This population-based study quantifies the association of attending surgeon attitudes with rates of genetic testing after diagnosis of breast cancer using information from patient and surgeon surveys merged to SEER and genetic testing data.
Key Points
Question
To what extent is attending surgeon associated with receipt of genetic testing after diagnosis of breast cancer?
Findings
In this population-based study of 7810 women, the attending surgeon explained 17.4% of the variation in testing. If a patient at higher pretest risk saw a surgeon at the 5th percentile of the surgeon distribution, she would have a 26.3% probability of testing compared with 72.3% if she saw a surgeon at the 95th percentile.
Meaning
Attending surgeons have an association with variation in the receipt of genetic testing after diagnosis of breast cancer.
Abstract
Importance
Genetic testing after diagnosis of breast cancer is common, but little is known about the influence of the surgeon on the variation in testing.
Objectives
To quantify and explain the association of attending surgeon with rates of genetic testing after diagnosis of breast cancer.
Design, Setting, and Participants
This population-based study identified 7810 women with stages 0 to II breast cancer treated between July 1, 2013, and August 31, 2015, through the Surveillance, Epidemiology, and End Results registries for the state of Georgia, as well as Los Angeles County, California. Surveys were sent approximately 2 months after surgery. Also surveyed were 488 attending surgeons identified by the patients.
Main Outcomes and Measures
The study examined the association of surgeon with variation in the receipt of genetic testing using information from patient and surgeon surveys merged to Surveillance, Epidemiology, and End Results and genetic testing data obtained from 4 laboratories.
Results
In total, 5080 women (69.6%) of 7303 who were eligible (mean [SD] age, 61.4 [0.8] years) and 377 surgeons (77.3%) of 488 (mean [SD] age, 53.8 [10.7] years) responded to the survey. Approximately one-third (34.5% [1350 of 3910] of patients had an elevated risk of mutation carriage, and 27.0% (1056 of 3910) overall had genetic testing. Surgeons had practiced a mean (SE) of 20.9 (0.6) years, and 28.9% (107 of 370) treated more than 50 cases of new breast cancer per year. The odds of a patient receiving genetic testing increased more than 2-fold (odds ratio, 2.48; 95% CI, 1.85-3.31) if she saw a surgeon with an approach 1 SD above that of a surgeon with the mean test rate. Approximately one-third (34.1%) of the surgeon variation was explained by patient volume and surgeon attitudes about genetic testing and counseling. If a patient with higher pretest risk saw a surgeon at the 5th percentile of the surgeon distribution, she would have a 26.3% (95% CI, 21.9%-31.2%) probability of testing compared with 72.3% (95% CI, 66.7%-77.2%) if she saw a surgeon at the 95th percentile.
Conclusions and Relevance
In this study, the attending surgeon was associated with the receipt of genetic testing after a breast cancer diagnosis. Variation in surgeon attitudes about genetic testing and counseling may explain a substantial amount of this association.
Introduction
Approximately one-third of patients receive genetic testing after diagnosis of breast cancer, and testing with a multigene panel is rapidly replacing tests based on only BRCA1 and BRCA2 in clinical practice.1,2,3,4 Surgeons have an important role in whether patients get tested because virtually all patients see a surgeon shortly after diagnosis, surgeons direct locoregional management, and genetic testing results inform the surgical treatment options. Guidelines recommend genetic testing for patients diagnosed as having breast cancer who have an elevated risk of a pathogenic mutation based on age, family history of cancer, and tumor characteristics.5 However, substantial variability has previously been documented in surgeon attitudes and practices related to genetic testing.1,2 Therefore, it is plausible that patients newly diagnosed as having breast cancer with similar indications for testing may have different probabilities of getting tested depending on the surgeon who treats them. This has important implications for patient care. The variability in testing that is attributable to the surgeon rather than clinical indication might prompt efforts to educate surgeons about genetic risk evaluation or motivate patients to seek a second opinion.6 However, no studies to date have estimated the extent to which genetic testing rates vary at the level of the surgeon or what the patient or surgeon correlates of that variation are. We examined the association of the attending surgeon with the receipt of genetic testing in a large diverse contemporary sample of patients newly diagnosed as having breast cancer. Our hypothesis was that the individual attending surgeon might explain a large amount of the variability in testing and that surgeon attitudes about genetic testing and counseling would explain a substantial amount of that surgeon-level variability.
