supplement). No identifiable driver alteration was identified in 5.7% of patient samples. There was a mean of 4 driver alterations (range, 0-15; interquartile range, 2-6) per tumor. Driver mutations were uncommon for AKT1 (Entrez 207) (4%), ERBB2 (Entrez 2064) (2%), and ESR1 (Entrez 2099) (0%). There were no recurrent structural rearrangements.
Figure 2. The Landscape of Somatic Driver Alterations in the Breast International Group 1-98 Trial.
Only somatic driver alterations with a weighted frequency of 4% or greater in the whole cohort are displayed. All percentages shown are weighted frequencies with the exception of estrogen receptor (ER) and progesterone receptor (PR) expression. Luminal-like status was determined using the published St Gallen 2013 consensus.19 Thirteen patients were luminal-like status unknown because of missing data. Numbers in parentheses indicate absolute numbers of patients per cohort and subtype. Indels indicates insertions and deletions; LN, lymph node.
Association With Clinicopathologic Characteristics
The associations between driver alterations and clinicopathologic variables are summarized (eFigures 5 and 6 and eTable 4 in the Supplement). MAP3K1 (Entrez 4214), SPEN (Entrez 23013), and PTPN11 (Entrez 5781) mutations were significantly associated with larger tumor size. MAP2K4 (Entrez 6416) mutations were significantly associated with age older than 65 years. There were no significant associations of driver alterations with nodal status. PIK3CA and MAP3K1 mutations were significantly associated with lower tumor grade and lower Ki-67 level. Conversely, TP53 mutations and several focal gene amplifications (CCND1, FGFR1 [Entrez 2260], MYC [Entrez 4609], and ZNF703 [Entrez 80139]) were significantly associated with higher tumor grade and higher Ki-67 levels. We observed that PIK3CA-mutated tumors were particularly heterogeneous in terms of coexisting alterations and their Ki-67 levels (eFigure 5 in the Supplement).
Coexistence and Mutual Exclusivity Between Somatic Alterations
To understand biological relationships between driver alterations, we examined for pairwise interactions (eFigure 7 and eTable 5 in the Supplement). TP53 mutations were significantly associated with the coexistence of several gene amplifications (MYC, ERBB2, FGFR1, ZNF703, and ZNF217 [Entrez 7764]). Strong mutual coexistence was observed between several gene amplifications consistent with coamplification of the same amplicon (CCND1 [Entrez 14174], FGF3, FGF4 [Entrez 14175], FGF19 [Entrez 9965], and EMSY [Entrez 56946] on 11q13; FGFR1, ZNF703, and GPR124 [Entrez 25960] on 8p11; and GNAS [Entrez 2778] and ZNF217 on 20q13). These gene amplifications were subsequently combined and analyzed per amplicon for prognostic associations.
Associations With Prognosis
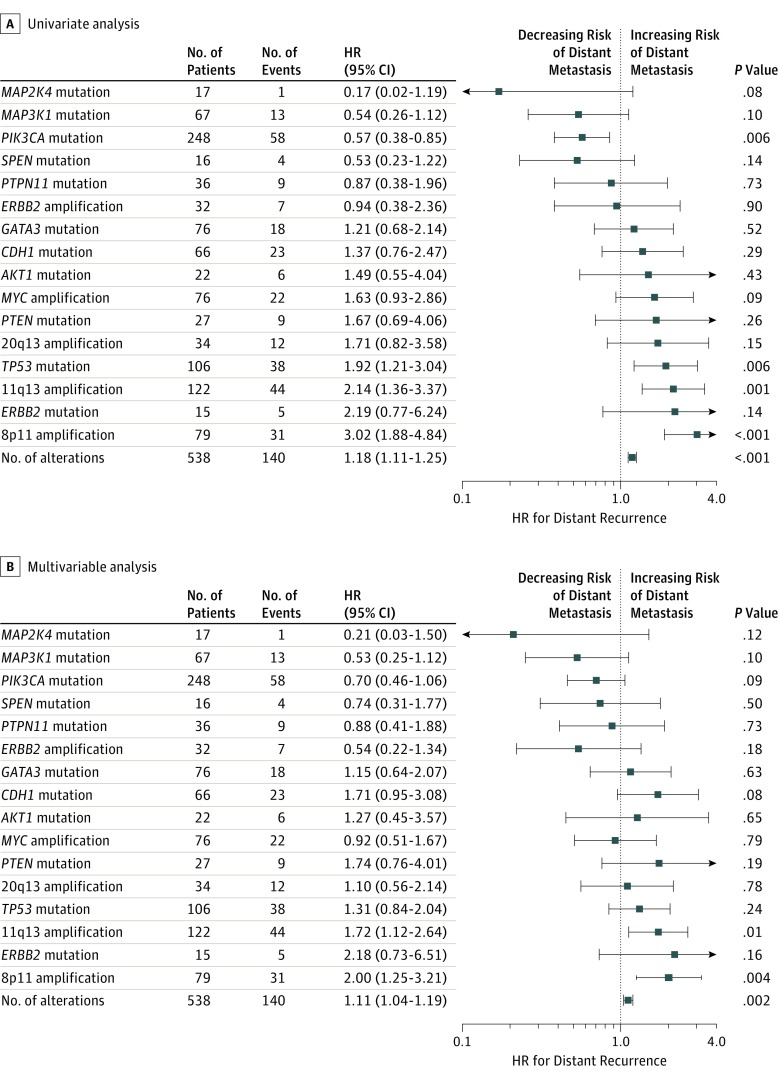
We evaluated associations between frequent driver alterations (≥5% frequency) and prognosis in both univariate and multivariable models (Figure 3). In univariate analysis, PIK3CA mutations were significantly associated with reduced risk of distant recurrence (HR, 0.57; 95% CI, 0.38-0.85; P = .006). Analysis of PIK3CA mutations by protein domain vs wild-type PIK3CA demonstrated favorable outcomes for all subgroups (kinase domain, helical domain, and other) (eTable 6 in the Supplement). Conversely TP53 mutations (HR, 1.92; 95% CI, 1.21-3.04; P = .006), 11q13 amplifications (HR, 2.14; 95% CI, 1.36-3.37; P = .001), and 8p11 amplifications (HR, 3.02; 95% CI, 1.88-4.84; P < .001) were associated with significantly increased risk of distant recurrence (eFigure 8 in the Supplement). In multivariable analysis adjusting for age, tumor size, nodal status, and grade, only 11q13 amplifications (HR, 1.72; 95% CI, 1.12-2.64; P = .01) and 8p11 amplifications (HR, 2.00; 95% CI, 1.25-3.21; P = .004) remained statistically significant. Increasing number of somatic driver alterations per tumor was also associated with worse outcomes in both univariate (HR, 1.18; 95% CI, 1.11-1.25; P < .001) and multivariable (HR, 1.11; 95% CI, 1.04-1.19; P = .002) analysis.
Figure 3. Prognostic Analyses for Distant Recurrence.
Weighted univariate and multivariable Cox proportional hazards regression analyses are shown for distant recurrence-free interval. The hazard ratio (HR) compares the mutated or amplified status vs the wild-type or nonamplified status of the gene (or amplicon), respectively. Number of alterations was evaluated as a continuous variable per additional driver alteration. Number of events indicates the observed number of distant recurrences in the analysis cohort. The univariate Cox proportional hazards regression model was adjusted for treatment arm, and the multivariable model was adjusted for treatment arm, age, tumor size, nodal status, and grade as described in the Methods section. Focal gene amplifications on chromosomes 20q13, 11q13, and 8p11 are described in the Results section.