Methods
Study Design
Patient Sample and Data Collection
The iCanCare study,7,8 broadly focused on treatment quality in patients with favorable breast cancer diagnoses, identified women who were aged 20 to 79 years, were diagnosed as having ductal carcinoma in situ or invasive breast cancer, and were reported to the Georgia or Los Angeles County (California) Surveillance, Epidemiology, and End Results (SEER) registry. Surveys were sent on a monthly basis approximately 2 months after surgery to 7810 women with stages 0 to II breast cancer treated between July 1, 2013, and August 31, 2015. Details of the survey procedures have been published elsewhere,9 and the survey sampling is shown in eFigure 1 in the Supplement. Information Management Services, Inc (Rockville, Maryland) merged survey responses and SEER clinical data with genetic testing information obtained from 4 laboratories (Ambry Genetics [Aliso Viejo, California], GeneDx [Gaithersburg, Maryland], Invitae [San Francisco, California], and Myriad Genetics [Salt Lake City, Utah]) that tested patients in the study regions and sent a deidentified data set to the University of Michigan, Ann Arbor, for analysis.10 The collaboration was covered under data use agreements between the University of Michigan, Information Management Services, Inc, and the genetic laboratories. The research was approved by institutional review boards of the University of Michigan (Ann Arbor), Emory University (Atlanta, Georgia), University of Southern California (Los Angeles), Georgia Department of Public Health (Atlanta), California State Committee for the Protection of Human Subjects, and California Cancer Registry. The institutional review boards granted a waiver of signed informed consent for the patient and clinician surveys. Patient consent for analyses of linked data was not required because all database records were deidentified.
Patient and Surgeon Sample
Almost all respondent patients (98.0% [4980 of 5080]) identified an attending surgeon. Surveys were sent to surgeons toward the end of the patient data collection period (488 attending surgeons identified by the patients), and 377 completed them (response rate, 77.3%). We linked 3910 respondent patients (53.5% of the 7303 patients sent questionnaires) to 370 respondent surgeons. On average, there were 10.5 (range, 1-84) patients per surgeon. We excluded 218 patients from the multivariable analyses because of missing surgeon-level information. We assessed potential bias due to missing data in sensitivity analyses using multiply imputed surgeon data and found model estimates of similar magnitude, direction, and 95% CI width (eAppendix in the Supplement).
Measures
The dependent variable was the receipt of genetic testing based on the laboratory-linked information. Patient covariates included an indicator of elevated pretest risk of a pathogenic mutation following National Comprehensive Cancer Network guidelines (based on patient report of age, tumor characteristics, and detailed family history of cancer),11 as well as race/ethnicity, insurance status, and geographic location.
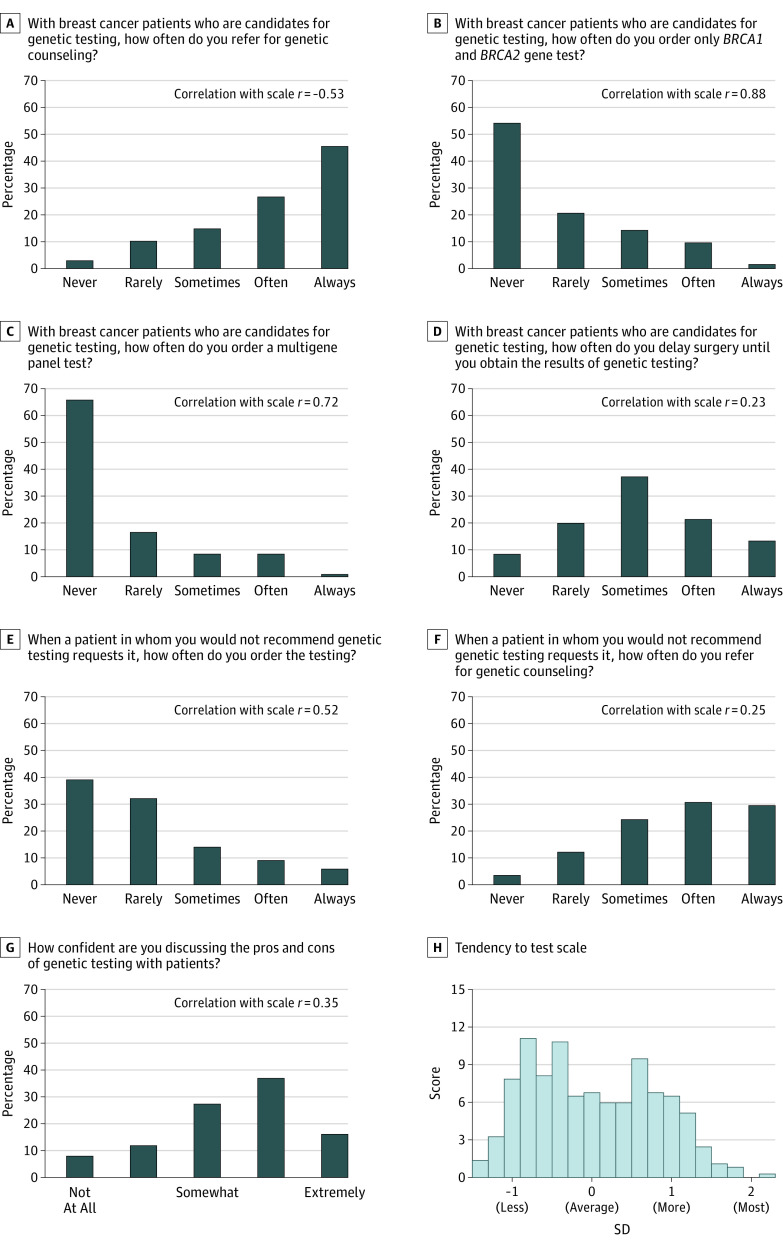