Interaction With Treatment: Letrozole vs Tamoxifen
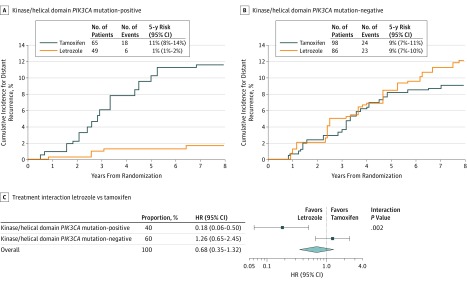
We next investigated whether PIK3CA mutations were associated with a significant treatment interaction. Consistent with our hypothesis, patients with tumors harboring a PIK3CA mutation (kinase or helical, n = 114) derived a greater magnitude of benefit with adjuvant letrozole over tamoxifen (HR, 0.18; 95% CI, 0.06-0.50) than patients whose tumors did not (n = 184) (HR, 1.26; 95% CI, 0.65-2.45; P interaction = .002) (Figure 4; eTable 7 in the Supplement). Further sensitivity analysis by PIK3CA mutation hotspot demonstrated similar results (eTable 8 in the Supplement). Of these patients, 36% were node-positive and 25% had received adjuvant chemotherapy. Patients whose tumors harbored a kinase or helical domain PIK3CA mutation and received letrozole monotherapy had an excellent prognosis, with an estimated 5-year distant recurrence-free proportion of 99% (95% CI, 98%-99%). A statistically significant difference in treatment effect (P interaction = .01) was also observed in the subgroup of no chemotherapy.
Figure 4. Treatment Interactions.
A and B, Weighted cumulative incidence curves demonstrating the prognostic effect of letrozole or tamoxifen monotherapy on the risk of distant recurrence by PIK3CA mutation status. Number of events indicates the observed number of distant recurrences per subgroup. The 5-year estimates for distant recurrence and 95% CIs were calculated with the use of sampling weights. C, Hazard ratio (HR) for letrozole monotherapy vs tamoxifen monotherapy according to PIK3CA mutation status. These data were derived with a weighted univariate Cox proportional hazards regression model. The interaction P value represents the interaction between PIK3CA mutation status and treatment benefit. The observed number of patients (number of events) was 114 (24) for the PIK3CA mutation-positive group and 184 (47) for PIK3CA mutation-negative group.
Higher Ki-67 levels have been reported to be predictive of increased benefit to adjuvant letrozole.25 As an exploratory analysis, we investigated the association between Ki-67 level and the presence of kinase/helical domain PIK3CA mutations as predictive biomarkers.25 In a Cox proportional hazards multivariable model that included adjustment for Ki-67 level, the treatment interaction with PIK3CA mutation status remained statistically significant (P = .03) (eTable 9 in the Supplement). We further performed a STEPP analysis to examine the association between Ki-67 level as a continuous variable and disease outcome, adjusted for PIK3CA mutation status (eFigure 9 in the Supplement). As illustrated, the increased magnitude of benefit of adjuvant letrozole appeared to be driven by PIK3CA mutation status independent of Ki-67 levels.
Discussion
In this analysis of primary tumor samples from postmenopausal women with ER+/HER2− BC from the randomized phase 3 BIG 1-98 study, we have described the landscape of driver alterations, their association with clinicopathologic factors, and prognostic associations for distant recurrence-free interval. Furthermore, we report that patients with tumors harboring kinase or helical domain PIK3CA mutations derived a greater magnitude of benefit with letrozole over tamoxifen therapy and had long-term freedom from distant recurrence. To our knowledge, this is the first study to report a DNA-based predictive biomarker in this setting. Given that relapse from ER+/HER2− disease is the major cause of death from BC, our results are important in understanding the biological mechanisms of aggressive ER+/HER2− disease.
Similar to previous studies in heterogeneously selected BCs, we observed a diverse driver alteration landscape.1,3,4,26 PIK3CA mutations were the most common, followed by TP53 mutations, with many other mutations occurring at lesser frequencies. One difference in the BIG 1-98 data set was that recurrent mutations in PTPN11 and SPEN were detected, which have only recently been described in BC,27,28,29 representing potential new drug targets as well as novel biology. Amplifications were frequent, with recurrent amplicons on chromosomes 11 (11q13/14), 8 (8p11), and 20 (20q13) detected.3 We observed significant coexistence associations between TP53 mutations and several focal gene amplifications (CCND1, FGFR1, MYC, and ZNF703), higher grade and Ki-67, suggesting that these alterations cooperate synergistically to produce a genomically unstable and aggressive ER+ BC phenotype.4,26
With respect to risk of distant recurrence, TP53 mutations and amplifications on 11q13 and 8p11 were associated with significantly poorer outcome. This outcome has been shown previously,3,4,30,31 with higher proliferation rates and promotion of endocrine resistance. Our unique data set lends support to their prognostic implications in early-stage postmenopausal ER+ BC. A novel finding was that progressively poorer outcomes were observed as numbers of driver alterations increased. This has been shown previously in myelodysplasia33 and suggests that driver alterations have significant implications even if they are subclonal, consistent with an adverse prognostic role described for intratumor heterogeneity34 and the presence of subclonal drivers35 observed in other cancer types.
As expected, tumor PIK3CA mutations were associated with a better outcome but did not remain significant in the multivariable model owing to associations with good prognosis variables.15,22,32,36 Our data, however, highlight the diversity in coexisting driver alterations with PIK3CA. This variation may result in a heterogeneous clinical phenotype but also suggests the need for future clinical studies in this setting to take genomic context into consideration.
We also report the novel finding of a predictive role for PIK3CA genotype in identifying patients who derived greater benefit from 5 years of letrozole adjuvant therapy. In patients with tumors harboring kinase/helical PIK3CA mutations, the estimated proportion of patients without a distant recurrence at 5 years for those who received 5 years of letrozole was 99%. This finding is biologically plausible15,16 and important as PIK3CA mutations are enriched in ER+/HER2− BC. Further studies to establish the exact mechanisms underlying this observation and validate our finding are warranted. If confirmed, PIK3CA genotyping could be easier to implement in the clinic because of the ease of interpreting a binary result (mutated vs not) and reproducibility of a DNA-based test.
Higher Ki-67 levels have previously been reported to be predictive of increased benefit to adjuvant letrozole.25 However, to our knowledge, no prior study has established the interaction between Ki-67 and PIK3CA mutation status on treatment effect comparing letrozole with tamoxifen. The interaction between PIK3CA status and treatment assignment remained statistically significant in a multivariable model, suggesting the genotype had independent predictive value even after adjustment for Ki-67 level. Moreover, using STEPP analysis (eFigure 9 in the Supplement), the magnitude of increased benefit of letrozole at higher Ki-67 levels appeared to be limited to the PIK3CA-mutated subgroup, suggesting that PIK3CA genotype provided additional predictive value above Ki-67 alone.
Although retrospective, our data are strengthened by the use of a clinical trial data set with prospective treatment randomization and robust recurrence data with a median follow-up of 8 years. The strength of biomarker analyses in this context has been previously reported.37
Limitations
Our study had a limited ability to examine subgroups and infrequent driver alterations. While we used only 20% of the samples from the total trial cohort, the sampling design and weighted analysis is a financially efficient, well-accepted, and methodologically sound approach for a biomarker study (eTable 2 in the Supplement).23,38 For example, the lobular histologic subtype has also been reported to derive increased benefit from adjuvant letrozole.39 While lobular carcinomas have been recently reported to harbor high frequencies of PIK3CA mutations,40 our study was underpowered to confirm that PIK3CA mutations are independently driving this outcome in the lobular carcinomas.
Conclusions
Our study highlights the feasibility of DNA sequencing of formalin-fixed, paraffin-embedded samples,18 especially as next-generation sequencing technologies become increasingly accessible as costs reduce. We propose that DNA-based biomarkers have advantages compared with gene expression–based prognostic scores7,8,9 in that categorical findings are achieved rather than continuous scores, reducing the uncertainty of intermediate values as well as the chance to identify therapeutic targets.