Surgeon variables considered included a unique surgeon identifier, years in practice, sex, and report of the annual volume of newly diagnosed breast cancer cases treated. We hypothesized that the patients of surgeons who reported that they were more likely to order genetic testing across a variety of indications and situations would be more likely to receive genetic testing. We used item response theory12,13 to develop a Surgeon Tendency to Order Genetic Testing Scale (hereafter referred to as the Tendency to Test Scale) for each surgeon based on 7 questions that assessed their likelihood of ordering genetic testing and counseling in different scenarios. Figure 1 shows 7 items that comprised the scale: 4 items refer to a scenario in which a patient is deemed by the surgeon respondent to be a candidate for testing, 2 items refer to a scenario in which the surgeon respondent does not recommend testing but the patient requests it, and 1 item assesses the surgeon’s confidence in discussing genetic testing. We used a graded response model to develop the surgeon Tendency to Test Scale to incorporate hierarchical categorical responses to each of the 7 items and estimate a corresponding latent trait for each surgeon. The estimated value of the latent trait ranged from −1.4 to 2.3 for the surgeons in our study and was standardized to have a mean (SD) of 0 (1). The correlations reported on the graphs quantify how each item contributes to the scale score, representing surgeon tendency to order genetic testing.
Figure 1. Surgeon Attitudes About Genetic Testing and Counseling.
A-G, The Tendency to Test Scale, a scale developed by the authors, was constructed from 7 survey items. The correlations with the overall surgeon Tendency to Test Scale reported on each panel provide some quantification of how each item contributes to the scale score, which represents the tendency to order genetic testing. H, The scale distribution from −2 (strongest tendency against testing) to 2 (strongest tendency for testing) is shown based on the item response model using the 7 items. The mean (SD) scale score was near zero at 0.3 (0.8).
Statistical Analysis
We first described the distribution of the patient and surgeon characteristics. We then used a nonlinear mixed model to estimate the variation across surgeons in the probability of ordering genetic testing, adjusting for other factors. Models included patient pretest risk of a pathogenic mutation based on National Comprehensive Cancer Network guidelines5 for use of germline genetic testing because this would be expected to be the primary determinant of testing. We next considered surgeon factors in addition to our Tendency to Test Scale (eg, years in practice, sex, and annual patient volume) by examining the univariate association of several surgeon-level practice and demographic variables with genetic testing. Among these, the surgeon volume explained the highest proportion of the variation in testing and was added to the variable set. Finally, we included patient race/ethnicity and insurance status as potential covariates, which we found to be associated with receipt of genetic testing and which we infer to be individual-level proxies for access to testing. Patient geographic site was also included to account for potential regional differences. We built 3 nested multilevel logistic regression models of increasing complexity. Within each model, we used a surgeon identifier to define the second level and used the patient as the primary unit of observation.