We have described the prognostic and predictive relevance of somatic driver alterations in ER+/HER2− early BC in postmenopausal women from a large, phase 3 adjuvant randomized study. DNA-based classifications have promise to add to current prognostic markers and aid our adjuvant treatment decisions in ER+/HER2− disease.
eTable 1. DNA Sequencing Target Gene Panel
eTable 2. Characteristics of the Entire Eligible BIG 1-98 Cohort and of the Analysis Population
eTable 3. Somatic Driver Alterations and Weighted Frequencies
eTable 4. Association With Clinicopathologic Characteristics
eTable 5. Pairwise Aanalysis – q Values
eTable 6. Prognostic Associations by Affected PIK3CA Protein Domain
eTable 7. Predictive Associations by Affected PIK3CA Protein Domain
eTable 8. Predictive Associations by Affected PIK3CA Mutation Hotspot
eTable 9. Treatment Interaction With PIK3CA Mutation Status in a Multivariate Cox Proportional Hazards Model
eFigure 1. Coexistent Alterations in Tumors With a PIK3CA Mutation
eFigure 2. PIK3CA Mutations and Affected Protein Domains
eFigure 3. Coalterations by Affected PIK3CA Protein Domain
eFigure 4. Coalteration Frequencies by PIK3CA Genotype
eFigure 5. Associations With Ki-67 (%) Levels
eFigure 6. Association of Pathological Characteristics by Affected PIK3CA Protein Domain
eFigure 7. Pairwise Analysis
eFigure 8. Prognostic Association of Frequent Amplicons With DRFI
eFigure 9. STEPP Analysis by PIK3CA Mutation Status
References
- 1.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens PJ, Tarpey PS, Davies H, et al. ; Oslo Breast Cancer Consortium (OSBREAC) . The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486(7403):400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis C, Shah SP, Chin SF, et al. ; METABRIC Group . The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira B, Chin SF, Rueda OM, et al. . The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nik-Zainal S, Davies H, Staaf J, et al. . Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin MB, Edge S, Greene F, et al. . AJCC Cancer Staging Manual. 8th ed Cham, Switzerland: Springer International Publishing; 2017. [Google Scholar]
- 7.Paik S, Shak S, Tang G, et al. . A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817-2826. [DOI] [PubMed] [Google Scholar]
- 8.van de Vijver MJ, He YD, van’t Veer LJ, et al. . A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999-2009. [DOI] [PubMed] [Google Scholar]
- 9.Parker JS, Mullins M, Cheang MC, et al. . Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360(8):790-800. [DOI] [PubMed] [Google Scholar]
- 11.Thürlimann B, Keshaviah A, Coates AS, et al. ; Breast International Group (BIG) 1-98 Collaborative Group . A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747-2757. [DOI] [PubMed] [Google Scholar]
- 12.Mouridsen H, Giobbie-Hurder A, Goldhirsch A, et al. ; BIG 1-98 Collaborative Group . Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361(8):766-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regan MM, Neven P, Giobbie-Hurder A, et al. ; BIG 1-98 Collaborative Group; International Breast Cancer Study Group (IBCSG) . Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol. 2011;12(12):1101-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner NC, Neven P, Loibl S, Andre F. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet. 2017;389(10087):2403-2414. [DOI] [PubMed] [Google Scholar]
- 15.Loi S, Haibe-Kains B, Majjaj S, et al. . PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor–positive breast cancer. Proc Natl Acad Sci U S A. 2010;107(22):10208-10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tikoo A, Roh V, Montgomery KG, et al. . Physiological levels of Pik3ca(H1047R) mutation in the mouse mammary gland results in ductal hyperplasia and formation of ERα-positive tumors. PLoS One. 2012;7(5):e36924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colleoni M, Giobbie-Hurder A, Regan MM, et al. . Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol. 2011;29(9):1117-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frampton GM, Fichtenholtz A, Otto GA, et al. . Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Winer EP, Coates AS, et al. ; Panel members . Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai J, Zeng D. Sample size/power calculation for case-cohort studies. Biometrics. 2004;60(4):1015-1024. [DOI] [PubMed] [Google Scholar]
- 21.Samuels Y, Wang Z, Bardelli A, et al. . High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. [DOI] [PubMed] [Google Scholar]