Our base model included only pretest risk (average or higher). We show the probability of receiving genetic testing across our sample of surgeons separately for patients at average and high pretest risk using estimates from the base model. We used nonparametric methods to estimate 95% CIs of these probabilities. Our second model added surgeon-level variables (annual patient volume and the Tendency to Test Scale score). Finally, model 3 estimated the probability of genetic testing, including the additional patient-level variables race/ethnicity, insurance status, and geographic site in addition to the pretest risk and surgeon-level variables. Likelihood ratio tests were significant for the comparisons of the nested models, including the first vs second model (χ2 = 39; P < .001) and the second vs third model (χ2 = 101; P < .001). We further examined how the results would change if the dichotomous pretest risk variable was broken into its component parts and included in the models. While the overall model fit improved with the components of pretest risk included separately, the estimates of surgeon variation and the direction and magnitude of the other covariates changed little, so only the results for the dichotomous risk variable are presented. eFigure 2 in the Supplement describes the analysis of testing by individual components of pretest risk.
All models incorporated patient survey nonresponse weights and physician weights so that the statistical inference was representative of our target population. Surgeon survey descriptive results incorporated nonresponse weights based on both physician and average patient characteristics. We calculated variance-explained estimates.14 Analyses using multiply imputed data were consistent with the reported results. We used Proc GLIMMIX (SAS, version 9.4; SAS Institute Inc) for the regression analyses and grm package (R, version 3.4.1; R Foundation for Statistical Computing) for the item response theory analyses. P < .05 was considered statistically significant. All statistical tests were 2-sided.
Results
In total, 5080 women (69.6% of 7303 who were eligible; mean [SD] age, 61.4 [0.8] years) and 377 surgeons (77.3% of 488; mean [SD] age, 53.8 [10.7] years) responded to the survey. The Table summarizes the distribution of patient and surgeon characteristics. Approximately one-third of patients (34.5% [1350 of 3910]) had an elevated risk of mutation carriage; 27.0% (1056 of 3910) of the total patient sample had genetic testing (13.8% [353 of 2560] of women with average pretest risk and 52.1% [703 of 1350] of women with higher pretest risk). The mean (SE) number of years in practice of surgeon respondents was 20.9 (0.6). Approximately one-quarter (24.3% [90 of 370]) of surgeons were female, and approximately one-quarter (28.9% [107 of 370]) of surgeons treated more than 50 new breast cancer cases per year.
Table. Characteristics of 3910 Patients and 370 Surgeons.
| Characteristic | Value |
|---|---|
| Patients, No. (%) | |
| Pretest risk | |
| Average risk | 2560 (65.5) |
| High risk | 1350 (34.5) |
| Age, y | |
| <45 | 458 (11.7) |
| 45 to 60 | 1518 (38.8) |
| >60 to 70 | 1276 (32.6) |
| >70 | 658 (16.8) |
| Triple-negative disease | |
| No/not known | 3637 (93.0) |
| Yes | 273 (7.0) |
| Family history and Jewish ancestry | |
| No/not known | 3019 (77.2) |
| Yes | 891 (22.8) |
| Race/ethnicity | |
| White | 2081 (53.2) |
| Black | 729 (18.6) |
| Latina | 663 (17.0) |
| Asian | 345 (8.8) |
| Other/unknown/missing | 92 (2.4) |
| Insurance status | |
| None or missing | 408 (10.4) |
| Medicaid | 460 (11.8) |
| Medicare or VA | 1084 (27.7) |
| Private | 1958 (50.1) |
| Geographic site | |
| Emory | 2125 (54.3) |
| USC | 1785 (45.7) |
| Receipt of genetic testing | |
| No | 2854 (73.0) |
| Yes | 1056 (27.0) |
| Surgeons | |
| Time in practice, mean (SE), y | 20.9 (0.6) |
| Sex, No. (%) | |
| Male | 273 (73.8) |
| Female | 90 (24.3) |
| Missing | 7 (1.9) |
| New breast cancer cases per year, No. (%) | |
| 1-10 | 49 (13.2) |
| 11-20 | 85 (23.0) |
| 21-50 | 112 (30.3) |
| 51-100 | 56 (15.1) |
| >100 | 51 (13.8) |
| Missing | 17 (4.6) |
Abbreviations: Emory, Emory University (Atlanta, Georgia); USC, University of Southern California (Los Angeles); VA, Veterans Affairs.
Figure 1 shows the frequency of responses for the 7 individual items that comprised the surgeon Tendency to Test Scale, the correlation between each item and the scale, and the distribution of the scale. Figure 1B, C, and E, which ask directly about how often surgeons order genetic testing, are correlated with the Tendency to Test Scale, whereas surgeons who report that they are more likely to refer patients for genetic counseling scored lower on the Tendency to Test Scale (negatively correlated with the scale). Surgeons who would often or always delay surgery to obtain genetic testing (35.2% [130 of 370]) and who are quite or extremely confident in discussing genetic testing with their patients (50.2% [186 of 370) scored higher on the Tendency to Test Scale (positively correlated with the scale). Figure 1H shows the distribution of the estimated Tendency to Test Scale scores based on the item response model using Figure1A through G. The scale is presented in units of SDs from the mean, with −1 SD representing the tendency against testing and 1 SD representing the tendency for testing. Estimated scale scores varied broadly, with the suggestion of a bimodal distribution.
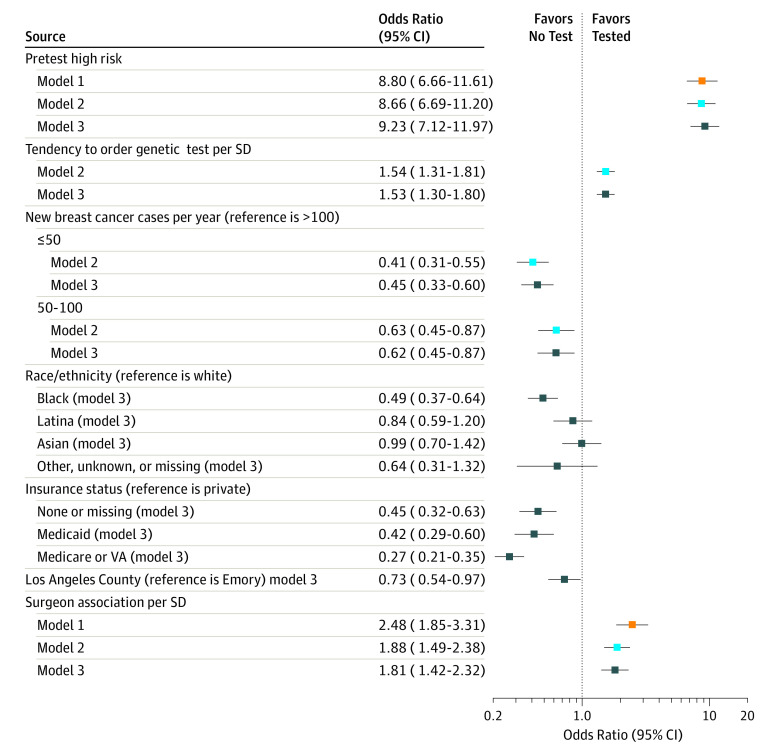
Figure 2 shows the results of the 3 successive multilevel models. The base model 1 (orange boxes) included the surgeon identifier and elevated pretest risk of mutation carriage, which (as expected) has a large association with testing, with an odds ratio of approximately 8.80 (95% CI, 6.66-11.61), and the model has an area under the curve of 0.84 (95% CI, 0.83-0.85). However, without the surgeon identifier, the area under the curve for a model that included only pretest risk was much lower at 0.72 (95% CI, 0.70-0.74). Overall, model 1 explained 37.6% of the total variability in the likelihood of the receipt of genetic testing: pretest risk of mutation explained approximately 20.3% of the variability in testing, and the surgeon identifier by itself explained approximately 17.3%. The odds of a patient receiving genetic testing would increase more than 2-fold (odds ratio, 2.48; 95% CI, 1.85-3.31) if she saw a surgeon with a genetic test ordering rate that was 1 SD above that of a surgeon with the mean test rate (independent of the patient’s pretest risk of mutation carriage). In model 2 (blue boxes), we added the surgeon volume and surgeon Tendency to Test Scale score. Relative to the highest-volume surgeons, patients seen by medium- and lower-volume surgeons were less likely to be tested. The odds of testing increased by 1.88 (95% CI, 1.49-2.38) for each 1-SD increase in the scale score. Cumulatively, the 2 surgeon-level variables included in the scale explained approximately one-third (34.1%) of the variation in testing associated with the surgeon. Model 3 (black boxes) shows the association of adding additional patient covariates (race/ethnicity, insurance status, and geographic site) that would not have any bearing on clinical indications for genetic testing but might reflect access to testing. Patients with no or public insurance or black race/ethnicity were less likely to get tested.