- 22.Sabine VS, Crozier C, Brookes CL, et al. . Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. J Clin Oncol. 2014;32(27):2951-2958. [DOI] [PubMed] [Google Scholar]
- 23.Gray RJ. Weighted analyses for cohort sampling designs. Lifetime Data Anal. 2009;15(1):24-40. [DOI] [PubMed] [Google Scholar]
- 24.Yip WK, Bonetti M, Cole BF, et al. . Subpopulation treatment effect pattern plot (STEPP) analysis for continuous, binary, and count outcomes. Clin Trials. 2016;13(4):382-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viale G, Giobbie-Hurder A, Regan MM, et al. ; Breast International Group Trial 1-98 . Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26(34):5569-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis MJ, Ding L, Shen D, et al. . Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486(7403):353-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Z, Wang X, Fang H, et al. . A tyrosine phosphatase SHP2 gain-of-function mutation enhances malignancy of breast carcinoma. Oncotarget. 2016;7(5):5664-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Légaré S, Cavallone L, Mamo A, et al. . The estrogen receptor cofactor SPEN functions as a tumor suppressor and candidate biomarker of drug responsiveness in hormone-dependent breast cancers. Cancer Res. 2015;75(20):4351-4363. [DOI] [PubMed] [Google Scholar]
- 29.Savas P, Teo ZL, Lefevre C, et al. . The subclonal architecture of metastatic breast cancer: results from a Prospective Community-Based Rapid Autopsy Program “CASCADE.” PLoS Med. 2016;13(12):e1002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller LD, Smeds J, George J, et al. . An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005;102(38):13550-13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bièche I, Olivi M, Noguès C, Vidaud M, Lidereau R. Prognostic value of CCND1 gene status in sporadic breast tumours, as determined by real-time quantitative PCR assays. Br J Cancer. 2002;86(4):580-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalinsky K, Jacks LM, Heguy A, et al. . PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res. 2009;15(16):5049-5059. [DOI] [PubMed] [Google Scholar]
- 33.Papaemmanuil E, Gerstung M, Malcovati L, et al. ; Chronic Myeloid Disorders Working Group of the International Cancer Genome Consortium . Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerlinger M, Rowan AJ, Horswell S, et al. . Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landau DA, Carter SL, Stojanov P, et al. . Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152(4):714-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson TR, Yu J, Lu X, et al. . The molecular landscape of high-risk early breast cancer: comprehensive biomarker analysis of a phase III adjuvant population. NPJ Breast Cancer. 2016;2:16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101(21):1446-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165-1172. [DOI] [PubMed] [Google Scholar]
- 39.Metzger Filho O, Giobbie-Hurder A, Mallon E, et al. . Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1-98 Trial. J Clin Oncol. 2015;33(25):2772-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desmedt C, Zoppoli G, Gundem G, et al. . Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol. 2016;34(16):1872-1881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. DNA Sequencing Target Gene Panel
eTable 2. Characteristics of the Entire Eligible BIG 1-98 Cohort and of the Analysis Population
eTable 3. Somatic Driver Alterations and Weighted Frequencies
eTable 4. Association With Clinicopathologic Characteristics
eTable 5. Pairwise Aanalysis – q Values
eTable 6. Prognostic Associations by Affected PIK3CA Protein Domain
eTable 7. Predictive Associations by Affected PIK3CA Protein Domain
eTable 8. Predictive Associations by Affected PIK3CA Mutation Hotspot
eTable 9. Treatment Interaction With PIK3CA Mutation Status in a Multivariate Cox Proportional Hazards Model
eFigure 1. Coexistent Alterations in Tumors With a PIK3CA Mutation
eFigure 2. PIK3CA Mutations and Affected Protein Domains
eFigure 3. Coalterations by Affected PIK3CA Protein Domain
eFigure 4. Coalteration Frequencies by PIK3CA Genotype
eFigure 5. Associations With Ki-67 (%) Levels
eFigure 6. Association of Pathological Characteristics by Affected PIK3CA Protein Domain
eFigure 7. Pairwise Analysis
eFigure 8. Prognostic Association of Frequent Amplicons With DRFI
eFigure 9. STEPP Analysis by PIK3CA Mutation Status