Figure 2. Estimated Odds Ratios for 3 Successive Multilevel Regression Models.
Model 1 (orange boxes) includes elevated pretest risk of mutation carriage and the surgeon identifier as the only variables. Model 2 (blue boxes) adds the surgeon volume and the Tendency to Test Scale score that measured “tendency to order genetic testing.” Model 3 (black boxes) adds sociodemographic characteristics likely to reflect access to testing of patients. All models include a surgeon identifier and quantify the amount of residual variation attributable to the surgeon (surgeon variation in testing) that remains after the inclusion of the variables in each respective model. The odds ratio listed for the surgeon represents the amount by which a patient’s odds of having a genetic test are multiplied if she sees a surgeon with an observed propensity to test that is 1 SD above the average surgeon’s (in other words, a surgeon in the 84th percentile as opposed to the 50th percentile for propensity to test). Emory indicates Emory University (Atlanta, Georgia); USC, University of Southern California (Los Angeles); and VA, Veterans Affairs.
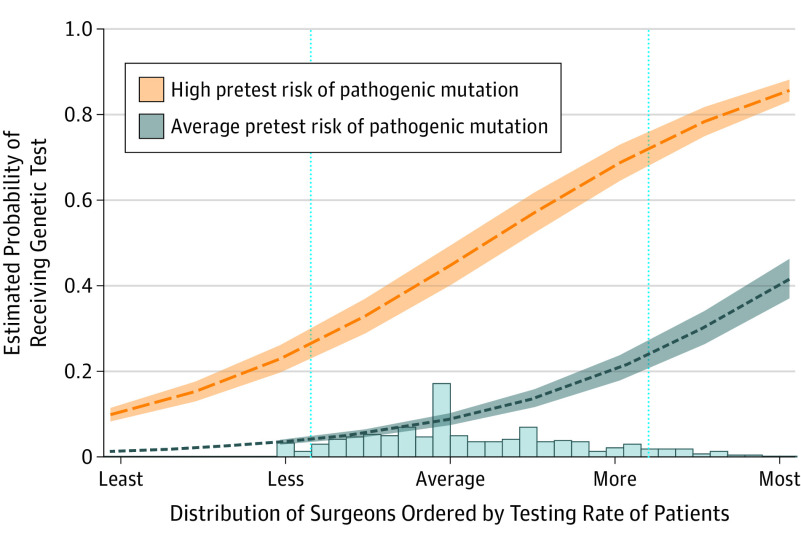
Figure 3 shows the estimated marginal probability of receiving genetic testing by patient pretest risk of mutation across different surgeons for the base model. The red band shows a change in testing probability for patients with high pretest risk that was associated with being seen by different surgeons, and the blue band shows this trend for patients with average pretest risk. The x-axis modifiers “Least” to “Most” refer to −2 SD or 2 SDs, respectively, from the mean (average) rate of testing observed for the surgeons. For a patient with a specified pretest risk (average or high), the surgeons on the left side of the horizontal scale were less likely to order testing than the surgeons on the right side. The histogram at the bottom of Figure 3 shows the distribution of the surgeons in our sample. If a patient with higher pretest risk saw a surgeon at the 5th percentile of the surgeon distribution, she would have a 26.3% (95% CI, 21.9%-31.2%) probability of undergoing testing compared with 72.3% (95% CI, 66.7%-77.2%) if she saw a surgeon at the 95th percentile. The probabilities for average-risk patients were 4.1% (95% CI, 2.9%-5.6%) and 23.8% (95% CI, 20.0%-27.9%), respectively, for the 5th and 95th percentiles.
Figure 3. Estimated Marginal Probability and Corresponding 95% CIs of the Receipt of Genetic Testing Across Different Surgeons.
The data are shown for patients with average (blue) and higher (orange) pretest risk of pathogenic mutation carriage. These estimates are from a model in which pretest risk is the only covariate. On the x-axis is the distribution of surgeons ordered by testing rate from those surgeons whose patients had the “least” to “most” testing, referring to −2 or 2 SDs on either side of the surgeon mean (average) rate of testing for the population. Blue vertical lines reference 5th and 95th percentiles of the observed surgeon distribution.
Discussion
In this contemporary population-based sample of patients with breast cancer, the individual attending surgeon explained a large amount of the variation in the receipt of germline genetic testing, almost equal to the amount explained by National Comprehensive Cancer Network guidelines–concordant clinical indications for formal genetic risk evaluation.11 Individual surgeon testing rates for patients with guideline-concordant indications varied from 26.3% for a surgeon at the 5th percentile below the average testing rate to 72.3% for a surgeon at the 95th percentile above the average testing rate. Surgeon attitudes about testing, as measured by the Tendency to Test Scale that we developed, explained a substantial amount of the association with testing.
At the surgeon level, we asked several other questions about testing. Surgeons’ confidence in discussing the pros and cons of testing markedly varied. In reference to genetic counseling, we found that a substantial minority of surgeons would order genetic testing without referral to a genetic counselor in response to a clinical vignette in which a patient was deemed by the surgeon respondent to be a candidate for genetic testing. In a second clinical vignette in which a surgeon did not recommend testing but the patient requests it, surgeons’ responses about test use and referral to genetic counseling also varied widely. Finally, we found that many surgeons reported that they rarely or never delay surgery to obtain genetic test results. Whether variation in these attitudes reflects surgeon or patient preference or clinical circumstances related to testing and choice of surgical procedure is unknown. Guidelines published after this study was conducted recommend pretest genetic counseling and acknowledge that the complexities in interpretation of modern panel testing require that providers (eg, laboratories, oncology physicians, and counselors) with particular expertise in cancer risk assessment be involved in their ordering and interpretation.5,15
In addition to surgeons’ attitudes, their annual patient volume explained some of the variation in use of testing. This may reflect that surgeons with greater specialization in breast cancer have more resources to offer testing, including timely access to genetic counselors. Patient factors, such as race/ethnicity and insurance status, were associated with testing, suggesting lingering disparities and access barriers. However, these barriers do not seem to be differentially distributed across surgeons because they explained a small amount of the surgeon variation when added to the model. Figure 2 shows the similar odds ratios for the surgeon association with testing of 1.88 vs 1.81.
We infer that the strong surgeon association with variation in testing reflects a lack of consensus regarding guidelines for testing and the approach to genetic risk evaluation of patients for whom testing is indicated. Patient volume had a strong association even after controlling for surgeons’ tendency to order testing: this may mean that volume is a proxy for testing access or might reflect surgeon location in multispecialty practice with other staff or specialists who recommend or implement testing. In the absence of further explanatory surgeon-level variables, we can only speculate about causes of the remaining variation, but it may reflect broader practice or practice style characteristics not captured in our Tendency to Test Scale score.
Strengths and Limitations
Strengths of our study include the following: a large, population-based, contemporary sample of patients who were surveyed shortly after initial surgical treatment; genetic test information linked directly from the testing laboratories; a valid measure of clinical indication for testing; almost complete identification of the attending surgeons and a high surgeon response rate; and surgeon measures relevant to clinical practice. However, some details of pretest risk of mutation carriage based on family history may have been missed. We could not measure directly whether attending surgeons appropriately recommended testing or counseling in a given patient encounter, although substantial gaps in the timing and receipt of genetic testing have been reported elsewhere.1 Other potential weaknesses include decay in the sample because of nonresponse of patients and surgeons, although our results were largely unchanged when we used multiple imputation to address missing surgeon data (eAppendix in the Supplement). We may have missed some testing in the linkage process. Because the results are from 2 states only, they may not generalize to the broader United States; however, the large size and diversity of the population in the regions studied are strengths.
Conclusions
Genetic risk evaluation of patients with breast cancer with higher risk of pathogenic mutation carriage is important because it informs both cancer treatment and risk reduction decisions for patients and targeted cancer risk reduction in relatives. The surge of genetic testing into cancer care is a major challenge for surgeons because there is legitimate uncertainty about its clinical utility, particularly with genes for which cancer risks are not well defined.16 For the few patients with pathogenic mutations, integrating the risk of future cancers into management of the current cancer is nontrivial.17 In addition, the replacement of testing based only on BRCA1 and BRCA2 with a multigene panel has markedly increased the proportion of patients with a variant of unknown significance result that foments uncertainty for patients and physicians. However, timely presurgical genetic counseling is increasingly hard to obtain as demand rapidly outpaces supply.18 These factors underscore the wide variability that we observed in surgeon attitudes about the role of genetic testing and counseling after cancer diagnosis. Our results highlight the need for greater outreach to surgeons in the community to build consensus about approaches to genetic risk evaluation and results management for patients with breast cancer, particularly as evidence emerges about the utility of genetic testing in clinical and demographic subgroups.
eFigure 1. Survey Sampling
eAppendix. Replication of Figure 2 Model (in Manuscript) That Estimated ORs for 3 Successive Multilevel Logistic Regression Models (MI)
eFigure 2. Distribution of Clinical Factors That Define Pretest Risk and Prevalence of Test by Different Groups of Patients
References
- 1.Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232-2239. doi: 10.1200/JCO.2016.71.6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurian AW, Griffith KA, Hamilton AS, et al. Genetic testing and counseling among patients with newly diagnosed breast cancer. JAMA. 2017;317(5):531-534. doi: 10.1001/jama.2016.16918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically-indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi: 10.1200/JCO.2017.76.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurian AW, Ward KC, Hamilton AS, et al. Uptake, results, and outcomes of germline multiple-gene sequencing after diagnosis of breast cancer. JAMA Oncol. 2018. doi: 10.1001/jamaoncol.2018.0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly MB, Pilarski R, Berry M, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J Natl Compr Canc Netw. 2017;15(1):9-20. doi: 10.6004/jnccn.2017.0003 [DOI] [PubMed] [Google Scholar]
- 6.Kurian AW, Friese CR, Bondarenko I, et al. Second opinions from medical oncologists for early-stage breast cancer: prevalence, correlates, and consequences. JAMA Oncol. 2017;3(3):391-397. doi: 10.1001/jamaoncol.2016.5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurian AW, Bondarenko I, Jagsi R, et al. Recent trends in chemotherapy use and oncologists’ treatment recommendations for early-stage breast cancer. J Natl Cancer Inst. 2018;110(5):493-500. doi: 10.1093/jnci/djx239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrow M, Abrahamse P, Hofer TP, et al. Trends in reoperation after initial lumpectomy for breast cancer: addressing overtreatment in surgical management. JAMA Oncol. 2017;3(10):1352-1357. doi: 10.1001/jamaoncol.2017.0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz SJ, Hawley ST, Hamilton AS, et al. Surgeon influence on variation in receipt of contralateral prophylactic mastectomy for women with breast cancer. JAMA Surg. 2017. doi: 10.1001/jamasurg.2017.0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services. Guidance regarding methods for de-identification of protected health information in accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html#. Published 2015. Accessed July 24, 2017.
- 11.Daly MB, Pilarski R, Axilbund JE, et al. Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2015. J Natl Compr Canc Netw. 2016;14(2):153-162. doi: 10.6004/jnccn.2016.0018 [DOI] [PubMed] [Google Scholar]
- 12.Lord FM. Applications of Item Response Theory to Practical Testing Problems. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 1980. [Google Scholar]
- 13.Mellenbergh GJ. Generalized linear item response theory. Psychol Bull. 1994;15:300-307. doi: 10.1037/0033-2909.115.2.300 [DOI] [Google Scholar]
- 14.Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. 2nd ed. London, England: Sage Publishers; 2012. [Google Scholar]
- 15.Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660-3667. doi: 10.1200/JCO.2015.63.0996 [DOI] [PubMed] [Google Scholar]
- 16.Kurian AW, Ford JM. Multigene panel testing in oncology practice: how should we respond? JAMA Oncol. 2015;1(3):277-278. doi: 10.1001/jamaoncol.2015.28 [DOI] [PubMed] [Google Scholar]
- 17.Katz SJ, Kurian AW, Morrow M. Treatment decision making and genetic testing for breast cancer: mainstreaming mutations. JAMA. 2015;314(10):997-998. doi: 10.1001/jama.2015.8088 [DOI] [PubMed] [Google Scholar]
- 18.Delikurt T, Williamson GR, Anastasiadou V, Skirton H. A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet. 2015;23(6):739-745. doi: 10.1038/ejhg.2014.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Survey Sampling
eAppendix. Replication of Figure 2 Model (in Manuscript) That Estimated ORs for 3 Successive Multilevel Logistic Regression Models (MI)
eFigure 2. Distribution of Clinical Factors That Define Pretest Risk and Prevalence of Test by Different Groups of Patients